Serum Metabonomic Research of the Anti-Hypertensive Effects of Ogaja on Spontaneously Hypertensive Rats
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Extraction of Acanthopanax Sessiliflorus Fruits
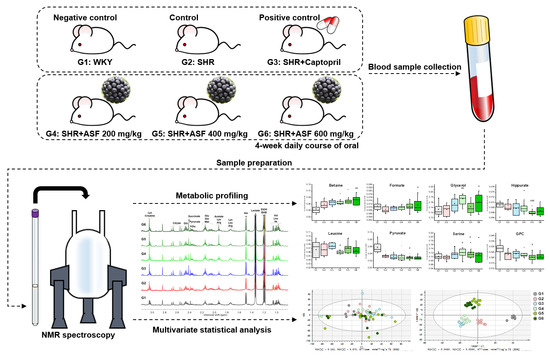
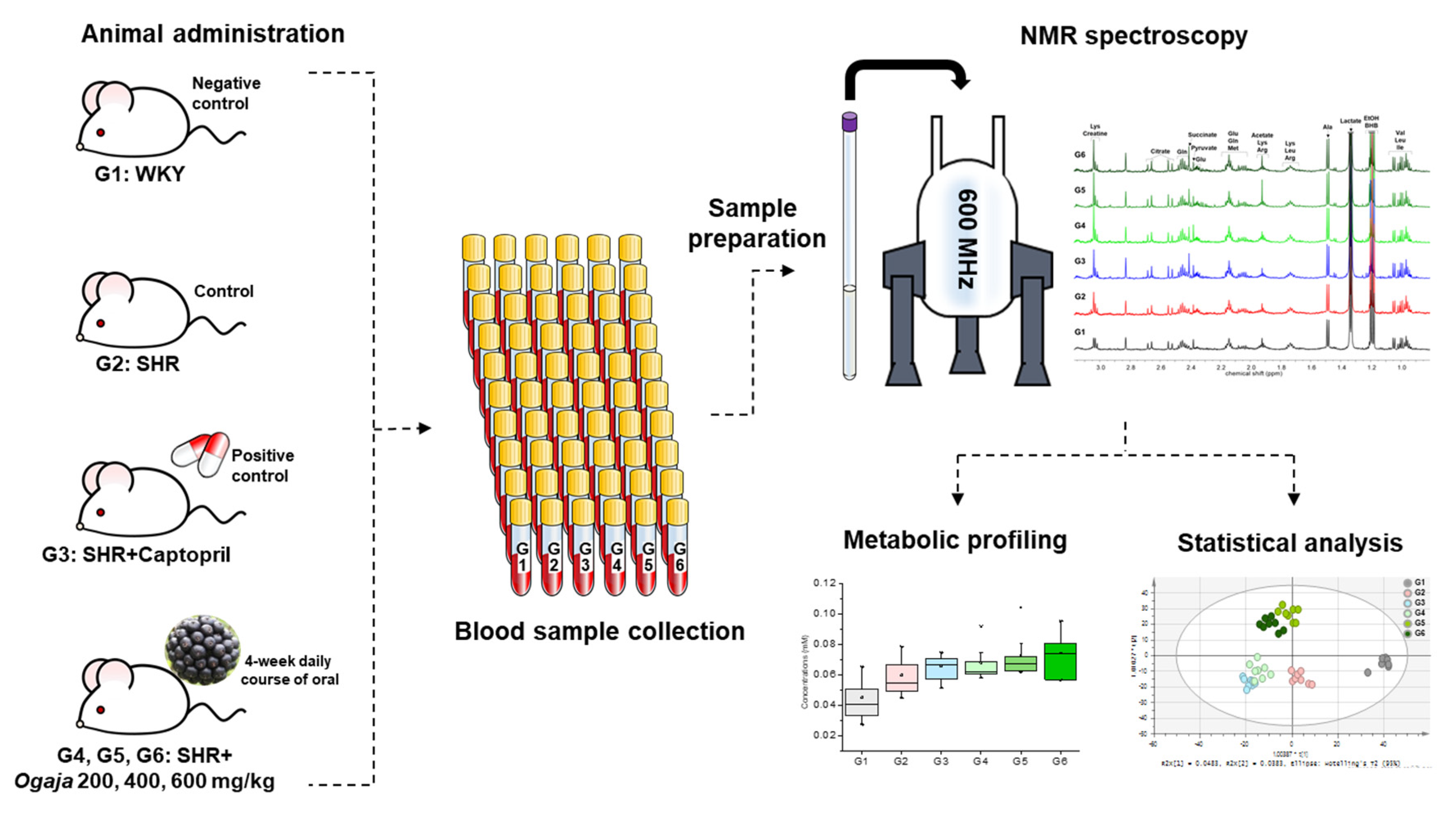
4.2. Animal Administration
4.3. Sample Preparation
4.4. NMR Data Acquisition and Data Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Noto, A.; Fanos, V.; Dessi, A. Metabolomics in newborns. In Advances in Clinical Chemistry; Elsevier: London, UK, 2016; Volume 74, pp. 35–61. [Google Scholar]
- McHugh, C.E.; Flott, T.L.; Schooff, C.R.; Smiley, Z.; Puskarich, M.A.; Myers, D.D.; Younger, J.G.; Jones, A.E.; Stringer, K.A. Rapid, Reproducible, Quantifiable NMR Metabolomics: Methanol and Methanol: Chloroform Precipitation for Removal of Macromolecules in Serum and Whole Blood. Metabolites 2018, 8, 93. [Google Scholar] [CrossRef]
- Huang, W.Y.; Davidge, S.T.; Wu, J. Bioactive natural constituents from food sources—Potential use in hypertension prevention and treatment. Crit. Rev. Food Sci. 2013, 53, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.A.; Schiffrin, E.L.; White, W.B.; Mann, S.; Lindholm, L.H.; Kenerson, J.G.; Flack, J.M.; Carter, B.L.; Materson, B.J.; Ram, C.V.S.; et al. Clinical practice guidelines for the management of hypertension in the community: A statement by the American Society of Hypertension and the International Society of Hypertension. J. Clin. Hyertens. 2014, 16, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, B.K.; Cho, S.H.; Shin, K.H. Phytochemical constituents from the fruits of Acanthopanax sessiliflorus. Arch. Pharm. Res. 2002, 25, 280. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Food and Drug Safety (KR). Food Public Code; Ministry of Food and Drug Safety: Cheongju, Korea, 2018.
- Yang, C.; An, Q.; Xiong, Z.; Song, Y.; Yu, K.; Li, F. Triterpenes from Acanthopanax sessiliflorus fruits and their antiplatelet aggregation activities. Planta Med. 2009, 75, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Seo, K.H.; Jeong, R.H.; Lee, S.M.; Kim, G.S.; Noh, H.J.; Kim, S.Y.; Kim, G.W.; Kim, J.Y.; Baek, N.I. Anti-inflammatory lignans from the fruits of Acanthopanax sessiliflorus. Molecules 2013, 18, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, Y.S.; Jung, S.H.; Ji, J.; Shin, K.H.; Kim, B.K.; Kang, S.S. Antitumor and immunostimulating activities of Acanthopanax sessiliflorus fruits. Nat. Prod. Sci. 2003, 9, 112–116. [Google Scholar]
- Jung, I.H.; Kim, S.E.; Lee, Y.G.; Kim, D.H.; Kim, H.; Kim, G.S.; Baek, N.I.; Lee, D.Y. Antihypertensive effect of ethanolic extract from Acanthopanax sessiliflorus fruits and quality control of active compounds. Oxid. Med. Cell. Longev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Trippodo, N.C.; Frohlich, E.D. Similarities of genetic (spontaneous) hypertension. Man and rat. Circ. Res. 1981, 48, 309–319. [Google Scholar] [CrossRef]
- Akira, K.; Masu, S.; Imachi, M.; Mitome, H.; Hashimoto, M.; Hashimoto, T. 1H NMR-based metabonomic analysis of urine from young spontaneously hypertensive rats. J. Pharm. Biomed. 2008, 46, 550–556. [Google Scholar] [CrossRef]
- Kim, J.K.; Choi, S.R.; Lee, J.; Park, S.Y.; Song, S.Y.; Na, J.; Kim, S.W.; Kim, S.J.; Nou, I.S.; Lee, Y.H.; et al. Metabolic differentiation of diamondback moth (Plutella xylostella (L.)) resistance in cabbage (Brassica oleracea L. ssp. capitata). J. Agric. Food Chem. 2013, 61, 11222–11230. [Google Scholar] [CrossRef]
- Wiklund, S.; Johansson, E.; Sjöström, L.; Mellerowicz, E.J.; Edlund, U.; Shockcor, J.P.; Gottfries, J.; Moritz, T.; Trygg, J. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal. Chem. 2008, 80, 115–122. [Google Scholar] [CrossRef]
- Triba, M.N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P.; Rutledge, D.N.; Savarin, P. PLS/OPLS models in metabolomics: The impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Mol. Biosyst. 2015, 11, 13–19. [Google Scholar] [CrossRef]
- Qu, Y.Y.; Hui, J.; Wang, L.M.; Tang, N.; Zhong, H.; Liu, Y.M.; Li, Z.; Feng, Q.; He, F. Reduced Expression of the Extracellular Calcium-Sensing Receptor (CaSR) Is Associated with Activation of the Renin-Angiotensin System (RAS) to Promote Vascular Remodeling in the Pathogenesis of Essential Hypertension. PLoS ONE 2016, 11, e0157456. [Google Scholar] [CrossRef]
- Babior, B.M. NADPH oxidase: An update. Blood 1999, 93, 1464–1476. [Google Scholar] [CrossRef]
- Griendling, K.K.; Sorescu, D.; Ushio-Fukai, M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ. Res. 2000, 86, 494–501. [Google Scholar] [CrossRef]
- DeLano, F.A.; Schmid-Schönbein, G.W. Proteinase activity and receptor cleavage: Mechanism for insulin resistance in the spontaneously hypertensive rat. Hypertension 2008, 52, 415–423. [Google Scholar] [CrossRef][Green Version]
- Lee, J.W.; Baek, N.I.; Lee, D.Y. Inhibitory Effects of seco-Triterpenoids from Acanthopanax sessiliflorus Fruits on HUVEC Invasion and ACE Activity. Nat. Prod. Commun. 2015, 10, 1517–1520. [Google Scholar] [CrossRef]
- Vargas, S.L.; Toma, I.; Kang, J.J.; Meer, E.J.; Peti-Peterdi, J. Activation of the succinate receptor GPR91 in macula densa cells causes renin release. J. Am. Soc. Nephrol. 2009, 20, 1002–1011. [Google Scholar] [CrossRef]
- He, W.; Miao, F.J.P.; Lin, D.C.H.; Schwandner, R.T.; Wang, Z.; Gao, J.; Chen, J.L.; Tian, H.; Ling, L. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature 2004, 429, 188–193. [Google Scholar] [CrossRef]
- Nikolic, S.B.; Sharman, J.E.; Adams, M.J.; Edwards, L.M. Metabolomics in hypertension. J. Hypertens. 2014, 32, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, M.; Liu, W.; Li, X.; Chu, H.; Bai, Y.; Sun, Z.; Gao, C.; Zheng, L.; Yang, J. Association of betaine with blood pressure in dialysis patients. J. Clin. Hypertens. 2018, 20, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Schwab, U.; Törrönen, A.; Toppinen, L.; Alfthan, G.; Saarinen, M.; Aro, A.; Uusitupa, M. Betaine supplementation decreases plasma homocysteine concentrations but does not affect body weight, body composition, or resting energy expenditure in human subjects. Am. J. Clin. Nutr. 2002, 76, 961–967. [Google Scholar] [CrossRef]
- Skeete, J.; DiPette, D.J. Relationship between homocysteine and hypertension: New data add to the debate. J. Clin. Hypertens. 2017, 19, 1171–1172. [Google Scholar] [CrossRef] [PubMed]
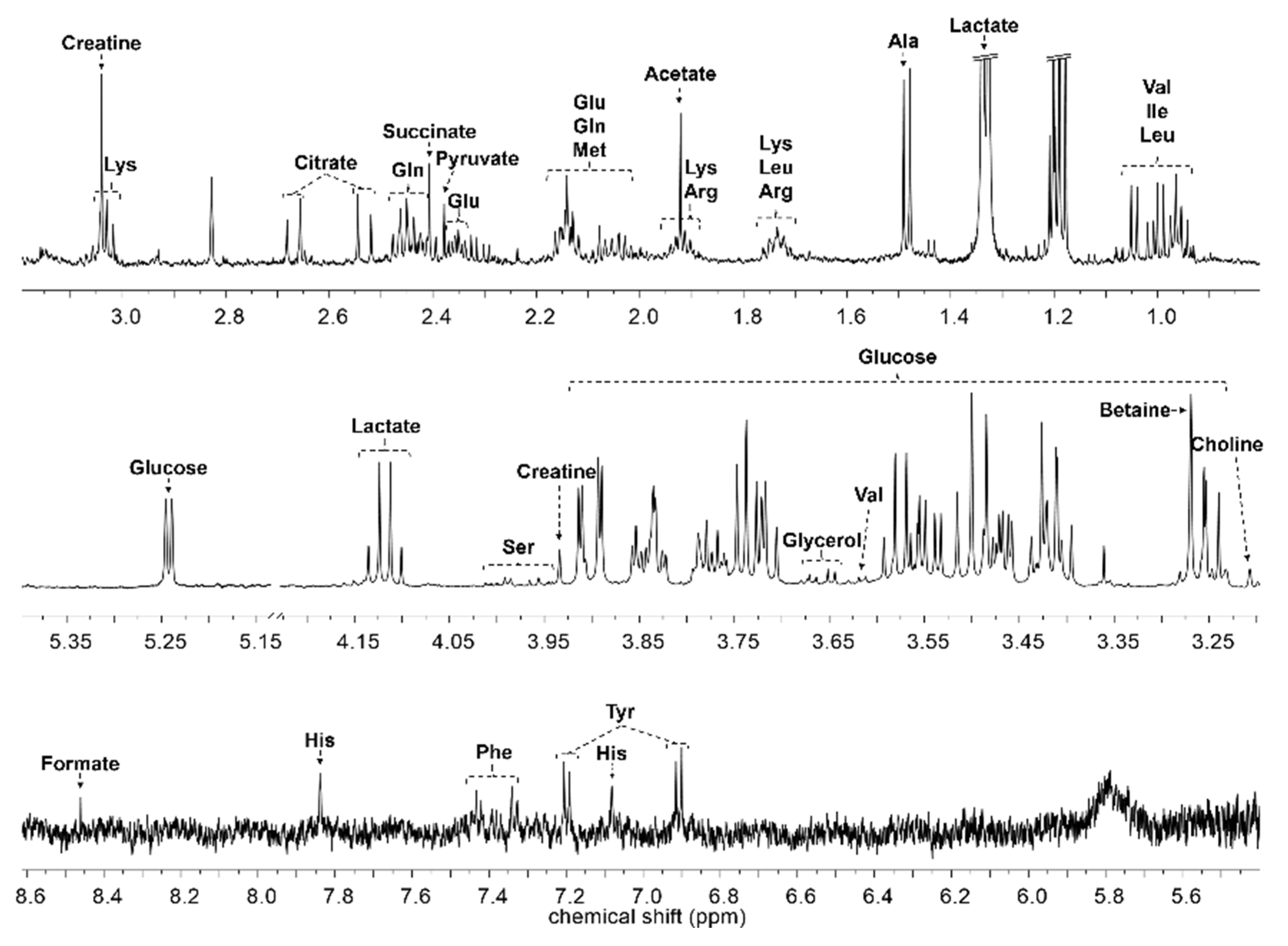
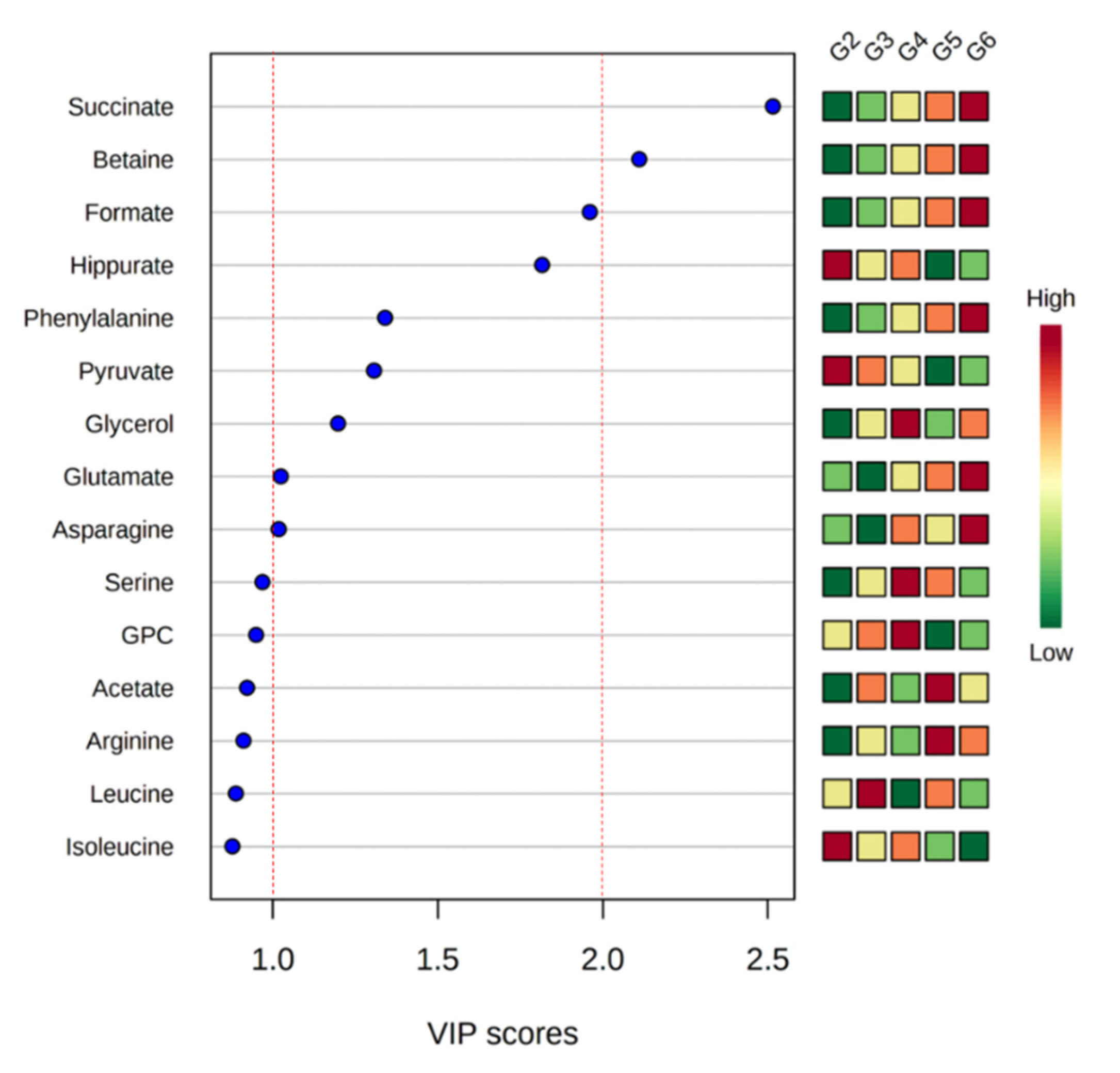
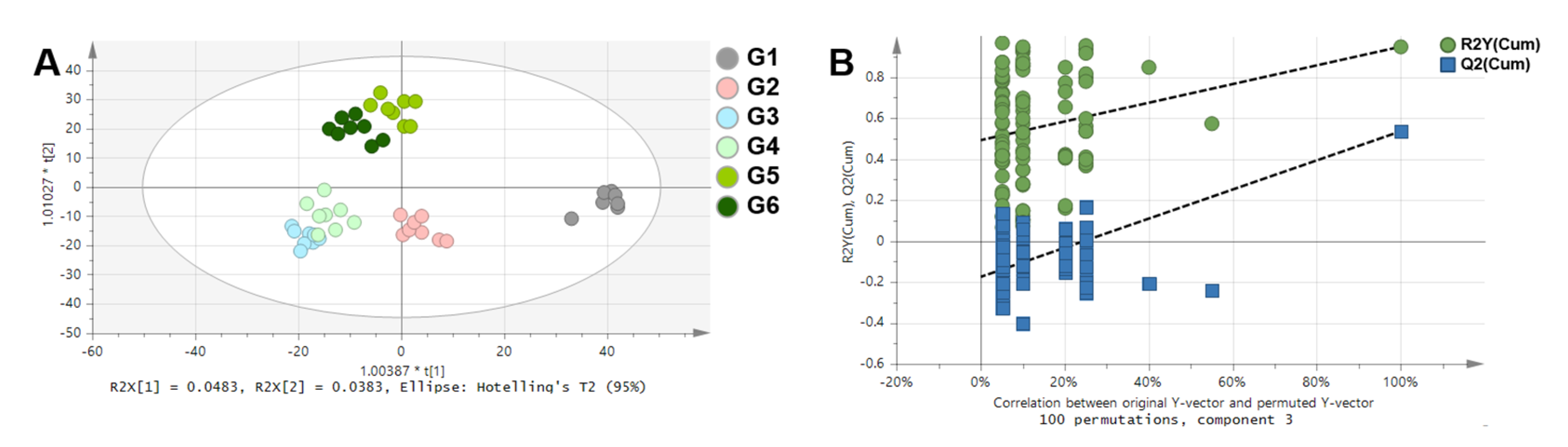
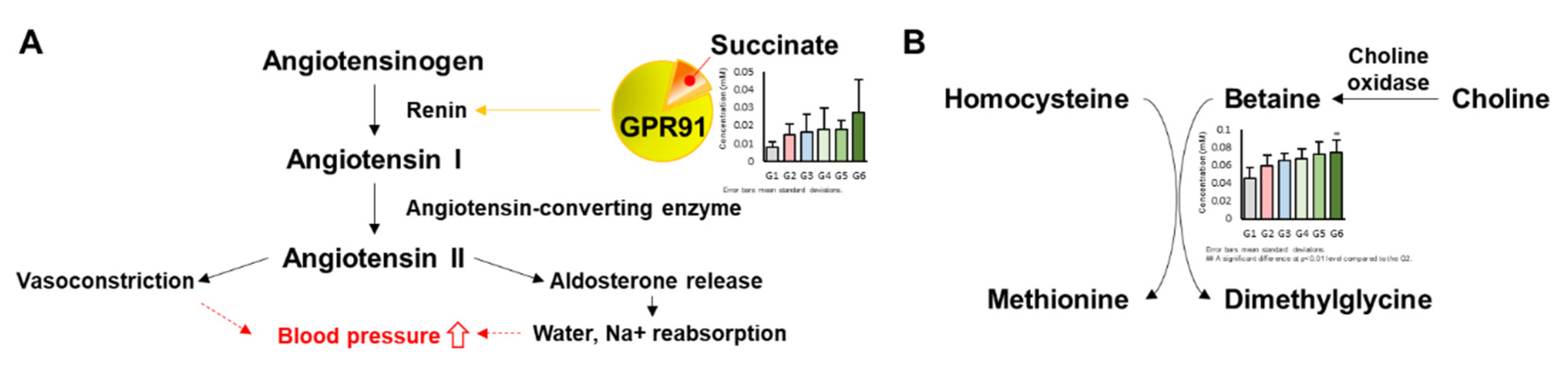
| Compound | Chemical Shifts (Multiplicities) (ppm) | G1 (mM) | G2 (mM) | G3 (mM) | G4 (mM) | G5 (mM) | G6 (mM) |
|---|---|---|---|---|---|---|---|
| 2-Oxoglutarate | 2.43 (t), 3.00 (t) | 0.014 ± 0.003 | 0.018 ± 0.002 | 0.019 ± 0.005 | 0.020 ± 0.003 | 0.018 ± 0.002 | 0.019 ± 0.004 |
| Acetate | 1.91 (s) | 0.042 ± 0.019 | 0.032 ± 0.010 | 0.038 ± 0.011 | 0.035 ± 0.009 | 0.038 ± 0.009 | 0.035 ± 0.007 |
| Alanine | 1.47 (d), 3.77 (q) | 0.193 ± 0.027 | 0.175 ± 0.028 | 0.169 ± 0.020 | 0.176 ± 0.024 | 0.180 ± 0.024 | 0.154 ± 0.022 |
| Arginine | 1.64–1.72 (m), 1.88–1.92 (m), 3.23 (t) | 0.078 ± 0.017 | 0.062 ± 0.025 | 0.062 ± 0.019 | 0.063 ± 0.020 | 0.077 ± 0.025 | 0.068 ± 0.036 |
| Asparagine | 2.85 (dd), 2.93 (dd) | 0.023 ± 0.007 | 0.028 ± 0.011 | 0.027 ± 0.005 | 0.031 ± 0.008 | 0.030 ± 0.006 | 0.030 ± 0.009 |
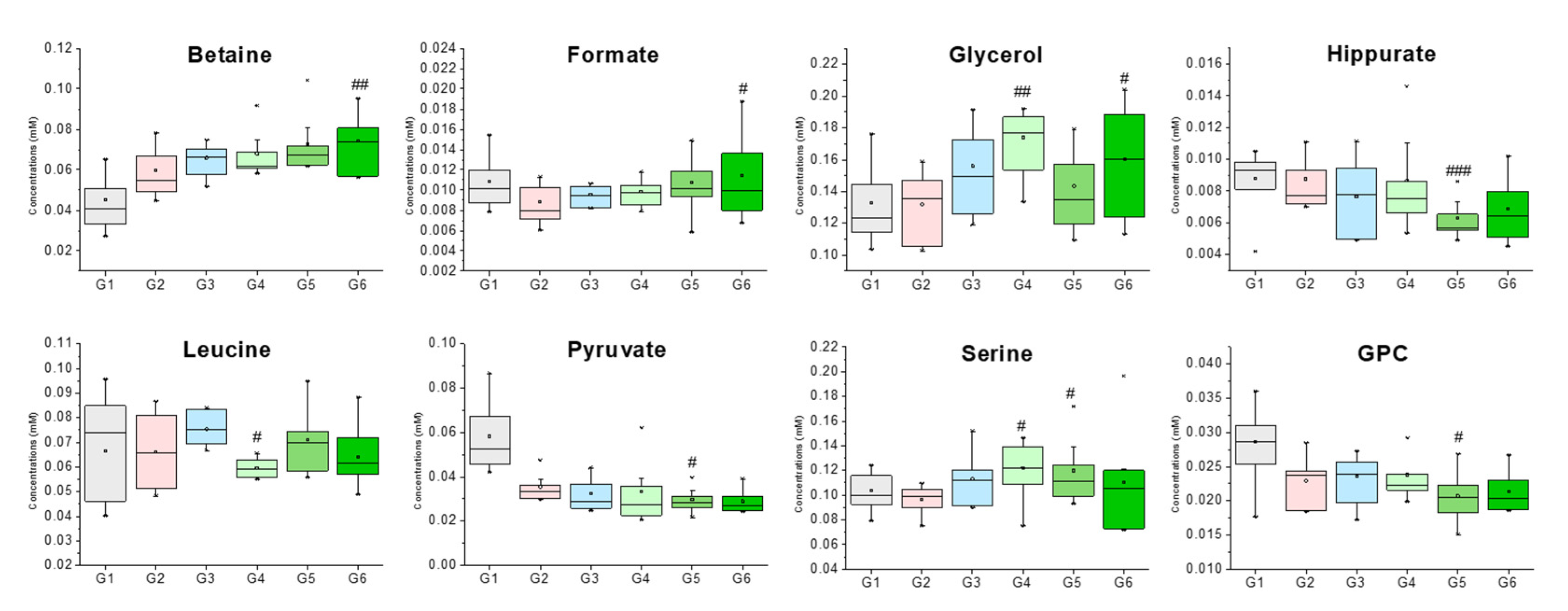
| Betaine | 3.25 (s), 3.89 (s) | 0.045 ± 0.012 | 0.060 ± 0.012 | 0.066 ± 0.008 | 0.068 ± 0.011 | 0.073 ± 0.014 | 0.074 ± 0.014 ## |
| Choline | 3.19 (s), 3.50 (dd), 4.05 (ddd) | 0.010 ± 0.001 | 0.017 ± 0.002 | 0.018 ± 0.002 | 0.019 ± 0.002 | 0.018 ± 0.002 | 0.017 ± 0.002 |
| Citrate | 2.52 (d), 2.65 (d) | 0.102 ± 0.015 | 0.111 ± 0.012 | 0.115 ± 0.012 | 0.116 ± 0.007 | 0.114 ± 0.016 | 0.113 ± 0.024 |
| Creatine | 3.02 (s), 3.92 (s) | 0.050 ± 0.007 | 0.077 ± 0.011 | 0.075 ± 0.016 | 0.068 ± 0.017 | 0.075 ± 0.012 | 0.067 ± 0.012 |
| Creatinine | 3.03 (s), 4.05 (s) | 0.007 ± 0.002 | 0.006 ± 0.000 | 0.006 ± 0.002 | 0.008 ± 0.002 | 0.006 ± 0.002 | 0.006 ± 0.001 |
| Formate | 8.44 (s) | 0.011 ± 0.002 | 0.009 ± 0.002 | 0.010 ± 0.001 | 0.010 ± 0.001 | 0.011 ± 0.003 | 0.011 ± 0.004 # |
| Glucose | 3.24 (m), 3.40–3.49 (m), 3.53 (dd), 3.70–3.89 (m), 4.64 (d), 5.23 (d) | 3.050 ± 0.223 | 2.758 ± 0.358 | 2.583 ± 0.404 | 2.525 ± 0.398 | 2.748 ± 0.205 | 2.717 ± 0.343 |
| Glutamate | 2.05−2.12 (m), 2.32−2.35 (m) | 0.069 ± 0.010 | 0.119 ± 0.028 | 0.118 ± 0.016 | 0.124 ± 0.010 | 0.130 ± 0.016 | 0.123 ± 0.020 |
| Glutamine | 2.11−2.14 (m), 2.42−2.46 (m), 3.76 (t) | 0.199 ± 0.014 | 0.230 ± 0.025 | 0.257 ± 0.028 | 0.243 ± 0.018 | 0.241 ± 0.042 | 0.229 ± 0.021 |
| Glycerol | 3.55 (dd), 3.64 (dd), 3.77 (m) | 0.133 ± 0.024 | 0.132 ± 0.022 | 0.156 ± 0.026 | 0.174 ± 0.020 ## | 0.143 ± 0.026 | 0.160 ± 0.034 # |
| Glycine | 3.55 (s) | 0.133 ± 0.013 | 0.127 ± 0.016 | 0.136 ± 0.010 | 0.130 ± 0.010 | 0.132 ± 0.015 | 0.123 ± 0.012 |
| GPC | 3.22 (s), 3.61 (m), 3.87 (m), 4.32 (m) | 0.009 ± 0.002 | 0.009 ± 0.002 | 0.008 ± 0.002 | 0.009 ± 0.003 | 0.006 ± 0.001 # | 0.007 ± 0.002 |
| Hippurate | 7.54 (t), 7.63 (t), 7.82 (d) | 0.026 ± 0.008 | 0.030 ± 0.006 | 0.028 ± 0.004 | 0.030 ± 0.003 | 0.032 ± 0.008 ### | 0.027 ± 0.006 |
| Histidine | 3.13 (dd), 3.98 (dd), 7.06 (s), 7.81 (s) | 0.005 ± 0.001 | 0.005 ± 0.002 | 0.006 ± 0.002 | 0.005 ± 0.001 | 0.005 ± 0.001 | 0.005 ± 0.001 |
| Isobutyrate | 1.06 (d), 2.38 (m) | 0.041 ± 0.012 | 0.047 ± 0.007 | 0.046 ± 0.003 | 0.047 ± 0.005 | 0.046 ± 0.006 | 0.042 ± 0.006 |
| Isoleucine | 0.93 (t), 1.00 (d), 1.25 (m), 1.46 (m), 1.97 (m), 3.66 (d) | 1.489 ± 0.185 | 1.694 ± 0.285 | 1.791 ± 0.385 | 1.811 ± 0.358 | 1.582 ± 0.218 | 1.726 ± 0.363 |
| Lacate | 1.32 (d), 4.10 (q) | 0.064 ± 0.020 | 0.067 ± 0.013 | 0.073 ± 0.009 | 0.058 ± 0.005 | 0.071 ± 0.012 | 0.060 ± 0.017 |
| Leucine | 0.95 (t), 1.67–1.74 (m), 3.73 (m) | 0.127 ± 0.013 | 0.119 ± 0.029 | 0.110 ± 0.012 | 0.122 ± 0.017 # | 0.126 ± 0.020 | 0.105 ± 0.017 |
| Lysine | 1.44–1.51 (m), 1.72 (m), 1.88 (m), 1.92 (m), 3.02 (t), 3.76 (t) | 0.021 ± 0.002 | 0.024 ± 0.004 | 0.024 ± 0.002 | 0.024 ± 0.002 | 0.024 ± 0.002 | 0.022 ± 0.003 |
| Methionine | 2.11 (m), 2.63 (t), 3.85 (dd) | 0.020 ± 0.003 | 0.020 ± 0.004 | 0.022 ± 0.002 | 0.022 ± 0.001 | 0.023 ± 0.003 | 0.022 ± 0.004 |
| Phenylalanine | 7.42 (m), 7.37 (t), 7.32 (dd) | 0.058 ± 0.015 | 0.035 ± 0.006 | 0.032 ± 0.007 | 0.033 ± 0.013 | 0.030 ± 0.005 | 0.029 ± 0.005 |
| Pyruvate | 2.36 (s) | 0.104 ± 0.016 | 0.097 ± 0.011 | 0.113 ± 0.020 | 0.122 ± 0.023 | 0.120 ± 0.026 # | 0.110 ± 0.041 |
| Serine | 3.84 (dd), 3.94 (dd), 3.98 (dd) | 0.008 ± 0.003 | 0.015 ± 0.006 | 0.017 ± 0.010 | 0.018 ± 0.012 # | 0.018 ± 0.005 # | 0.027 ± 0.018 |
| Succinate | 2.39 (s) | 0.031 ± 0.005 | 0.033 ± 0.005 | 0.031 ± 0.006 | 0.034 ± 0.007 | 0.038 ± 0.012 | 0.031 ± 0.008 |
| Tyrosine | 3.05 (dd), 3.19 (dd), 3.93 (dd), 6.89 (m), 7.18 (m) | 0.076 ± 0.023 | 0.083 ± 0.013 | 0.080 ± 0.008 | 0.084 ± 0.007 | 0.084 ± 0.015 | 0.075 ± 0.010 |
| Valine | 0.98 (d), 1.03 (d), 2.26 (m), 3.60 (d) | 0.025 ± 0.006 | 0.031 ± 0.008 | 0.029 ± 0.014 | 0.036 ± 0.012 | 0.027 ± 0.009 | 0.034 ± 0.013 |
| myo-Inositol | 3.27 (t), 3.52 (dd), 3.62 (t), 4.06 (t) | 0.029 ± 0.006 | 0.023 ± 0.003 | 0.024 ± 0.004 | 0.024 ± 0.003 | 0.021 ± 0.004 | 0.021 ± 0.003 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, D.; Choi, B.-R.; Lee, Y.-S.; Han, K.-S.; Kim, D.; Lee, D.Y. Serum Metabonomic Research of the Anti-Hypertensive Effects of Ogaja on Spontaneously Hypertensive Rats. Metabolites 2020, 10, 404. https://doi.org/10.3390/metabo10100404
Yoon D, Choi B-R, Lee Y-S, Han K-S, Kim D, Lee DY. Serum Metabonomic Research of the Anti-Hypertensive Effects of Ogaja on Spontaneously Hypertensive Rats. Metabolites. 2020; 10(10):404. https://doi.org/10.3390/metabo10100404
Chicago/Turabian StyleYoon, Dahye, Bo-Ram Choi, Young-Seob Lee, Kyung-Sook Han, Donghwi Kim, and Dae Young Lee. 2020. "Serum Metabonomic Research of the Anti-Hypertensive Effects of Ogaja on Spontaneously Hypertensive Rats" Metabolites 10, no. 10: 404. https://doi.org/10.3390/metabo10100404
APA StyleYoon, D., Choi, B.-R., Lee, Y.-S., Han, K.-S., Kim, D., & Lee, D. Y. (2020). Serum Metabonomic Research of the Anti-Hypertensive Effects of Ogaja on Spontaneously Hypertensive Rats. Metabolites, 10(10), 404. https://doi.org/10.3390/metabo10100404