Relationships between Follicle-Stimulating Hormone and Adiponectin in Postmenopausal Women
Abstract
:1. Introduction
2. Results
2.1. Demographic, Clinic, and Laboratory Data
2.2. Correlation between FSH, BMI, and the HOMA-IR Values
2.3. Multivariate Linear Regression Analyzes to Evaluate the Variables Associated with FSH
3. Discussion
4. Materials and Methods
4.1. Participants and Study Design
4.2. Anthropometric Measures
4.3. Measurement of Insulin and Adipocyte-Derived Hormones
4.4. Measurement of FSH, E2, and Biochemical Measures
4.5. Stastical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barbieri, R.L. The endocrinology of the menstrual cycle. Methods Mol. Biol. 2014, 1154, 145–169. [Google Scholar] [CrossRef]
- Ulloa-Aguirre, A.; Reiter, E.; Crepieux, P. FSH Receptor Signaling: Complexity of Interactions and Signal Diversity. Endocrinology 2018, 159, 3020–3035. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Ji, Y.; Yuen, T.; Rendina-Ruedy, E.; DeMambro, V.E.; Dhawan, S.; Abu-Amer, W.; Izadmehr, S.; Zhou, B.; Shin, A.C.; et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature 2017, 546, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Liu, P.; Yuen, T.; Haider, S.; He, J.; Romero, R.; Chen, H.; Bloch, M.; Kim, S.M.; Lizneva, D.; et al. Epitope-specific monoclonal antibodies to FSHbeta increase bone mass. Proc. Natl. Acad. Sci. USA 2018, 115, 2192–2197. [Google Scholar] [CrossRef] [Green Version]
- Lenton, E.A.; Sexton, L.; Lee, S.; Cooke, I.D. Progressive changes in LH and FSH and LH: FSH ratio in women throughout reproductive life. Maturitas 1988, 10, 35–43. [Google Scholar] [CrossRef]
- Agrinier, N.; Cournot, M.; Dallongeville, J.; Arveiler, D.; Ducimetiere, P.; Ruidavets, J.B.; Ferrieres, J. Menopause and modifiable coronary heart disease risk factors: A population based study. Maturitas 2010, 65, 237–243. [Google Scholar] [CrossRef]
- Perez-Lopez, F.R.; Chedraui, P.; Gilbert, J.J.; Perez-Roncero, G. Cardiovascular risk in menopausal women and prevalent related co-morbid conditions: Facing the post-Women’s Health Initiative era. Fertil. Steril. 2009, 92, 1171–1186. [Google Scholar] [CrossRef] [PubMed]
- Lizneva, D.; Rahimova, A.; Kim, S.M.; Atabiekov, I.; Javaid, S.; Alamoush, B.; Taneja, C.; Khan, A.; Sun, L.; Azziz, R.; et al. FSH Beyond Fertility. Front. Endocrinol. 2019, 10, 136. [Google Scholar] [CrossRef]
- Randolph, J.F., Jr.; Sowers, M.; Gold, E.B.; Mohr, B.A.; Luborsky, J.; Santoro, N.; McConnell, D.S.; Finkelstein, J.S.; Korenman, S.G.; Matthews, K.A.; et al. Reproductive hormones in the early menopausal transition: Relationship to ethnicity, body size, and menopausal status. J. Clin. Endocrinol. Metab. 2003, 88, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Stefanska, A.; Cembrowska, P.; Kubacka, J.; Kuligowska-Prusinska, M.; Sypniewska, G. Gonadotropins and Their Association with the Risk of Prediabetes and Type 2 Diabetes in Middle-Aged Postmenopausal Women. Dis. Markers 2019, 2019, 2384069. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Kuang, L.; Han, B.; Li, Q.; Chen, Y.; Zhu, C.; Chen, Y.; Xia, F.; Cang, Z.; Zhu, C.; et al. Follicle-stimulating hormone associates with prediabetes and diabetes in postmenopausal women. Acta Diabetol. 2016, 53, 227–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, H.; Pessin, J.E. Adipokines mediate inflammation and insulin resistance. Front. Endocrinol. 2013, 4, 71. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Jaramillo, P.; Gomez-Arbelaez, D.; Lopez-Lopez, J.; Lopez-Lopez, C.; Martinez-Ortega, J.; Gomez-Rodriguez, A.; Triana-Cubillos, S. The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm. Mol. Biol. Clin. Investig. 2014, 18, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Sowers, M.R.; Wildman, R.P.; Mancuso, P.; Eyvazzadeh, A.D.; Karvonen-Gutierrez, C.A.; Rillamas-Sun, E.; Jannausch, M.L. Change in adipocytokines and ghrelin with menopause. Maturitas 2008, 59, 149–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Zazzo, E.; Polito, R.; Bartollino, S.; Nigro, E.; Porcile, C.; Bianco, A.; Daniele, A.; Moncharmont, B. Adiponectin as Link Factor between Adipose Tissue and Cancer. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polito, R.; Nigro, E.; Fei, L.; de Magistris, L.; Monaco, M.L.; D’Amico, R.; Naviglio, S.; Signoriello, G.; Daniele, A. Adiponectin Is Inversely Associated With Tumour Grade in Colorectal Cancer Patients. Anticancer. Res. 2020, 40, 3751–3757. [Google Scholar] [CrossRef]
- Ye, J.; Jia, J.; Dong, S.; Zhang, C.; Yu, S.; Li, L.; Mao, C.; Wang, D.; Chen, J.; Yuan, G. Circulating adiponectin levels and the risk of breast cancer: A meta-analysis. Eur. J. Cancer Prev. 2014, 23, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shin, H.J.; Ding, E.L.; van Dam, R.M. Adiponectin levels and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 2009, 302, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Park, S.K.; Harlow, S.D.; Zheng, H.; Karvonen-Gutierrez, C.; Thurston, R.C.; Ruppert, K.; Janssen, I.; Randolph, J.F., Jr. Association between changes in oestradiol and follicle-stimulating hormone levels during the menopausal transition and risk of diabetes. Diabet. Med. 2017, 34, 531–538. [Google Scholar] [CrossRef]
- Zaidi, M.; Lizneva, D.; Kim, S.M.; Sun, L.; Iqbal, J.; New, M.I.; Rosen, C.J.; Yuen, T. FSH, Bone Mass, Body Fat, and Biological Aging. Endocrinology 2018, 159, 3503–3514. [Google Scholar] [CrossRef] [Green Version]
- Sowers, M.; Zheng, H.; Tomey, K.; Karvonen-Gutierrez, C.; Jannausch, M.; Li, X.; Yosef, M.; Symons, J. Changes in body composition in women over six years at midlife: Ovarian and chronological aging. J. Clin. Endocrinol. Metab. 2007, 92, 895–901. [Google Scholar] [CrossRef] [Green Version]
- Seth, B.; Arora, S.; Singh, R. Association of obesity with hormonal imbalance in infertility: A cross-sectional study in north Indian women. Indian J. Clin. Biochem. 2013, 28, 342–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gourlay, M.L.; Specker, B.L.; Li, C.; Hammett-Stabler, C.A.; Renner, J.B.; Rubin, J.E. Follicle-stimulating hormone is independently associated with lean mass but not BMD in younger postmenopausal women. Bone 2012, 50, 311–316. [Google Scholar] [CrossRef] [Green Version]
- Ecochard, R.; Marret, H.; Barbato, M.; Boehringer, H. Gonadotropin and body mass index: High FSH levels in lean, normally cycling women. Obstet. Gynecol. 2000, 96, 8–12. [Google Scholar] [CrossRef]
- Caillon, H.; Freour, T.; Bach-Ngohou, K.; Colombel, A.; Denis, M.G.; Barriere, P.; Masson, D. Effects of female increased body mass index on in vitro fertilization cycles outcome. Obes. Res. Clin. Pract. 2015, 9, 382–388. [Google Scholar] [CrossRef] [PubMed]
- De Pergola, G.; Maldera, S.; Tartagni, M.; Pannacciulli, N.; Loverro, G.; Giorgino, R. Inhibitory effect of obesity on gonadotropin, estradiol, and inhibin B levels in fertile women. Obesity 2006, 14, 1954–1960. [Google Scholar] [CrossRef] [Green Version]
- Tepper, P.G.; Randolph, J.F., Jr.; McConnell, D.S.; Crawford, S.L.; El Khoudary, S.R.; Joffe, H.; Gold, E.B.; Zheng, H.; Bromberger, J.T.; Sutton-Tyrrell, K. Trajectory clustering of estradiol and follicle-stimulating hormone during the menopausal transition among women in the Study of Women’s Health across the Nation (SWAN). J. Clin. Endocrinol. Metab. 2012, 97, 2872–2880. [Google Scholar] [CrossRef] [PubMed]
- Ausmanas, M.K.; Tan, D.A.; Jaisamrarn, U.; Tian, X.W.; Holinka, C.F. Estradiol, FSH and LH profiles in nine ethnic groups of postmenopausal Asian women: The Pan-Asia Menopause (PAM) study. Climacteric 2007, 10, 427–437. [Google Scholar] [CrossRef]
- Simoncig Netjasov, A.; Tancic-Gajic, M.; Ivovic, M.; Marina, L.; Arizanovic, Z.; Vujovic, S. Influence of obesity and hormone disturbances on sexuality of women in the menopause. Gynecol. Endocrinol. 2016, 32, 762–766. [Google Scholar] [CrossRef]
- Karvonen-Gutierrez, C.; Kim, C. Association of Mid-Life Changes in Body Size, Body Composition and Obesity Status with the Menopausal Transition. Healthcare 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Jull, J.; Stacey, D.; Beach, S.; Dumas, A.; Strychar, I.; Ufholz, L.A.; Prince, S.; Abdulnour, J.; Prud’homme, D. Lifestyle interventions targeting body weight changes during the menopause transition: A systematic review. J. Obes. 2014, 2014, 824310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapoor, E.; Collazo-Clavell, M.L.; Faubion, S.S. Weight Gain in Women at Midlife: A Concise Review of the Pathophysiology and Strategies for Management. Mayo. Clin. Proc. 2017, 92, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- De Vos, F.Y.; van Laarhoven, H.W.; Laven, J.S.; Themmen, A.P.; Beex, L.V.; Sweep, C.G.; Seynaeve, C.; Jager, A. Menopausal status and adjuvant hormonal therapy for breast cancer patients: A practical guideline. Crit. Rev. Oncol. Hematol. 2012, 84, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Rossi, E.; Morabito, A.; Di Rella, F.; Esposito, G.; Gravina, A.; Labonia, V.; Landi, G.; Nuzzo, F.; Pacilio, C.; De Maio, E.; et al. Endocrine effects of adjuvant letrozole compared with tamoxifen in hormone-responsive postmenopausal patients with early breast cancer: The HOBOE trial. J. Clin. Oncol. 2009, 27, 3192–3197. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.C.; Stewart, R.B.; Banerji, M.A.; Gordon, D.H.; Kral, J.G. Relationships between tamoxifen use, liver fat and body fat distribution in women with breast cancer. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 296–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hesselbarth, N.; Pettinelli, C.; Gericke, M.; Berger, C.; Kunath, A.; Stumvoll, M.; Bluher, M.; Kloting, N. Tamoxifen affects glucose and lipid metabolism parameters, causes browning of subcutaneous adipose tissue and transient body composition changes in C57BL/6NTac mice. Biochem. Biophys. Res. Commun. 2015, 464, 724–729. [Google Scholar] [CrossRef]
- Sheean, P.M.; Hoskins, K.; Stolley, M. Body composition changes in females treated for breast cancer: A review of the evidence. Breast Cancer Res. Treat. 2012, 135, 663–680. [Google Scholar] [CrossRef] [Green Version]
- Karim, R.; Stanczyk, F.Z.; Brinton, R.D.; Rettberg, J.; Hodis, H.N.; Mack, W.J. Association of endogenous sex hormones with adipokines and ghrelin in postmenopausal women. J. Clin. Endocrinol. Metab. 2015, 100, 508–515. [Google Scholar] [CrossRef]
- Mankowska, A.; Nowak, L.; Sypniewska, G. Adiponectin and Metabolic Syndrome in Women at Menopause. EJIFCC 2009, 19, 173–184. [Google Scholar]
- Liu, X.M.; Chan, H.C.; Ding, G.L.; Cai, J.; Song, Y.; Wang, T.T.; Zhang, D.; Chen, H.; Yu, M.K.; Wu, Y.T.; et al. FSH regulates fat accumulation and redistribution in aging through the Galphai/Ca(2+)/CREB pathway. Aging Cell 2015, 14, 409–420. [Google Scholar] [CrossRef]
- Cui, H.; Zhao, G.; Liu, R.; Zheng, M.; Chen, J.; Wen, J. FSH stimulates lipid biosynthesis in chicken adipose tissue by upregulating the expression of its receptor FSHR. J. Lipid. Res. 2012, 53, 909–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.C.; Chung, Y.F.; Yeh, Y.T.; Chaung, H.C.; Kuo, F.C.; Fu, O.Y.; Chen, H.Y.; Hou, M.F.; Yuan, S.S. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 2006, 237, 109–114. [Google Scholar] [CrossRef]
- Sattar, N.; Wannamethee, S.G.; Forouhi, N.G. Novel biochemical risk factors for type 2 diabetes: Pathogenic insights or prediction possibilities? Diabetologia 2008, 51, 926–940. [Google Scholar] [CrossRef] [PubMed]
- Finucane, F.M.; Luan, J.; Wareham, N.J.; Sharp, S.J.; O’Rahilly, S.; Balkau, B.; Flyvbjerg, A.; Walker, M.; Hojlund, K.; Nolan, J.J.; et al. Correlation of the leptin:adiponectin ratio with measures of insulin resistance in non-diabetic individuals. Diabetologia 2009, 52, 2345–2349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fruhbeck, G.; Catalan, V.; Rodriguez, A.; Gomez-Ambrosi, J. Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte 2018, 7, 57–62. [Google Scholar] [CrossRef]
- Huang, W.Y.; Chang, C.C.; Chen, D.R.; Kor, C.T.; Chen, T.Y.; Wu, H.M. Circulating leptin and adiponectin are associated with insulin resistance in healthy postmenopausal women with hot flashes. PLoS ONE 2017, 12, e0176430. [Google Scholar] [CrossRef]


| Variables | Non-BrCa | BrCa | BrCa-Gn | pa Value | pb Value | pc Value |
|---|---|---|---|---|---|---|
| n | 261 | 88 | 59 | |||
| Age, years | 54 (52, 57) | 52 (47, 57) | 46 (43, 49) | <0.001 | <0.001 | <0.001 |
| MP_duration, years | 4 (1, 8) | 3 (2, 7) | 2 (1, 3) | <0.001 | 0.924 | <0.001 |
| SBP, mmHg | 115 (96, 118) | 114 (95, 116) | 112 (94, 109) | 0.212 | 0.287 | 0.074 |
| DBP, mmHg | 71 (65, 76) | 72 (63, 75) | 70 (62, 73) | 0.465 | 0.612 | 0.435 |
| BMI, kg/m2 | 23.1 (21.6, 25.5) | 23(21.05, 24.95) | 23.9 (21.5, 26.1) | 0.290 | 0.320 | 0.138 |
| Fasting glucose, mg/dL | 95 (90, 101) | 93.5 (88, 101) | 91 (86, 95) | 0.001 | 0.249 | 0.022 |
| Insulin, pg/mL | 270.3 (149, 426) | 376.5 (231, 510) | 323.5 (178, 572) | 0.001 | <0.001 | 0.661 |
| Hemoglobin A1c, % | 5.5 (5.3, 5.7) | 5.55 (5.2, 5.8) | 5.4 (5.2, 5.6) | 0.113 | 0.674 | 0.066 |
| FSH, mIU/mL | 67.9 (54, 80.4) | 36.17 (26.8, 56.4) | 3.19 (1.8, 5.9) | <0.001 | <0.001 | <0.001 |
| Estradiol, pg/mL | 20 (20, 20) | 20 (20, 20) | 20 (20, 20) | 0.508 | 0.315 | 0.993 |
| Total cholesterol, mg/dL | 206 (186, 234) | 193 (167, 216) | 180 (160, 206) | <0.001 | <0.001 | 0.054 |
| Triglyceride, mg/dL | 94.5 (67, 126) | 103.5 (74, 164) | 114 (79, 150) | 0.007 | 0.010 | 0.882 |
| HDL cholesterol, mg/dL | 57 (49, 68) | 52 (43, 64) | 52 (46, 61) | 0.008 | 0.014 | 0.958 |
| LDL cholesterol, mg/dL | 124.6 (104, 152) | 108.4 (82, 127) | 97.4 (82, 124) | <0.001 | <0.001 | 0.392 |
| Adiponectin, ug/mL | 30.6 (16.4, 45.6) | 23.7 (13.2, 39.4) | 17.8 (11.5, 31.4) | <0.001 | 0.049 | 0.048 |
| Resistin, ng/mL | 22.0 (16.1, 33.7) | 19.6 (16.2, 24.9) | 18.6 (13.5, 28.3) | 0.014 | 0.038 | 0.421 |
| Leptin, ng/mL | 7.7 (4.8, 14.1) | 10.9 (6.9, 15.7) | 12.7 (6.9, 22.5) | <0.001 | 0.003 | 0.222 |
| Leptin to Adiponectin ratio | 0.28 (0.14, 0.71) | 0.48 (0.25, 1.05) | 0.78 (0.27, 1.358) | <0.001 | 0.002 | 0.138 |
| HOMA-IR | 1.04 (0.53, 1.61) | 1.39 (0.79, 2.01) | 1.17 (0.61, 2.18) | 0.004 | 0.002 | 0.511 |
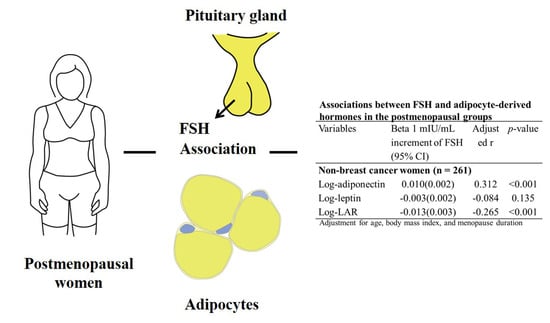
| Variables | Beta 1 mIU/mL Increment of FSH (95% CI) | Adjusted r | p-Value |
|---|---|---|---|
| Non-breast cancer women (n = 261) | |||
| Log-adiponectin | 0.010 (0.002) | 0.312 | <0.001 |
| Log-leptin | −0.003 (0.002) | −0.084 | 0.135 |
| Log-LAR | −0.013 (0.003) | −0.265 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-Y.; Chen, D.-R.; Kor, C.-T.; Chen, T.-Y.; Lin, P.-T.; Tseng, J.T.C.; Wu, H.-M. Relationships between Follicle-Stimulating Hormone and Adiponectin in Postmenopausal Women. Metabolites 2020, 10, 420. https://doi.org/10.3390/metabo10100420
Huang W-Y, Chen D-R, Kor C-T, Chen T-Y, Lin P-T, Tseng JTC, Wu H-M. Relationships between Follicle-Stimulating Hormone and Adiponectin in Postmenopausal Women. Metabolites. 2020; 10(10):420. https://doi.org/10.3390/metabo10100420
Chicago/Turabian StyleHuang, Wan-Yu, Dar-Ren Chen, Chew-Teng Kor, Ting-Yu Chen, Po-Te Lin, Joseph Ta Chien Tseng, and Hung-Ming Wu. 2020. "Relationships between Follicle-Stimulating Hormone and Adiponectin in Postmenopausal Women" Metabolites 10, no. 10: 420. https://doi.org/10.3390/metabo10100420