Abstract
Taxifolin is a natural polyphenol belonging to the class of flavonoids. The structure of this compound is characterized by the presence of two chiral centers. The spheroidal form of taxifolin (TAXs) has emerged as a promising modification due to enhanced solubility, higher safety profile, and long-term release from solid dosage forms. The study’s objective was to assess the diastereomeric content in TAXs and industrially produced samples of taxifolin. Considering the difference in the physico-chemical properties of diastereomers and based on the literature data, we developed a qualitative HPLC method. The chromatograms were recorded using a diode array detector at 290 nm and a mass spectrometer operated in negative ionization mode. Our data suggest that a biphenyl column and gradient elution using 0.1% formic acid in water and 0.2% formic acid in methanol, with the organic phase gradient from 7% to 21% and a flow rate of 0.65 mL/min for 15 min at 60 °C, provides the best conditions for the separation of taxifolin diastereomers. This method was validated for quantitative analysis. We discovered that the cis-isomer was present in all the analyzed samples, with its quantity ranging from 0.8% to 9.5%. TAXs can be considered a sample enriched with diastereomers.
1. Introduction
Taxifolin, also known as dihydroquercetin, is a natural polyphenol belonging to the class of flavonoids. It can be considered a “blockbuster” molecule due to the growing interest of the scientific community in this antioxidant. This heightened attention is primarily a result of the wide range of pharmacological activities of taxifolin [1,2,3]. This flavonoid showed antidiabetic [4,5], wound-healing [6,7,8], antitumor [9], cardioprotective [10], radioprotective [11], and neuroprotective [12,13] activity. Taxifolin is registered as an active pharmaceutical ingredient (API) in Russia and Kazakhstan and is also available in the European market as a supplement material.
Another advantage of taxifolin is the sustainable supply of raw materials, which facilitates industrial production. This flavonoid is found in various sources, including onions, tamarind, milk thistle, grapes, and citrus fruits [14,15,16]. Biotechnological methods for taxifolin production have been explored [17]. The utilization of other natural sources, such as Korean rosebay (Rhododendron mucroulatum Turcz.), is also described [18]. Nevertheless, the primary source of taxifolin remains the wood of larch spices (Larix sibirica Ledeb. and Larix dahurica Turcz.). Taxifolin is the major flavonoid component of larch wood, constituting up to 4% of its mass in different plant parts [19].
Several patented technologies for obtaining taxifolin exist. Lashin and Ostronkov, for instance, proposed extracting taxifolin from larch wood using a 75–85% ethanol solution in water at 45–50 °C, with a source-to-solvent mass ratio of 1:10 [20]. Levdanskij et al. offered another method involving boiling in a 10–25% ethanol solution in water for 1–2 h [21]. In general, aqueous ethanol solutions are the most commonly used extraction fluids for taxifolin [22,23], although ethyl acetate, isopropyl alcohol, acetone, and their aqueous solutions can also be employed [24,25,26].
One of the key factors hindering the development of drugs based on taxifolin is its low bioavailability. Previous efforts have sought to address this issue through phase modification [27,28]. The spheroidal form of taxifolin (TAXs) has emerged as a promising modification in our investigations [29]. This modification is characterized by enhanced solubility up to 2.2 times, a higher safety profile, and long-term release from solid dosage forms [30,31].
The process of drug development necessitates qualitative and quantitative control of stereoisomers in APIs. The structure of taxifolin is characterized by the presence of two chiral centers (Figure 1). Two enantiomers—2R,3R-taxifolin and 2S,3S-taxifolin—correspond to the trans-configuration of substitutes at the single bound between carbon atoms in positions 2 and 3. The other two molecules are associated with the cis-configuration, and they are enantiomers for each other. The structures in Figure 1a,b are diastereomers for molecules in Figure 1c,d. Safety and efficacy have been confirmed for Diquertin®—a remedy on the taxifolin basis [32]. According to X-ray crystallography and circular dichroism data, the reference sample obtained from Diquertin® is a 2R,3R-configuration of taxifolin, which corresponds to the structure of the trans-diastereomer [33]. Therefore, the study’s objective was to assess the diastereomeric content in TAXs and commercially available samples of taxifolin.
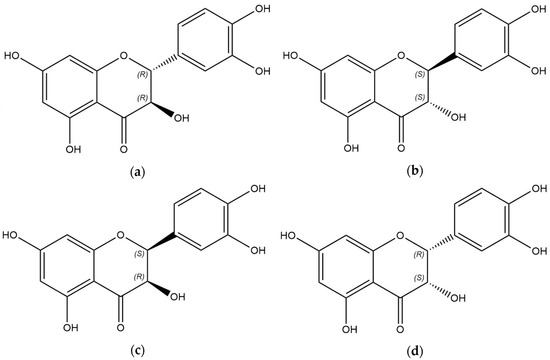
Figure 1.
Molecular models of taxifolin’s stereoisomers: (a) 2R,3R-configuration, (b) 2S,3S-configuration, (c) 2S,3R-configuration, (d) 2R,3S-configuration. Images were generated in Avogadro.
2. Materials and Methods
2.1. Materials
Commercially available samples of taxifolin were obtained from Ametis JSC (Lavitol, 99.1%, Blagoveshchensk, Russia), Taxifolia LLC (97%, Belgorod, Russia), and Robios LLC (VitaRost, >90%, Serpukhov, Russia). Additionally, a reference sample of taxifolin (99.99%; Ametis JSC, Blagoveshchensk, Russia) was utilized.
TAXs was generated as described in the literature [29,30] from Lavitol, mentioned as TAXr. In brief, a 40 L taxifolin water solution (T = 60 °C, C = 40 mg/mL) was subjected to spray drying using a GLP-60 centrifugal sprat. The inlet air temperature was 180 °C, and the outlet temperature was 80 °C. The relative centrifugal force of the disk atomizer was 22,700× g.
Methanol (HPLC grade, Chimmed Group, Moscow, Russia), ethanol (for spectroscopy, Merck KGaA, Darmstadt, Germany), acetonitrile (HPLC grade, Fisher Scientific, Loughborough, UK), acetone (for analysis, Chimmed Group, Moscow, Russia), and distilled deionized water were used as solvents. Formic acid (MS grade) and ortho-phosphoric acid (for analysis) were obtained from Honeywell-Fluka (Steinheim, Germany) and Merck KGaA (Darmstadt, Germany), respectively.
2.2. Moisture Content
Moisture content was measured using a moisture tester ML-50 (A&D, Tokyo, Japan). Each taxifolin sample was dried to a constant weight at 105 °C in triplicate and presented as the mean ± standard deviation.
2.3. HPLC
Stock solutions were prepared by dissolving 10.0 mg of the taxifolin samples in 1 mL of methanol, and then they were centrifuged for 10 min at 12,000 rpm using Centrifuge 5418 (Eppendorf AG, Hamburg, Germany). To obtain the working solutions, the supernatants (10 μL) were diluted in 990 μL of water/methanol solvent (2:8, vol.).
The separation was conducted using a chromatograph LC-30AD (Shimadzu, Kyoto, Japan) with a mass spectrometer LCMS-8040 (Shimadzu, Kyoto, Japan). The mass spectrometer operated in negative ionization mode. The chromatograms were recorded using a diode array detector SPD-M20A (Shimadzu, Kyoto, Japan) at 290 nm. The following columns were employed: Luna 5µ C18(2) 100 Å, 250 mm × 4.6 mm (Phenomenex, Torrance, CA, USA), Luna 5µ CN 100 Å, 250 mm × 4.6 mm (Phenomenex, Torrance, CA, USA), Kinetex® 2.6 μm Biphenyl 100 Å, 100 mm × 3.0 mm (Phenomenex, Torrance, CA, USA). Methanol, acetonitrile, and water in different ratios were used as eluents. The flow rate ranged from 0.65 to 1.00 mL/min, and the temperature varied from 25 to 60 °C. Formic acid and ortho-phosphoric acid served as modifiers. In the case of ortho-phosphoric acid, the mass spectrometer was not explored.
The quality of chromatographic separation was assessed, using tailing factor (TF), number of theoretical plates (NTP), and resolution factor (Rs), which were calculated as follows:
where W0.05 is peak width at 5% high, W0.5 is peak width at 50% high, RT is a retention time (min), and F is the leading edge of the peak.
2.4. Validation of Quantitative Analysis
Validation was performed in accordance with Russia State Pharmacopoeia 15 [34], which is harmonized with European Pharmacopoeia 11 [35].
To generate the calibration curve, 1.0, 5.0, 10.0, 15.0, and 20.0 μL of the stock solution of taxifolin reference sample were diluted in 999.0, 995.0, 990.0, 985.0, and 980.0 μL of water/methanol solvent (2:8, vol.), respectively. Each point was recorded in quintuplicate and presented as the mean ± standard deviation. The curve was graphed in triplicate on different days using fresh stock solutions.
We calculated the limit of detection (LD) and the limit of quantification (LQ) from compressibility data using Equations (4) and (5), respectively:
where s is the standard deviation of the analytical signal, and k is the slope of the calibration curve.
LD = 3.3 × s/k
LQ = 10 × s/k
2.5. Polarimetry
The optical rotation was determined using an automatic digital polarimeter UniPol 2020 (Schmidt + Haensch, Berlin, Germany). To prepare the working solutions, 0.5 mg of taxifolin samples were dissolved in 50 mL of ethanol or acetone and filtered. The specific optical rotations ([α]D) were calculated as follows:
where α is the optical rotation, C is the taxifolin concentration, and l is the path length of the cuvette (2.004 dm). All measurements were repeated in triplicate and presented as the mean ± standard deviation.
[α]D = α/(C × l)
3. Results
3.1. Analytical Method Development
To perform the qualitative analysis of taxifolin diastereomers in taxifolin samples, a valid method was needed. The process of analytical method development based on chromatography can be seen in Table 1.

Table 1.
Characteristics of chromatographic conditions.
In the majority of cases, we used reversed-phase HPLC with gradient elution, where solvent A is a water solution of acid, and B is the solution of the same acid in an organic solvent. Optimization included the selection of a stationary phase, gradient program, elution time, flow rate, and temperature. What stands out in this table is the better separation of diastereomers in cases where the stationary phase contains a phenyl group: the number of theoretical plates (NTP) and resolution (Rs) increased, primarily by 48.6 and 4.4 times, respectively. Methanol was more effective as an organic solvent than acetonitrile. The higher Rs was estimated when the percentage of water solvent in the eluent was not less than 75% during the entire gradient program. All other things being equal, a decrease in elution time resulted in the coalescence of diastereomers’ peaks. Also, a slower flow rate provided better separation, while temperature had little effect on the efficacy of chromatography.
The data presented in the table above suggest that the optimization of a biphenyl column and gradient elution using 0.1% formic acid in water and 0.2% formic acid in methanol, with the organic phase gradient from 7% to 21% and a flow rate of 0.65 mL/min for 15 min at 60 °C, provides the best conditions for the separation of taxifolin diastereomers. This method was validated for quantitative analysis. The relationship between the maximum peak area and concentration is linear (R2 = 0.9999) within the range of 0.01 to 0.20 mg/mL and can be described by the following formula:
f(x) = 2.54573 × 10⁷x + 12,419.5
The limits of detection and quantitation were calculated as 0.0016 and 0.0049 mg/mL, respectively. The relevant standard deviation (RSD) was 0.18%, while the intra-day and inter-day variations of this parameter were 0.24% and 0.93%, respectively.
3.2. Analysis of TAXs and TAXr
The developed analytical method based on HPLC was employed for the comparative analysis of the amount of taxifolin diastereomers in TAXs and TAXr.
Figure 2 presents the chromatograms of taxifolin samples recorded using a UV detector at a wavelength of 290 nm. They are characterized by the presence of three peaks with the following RTs: 10.1 min, 11.6 min, and 15.1 min. Notably, the second peak shows a 5.7-fold increase during the generation of TAXs.
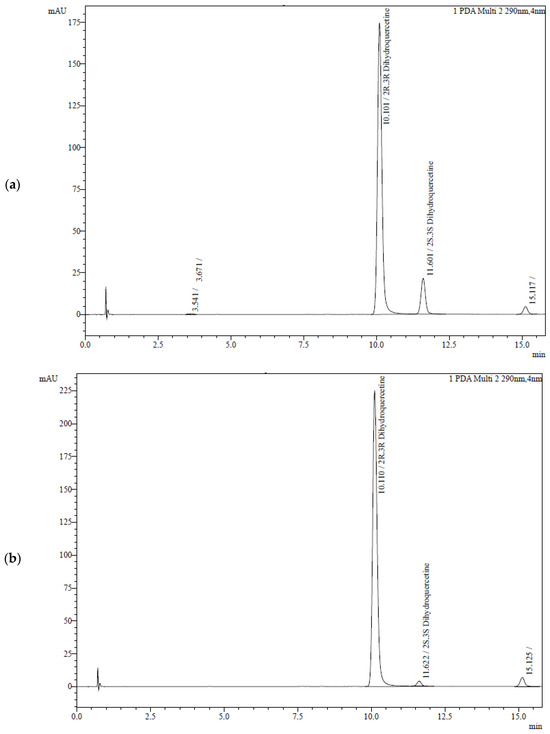
Figure 2.
Chromatograms of taxifolin samples: (a) TAXs; (b) TAXr. For chromatographic conditions, see Table 1, row 7.
To identify the components of the separated samples, UV spectroscopic data (Figure 3) and mass spectrometric data (Figure 4) were analyzed. All three peaks have a similar UV spectrum pattern with a λmax at 289 nm. However, the mass spectrum of the third peak contains a molecular ion peak with m/z 317, which is 14 higher than the other two components.
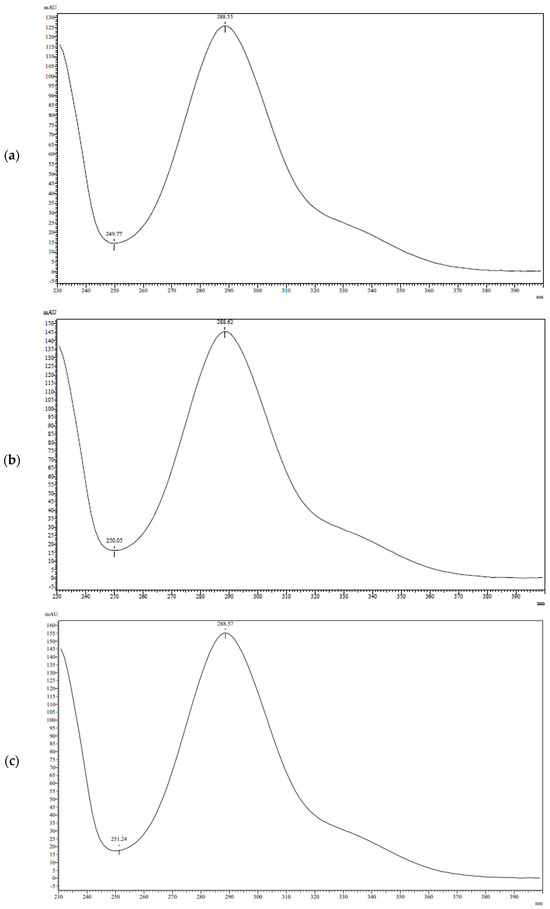
Figure 3.
UV spectra of peak with RT: (a) 10.1 min; (b) 11.6 min; (c) 15.1 min.
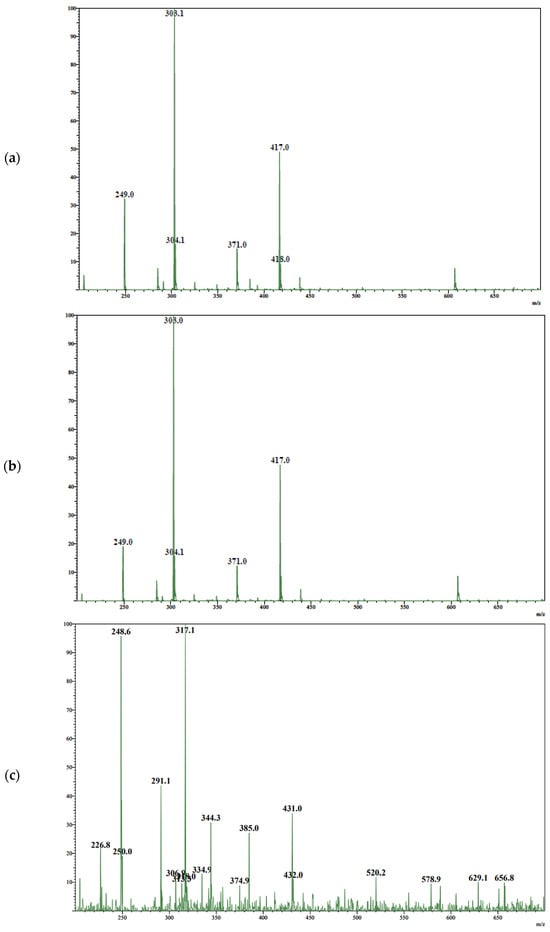
Figure 4.
Mass spectra of peak with RT: (a) 10.1 min; (b) 11.6 min; (c) 15.1 min.
The results indicate that the peaks with RT 10.1 and 11.6 are formed by two diastereomers of taxifolin. Trans-taxifolin was identified by comparison with the chromatographic and spectral characteristics of the reference sample. It is the major component of TAXs and TAXr.
3.3. Analysis of Commercially Available Samples of Taxifolin
In the final part of the research, the stereoisomer composition of commercially available samples of taxifolin was explored and compared with TAXs (Table 2), which has the biggest amount of the cis-diastereomer. It is observed that the content of the cis-isomer is not consistent in the taxifolin samples and ranges between 0.8% and 9.5%. Additionally, among industrially produced samples, the initial TAXr shows the highest concentration of the cis-isomer and the highest purity, while the sample provided by Robios LLC demonstrates the smallest amount of total taxifolin, but its diastereomeric composition is more homogenous than others.

Table 2.
Contents of taxifolin diastereomers in taxifolin samples.
To gather the stereochemical information about commercially available taxifolin samples, polarimetric measurements were performed (Table 3). The specific optical rotation of taxifolin solutions varies from 18.8 to 22.1 in ethanol and from 20.5 to 32.0 in acetone. In all cases, the solutions of TAXs are characterized by the highest value of this parameter. Nevertheless, the correlation between diastereomer content and optical rotation was moderate (r2 is 0.7743 and 0.5276 for ethanol and acetone solutions, respectively).

Table 3.
Optical rotation of different commercially available taxifolin samples.
These results in this section indicate that TAXs contains a far greater amount of cis-taxifolin than commercially available samples of this flavonoid. The next section, therefore, moves on to discuss the scientific implications of this discovery.
4. Discussion
As mentioned in the literature review, the structure of taxifolin is characterized by two asymmetric carbon atoms. Despite the importance of diastereomeric composition for the safety profile, there is a limited amount of information available in the current literature on this subject. The majority of studies in this field have primarily focused on glycosides of taxifolin stereoisomers [36,37,38,39].
This study aimed to assess the diastereomer containment in TAXs. The initial objective was the design of a qualitative analytical method for the identification of cis-taxifolin. Traditionally, differential spectrophotometry is used for flavonoid analysis in pharmacognosy [40,41,42,43]. However, this method can detect the presence of any flavonoid and is not applicable for identification. Because of this limitation, HPLC is increasingly being used for the analysis of flavonoids. To achieve the goals of qualitative and quantitative control, the reversed phase is employed, utilizing aqueous solutions of acetonitrile and alcohols as mobile phases [44,45,46,47]. The identification of flavonoids can be performed using reference samples or diode-array detectors and mass spectrometry [48,49,50]. Considering the difference in the physico-chemical properties of diastereomers and based on the literature data, we developed a qualitative HPLC method for taxifolin diastereomers. During the design process, it was observed that better separation of diastereomers was achieved when the stationary phase contained a phenyl group, and the water-methanol mixture was more effective than the water-acetonitrile solution. In the case of the best chromatography system, the NTP for peaks of trans- and cis-taxifolin was 20,531 and 26,285, respectively, and the Rs was 5.3. This method was validated and is suitable for pharmaceutical analysis [34,35].
The diastereomers of taxifolin in TAXs were identified through HPLC and spectroscopic parameters. It was known, that the reference sample of taxifolin consists of trans-diastereomers [33]. The chromatogram of the reference sample is characterized by only one peak with RT of 10.1 min, which was attributed to trans-taxifolin. All other analyzed samples demonstrate two peaks at RT 10.1 and 11.6 min. They are characterized by similar spectral parameters. The UV-spectra profiles of both compounds are characterized by a maximum at 289 nm, which correlates with previously published spectral data of taxifolin [51,52]. The mass spectra of the diastereomers obtained during electrospray ionization show the molecular ion peak at m/z 304 and an intense quasi-molecular ion peak at m/z 303. This is a common picture in soft ionization for the mass spectroscopy of taxifolin [53,54]. The minor peak at m/z 249 can be attributed to the loss of three water molecules from quasi-molecular ions. Simultaneously, the peak at m/z 371 can be explained by the formation of an association between quasi-molecular ions and sodium formate [M–H+HCOONa]−. Additionally, a peak at m/z 417 may be associated with the formation of a cluster with formic acid and its sodium salt [M–H+HCOOH+HCOONa]−. The suggestion about the cluster formation with the components of the mobile phase during LC-MS/MS analysis of taxifolin stays in line with the data published previously by Abad-Garcia et al. [55]. Taking into account the achiral conditions of chromatographic separation, two compounds with RT 10.1 and 11.6 min were considered as diastereomers. As the first one was identified as trans-taxifolin, the second component was cis-taxifolin, apparently.
The profile of the mass spectrum for the compound with RT 15.1 min differs significantly from the taxifolin one. The concentration of this component is smaller than that of taxifolin, so this mass spectrum is characterized by multiple peaks. Nevertheless, this compound can be identified as dihydroisorahmnetin—3,5,7-trihydroxy-2-(4-hydroxy-3-methoxyphenyl)-2,3-dihydro-4H-1-benzopyran-4-one. This suggestion is confirmed by the presence of peaks with m/z 335, 318, 317, 291, and 249 that can be attributed to [M–H+H2O]−, [M]−, [M–H]−, [M–H–C2H2]−, and [M–CH3–CO–C2H2]−, respectively.
When the appropriate method of diastereomeric analysis for taxifolin was designed, the scope of our interest was focused on the containment of the cis-isomer in commercially available samples. Despite having the smallest amount of taxifolin, the sample obtained from Robios LLC was diastereomerically homogenous. In contrast, the TAXr exhibited the highest purity of taxifolin but had the largest amount of cis-isomers. This may be associated with the use of high temperatures during the concentration of extracts [56]. Nevertheless, the percentage of cis-diastereomers in TAXs was 2.6 times higher compared with the initial sample, accounting for 9.5% of the mass. Therefore, TAXs can be considered a cis-isomer-enriched sample compared with the other ones.
The importance of stereoisomer control in APIs is evident, as stereochemistry significantly affects both the efficacy [57] and safety [58,59] of the remedy. Polarimetry may be the ideal method for routine quantitative analysis of taxifolin diastereomers. Unfortunately, the correlation between their contents and optical rotation was not suitable for pharmaceutical analysis. The optical rotation values of TAXr and TAXs solutions differed insignificantly, which was an unexpected outcome. This discrepancy could be attributed to the presence of optically active impurities of its related flavonoids. Taking into account the achiral stationary phase in the developed HPLC method, another possible explanation is that each diastereomer may contain two enantiomers with different optical rotation values. These findings may help explain the inconsistencies in the literature data. Gaggeri et al. [60] reported about [α]D20 +12.68° in methanol for trans-taxifolin, while in the work authored by Towatari et al. [61], this parameter was +6.3°. For cis-isomers, the optical rotation of the methanol solution varies from +30.5° [36] to +58.8° [62].
The impact of the cis-isomer on the biological activity of the taxifolin sample remains unclear. Considering the data of our research, we recommend conducting future studies on this topic.
5. Conclusions
The objective of this research was to assess the diastereomeric content of TAXs and commercially available samples of taxifolin. The HPLC methods we developed for this purpose can be applied to monitor the diastereomeric purity in taxifolin samples. We discovered that the cis-isomer was present in all the analyzed samples, with its quantity ranging from 0.8% to 9.5%. TAXs can be considered a sample enriched with cis-isomers compared with the other ones. To the best of our knowledge, it is the first research that is focused on the diastereomeric content of taxifolin substances, produced by different manufacturers. This study has raised important questions regarding the nature of taxifolin: Is this compound solely an antioxidant, or does it possess stereospecific properties? Further research on obtaining cis-taxifolin and investigation into its biological activity will provide answers to this question.
Author Contributions
Conceptualization, R.P.T. and I.A.S.; methodology, R.P.T. and E.S.M.; software, T.A.R.; validation, T.A.R., V.L.B. and I.A.S.; formal analysis, R.P.T. and E.S.M.; investigation, M.A.T., A.D.S. and D.I.P.; resources, A.K.Z. and E.S.M.; data curation, A.K.Z. and R.P.T.; writing—original draft preparation, R.P.T. and I.D.N.; writing—review and editing, R.P.T., E.S.M., T.A.R., V.L.B. and I.A.S.; visualization, M.A.T.; supervision, V.L.B. and I.A.S.; project administration, T.A.R. and I.A.S.; funding acquisition, R.P.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 23-75-01130.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank A.S. Gulenkov for his constructive help.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Das, A.; Baidya, R.; Chakraborty, T.; Samanta, A.K.; Roy, S. Pharmacological basis and new insights of taxifolin: A comprehensive review. Biomed. Pharmacother. 2021, 142, 112004. [Google Scholar] [CrossRef]
- Taldaev, A.; Terekhov, R.; Nikitin, I.; Zhevlakova, A.; Selivanova, I. Insights into the Pharmacological Effects of Flavonoids: The Systematic Review of Computer Modeling. Int. J. Mol. Sci. 2022, 23, 6023. [Google Scholar] [CrossRef]
- Jain, S.; Vaidya, A. Comprehensive review on pharmacological effects and mechanism of actions of taxifolin: A bioactive flavonoid. Pharmacol. Res.-Mod. Chin. Med. 2023, 7, 100240. [Google Scholar] [CrossRef]
- Alay, M.; Sonmez, M.G.; Sakin, A.; Atmaca, M.; Suleyman, H.; Yazici, G.N.; Coban, A.; Suleyman, B.; Bulut, S.; Altuner, D. The effects of taxifolin on neuropathy related with hyperglycemia and neuropathic pain in rats: A biochemical and histopathological evaluation. Adv. Clin. Exp. Med. 2022, 31, 427–435. [Google Scholar] [CrossRef]
- Kondo, S.; Adachi, S.I.; Yoshizawa, F.; Yagasaki, K. Antidiabetic Effect of Taxifolin in Cultured L6 Myotubes and Type 2 Diabetic Model KK-Ay/Ta Mice with Hyperglycemia and Hyperuricemia. Curr. Issues Mol. Biol. 2021, 43, 1293–1306. [Google Scholar] [CrossRef]
- Shevelev, A.B.; La Porta, N.; Isakova, E.P.; Martens, S.; Biryukova, Y.K.; Belous, A.S.; Sivokhin, D.A.; Trubnikova, E.V.; Zylkova, M.V.; Belyakova, A.V.; et al. In Vivo Antimicrobial and Wound-Healing Activity of Resveratrol, Dihydroquercetin, and Dihydromyricetin against Staphylococcus aureus, Pseudomonas aeruginosa, and Candida albicans. Pathogens 2020, 9, 296. [Google Scholar] [CrossRef]
- Terekhov, R.P.; Selivanova, I.A.; Anurova, M.N.; Zhevlakova, A.K.; Nikitin, I.D.; Cong, Z.; Ma, S.; Yang, F.; Dong, Z.; Liao, Y. Comparative Study of Wound-Healing Activity of Dihydroquercetin Pseudopolymorphic Modifications. Bull. Exp. Biol. Med. 2021, 170, 444–447. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, C.; Zhao, Y.; Zhang, J.; Ding, Q.; Zhang, S.; Wang, N.; Yang, J.; Xi, S.; Zhao, T.; et al. Sodium alginate/poly(vinyl alcohol)/taxifolin nanofiber mat promoting diabetic wound healing by modulating the inflammatory response, angiogenesis, and skin flora. Int. J. Biol. Macromol. 2023, 252, 126530. [Google Scholar] [CrossRef]
- Butt, S.S.; Khan, K.; Badshah, Y.; Rafiq, M.; Shabbir, M. Evaluation of pro-apoptotic potential of taxifolin against liver cancer. PeerJ 2021, 9, e11276. [Google Scholar] [CrossRef]
- Obeidat, H.M.; Althunibat, O.Y.; Alfwuaires, M.A.; Aladaileh, S.H.; Algefare, A.I.; Almuqati, A.F.; Alasmari, F.; Aldal’in, H.K.; Alanezi, A.A.; Alsuwayt, B.; et al. Cardioprotective Effect of Taxifolin against Isoproterenol-Induced Cardiac Injury through Decreasing Oxidative Stress, Inflammation, and Cell Death, and Activating Nrf2/HO-1 in Mice. Biomolecules 2022, 12, 1546. [Google Scholar] [CrossRef]
- Teselkin, Y.O.; Babenkova, I.V.; Tjukavkina, N.A.; Rulenko, I.A.; Kolesnik, Y.A.; Kolhir, V.K.; Eichholz, A.A. Influence of dihydroquercetin on the lipid peroxidation of mice during post-radiation period. Phytother. Res. 1998, 12, 517–519. [Google Scholar] [CrossRef]
- Iwasa, M.; Kato, H.; Iwashita, K.; Yamakage, H.; Kato, S.; Saito, S.; Ihara, M.; Nishimura, H.; Kawamoto, A.; Suganami, T.; et al. Taxifolin Suppresses Inflammatory Responses of High-Glucose-Stimulated Mouse Microglia by Attenuating the TXNIP–NLRP3 Axis. Nutrients 2023, 15, 2738. [Google Scholar] [CrossRef]
- Hu, Z.; Xuan, L.; Wu, T.; Jiang, N.; Liu, X.; Chang, J.; Wang, T.; Han, N.; Tian, X. Taxifolin attenuates neuroinflammation and microglial pyroptosis via the PI3K/Akt signaling pathway after spinal cord injury. Int. Immunopharmacol. 2023, 114, 109616. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Masuero, D.; Gasperotti, M.; Franceschi, P.; Caputi, L.; Viola, R.; Mattivi, F. A versatile targeted metabolomics method for the rapid quantification of multiple classes of phenolics in fruits and beverages. J. Agric. Food Chem. 2012, 60, 8831–8840. [Google Scholar] [CrossRef]
- Slimestad, R.; Fossen, T.; Vågen, I.M. Onions: A Source of Unique Dietary Flavonoids. J. Agric. Food Chem. 2007, 55, 10067–10080. [Google Scholar] [CrossRef]
- Schauss, A.G.; Tselyico, S.S.; Kuznetsova, V.A.; Yegorova, I. Toxicological and Genotoxicity Assessment of a Dihydroquercetin-Rich Dahurian Larch Tree (Larix gmelinii Rupr) Extract (Lavitol). Int. J. Toxicol. 2015, 34, 162–181. [Google Scholar] [CrossRef]
- Thuan, N.H.; Shrestha, A.; Trung, N.T.; Tatipamula, V.B.; Van Cuong, D.; Canh, N.X.; Van Giang, N.; Kim, T.S.; Sohng, J.K.; Dhakal, D. Advances in biochemistry and the biotechnological production of taxifolin and its derivatives. Biotechnol. Appl. Biochem. 2022, 69, 848–861. [Google Scholar] [CrossRef]
- Eun, C.S.; Min, P.S.; Hee, K.T.; Eun, K.Y.; Hwan, J.Y.; Seok, K.M. A Mathod for Extracting Taxifolin Glycoside with High Content and Taxifolin Non-Saccharide. KR 102380457 B1, 30 March 2022. [Google Scholar]
- Lee, S.B.; Cha, K.H.; Selenge, D.; Solongo, A.; Nho, C.W. The Chemopreventive Effect of Taxifolin Is Exerted through ARE-Dependent Gene Regulation. Biol. Pharm. Bull. 2007, 30, 1074–1079. [Google Scholar] [CrossRef]
- Lashin, S.A.; Ostronkov, V.S. Method of Dihydroquercetin Production. RU 2330677 C1, 8 October 2008. [Google Scholar]
- Levdanskij, V.A.; Levdanskij, A.V.; Kuznecov, B.N. Method for Producing Dihydroquercetin. RU 2454410 C1, 27 June 2011. [Google Scholar]
- Wiesbeck, F. Process for the Production of Taxifolin from Wood. EP 2809310 B1, 20 January 2016. [Google Scholar]
- Wiesbeck, F. Process for the Manufacture of Taxifolin from Wood. US 9073889 B2, 7 July 2015. [Google Scholar]
- Babkin, V.A.; Ostrouhova, L.A.; Babkin, D.V.; Yu, A.; Malkov. Method of Preparing Dihydroquercetin. RU 2158598 C2, 27 January 2000. [Google Scholar]
- Kislicyn, A.N.; Mal’chikov, E.L. Method of Isolation of Dihydroquercetin from Larchwood and Installation for Its Implementation. RU 2346941 C2, 20 February 2009. [Google Scholar]
- Hutoryanskij, V.A.; Bazhenov, B.N.; Sajbotalov, M.Y.U.; Tyukavkina, N.A. Method of Preparation of Dihydroquercetin. RU 96111031 A, 27 September 1997. [Google Scholar]
- Terekhov, R.P.; Selivanova, I.A.; Tyukavkina, N.A.; Shylov, G.V.; Utenishev, A.N.; Porozov, Y.B. Taxifolin tubes: Crystal engineering and characteristics. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2019, 75, 175–182. [Google Scholar] [CrossRef]
- Selivanova, I.A.; Terekhov, R.P. Crystal engineering as a scientific basis for modification of physicochemical properties of bioflavonoids. Russ. Chem. Bull. 2019, 68, 2155–2162. [Google Scholar] [CrossRef]
- Terekhov, R.P.; Selivanova, I.A.; Tyukavkina, N.A.; Ilyasov, I.R.; Zhevlakova, A.K.; Dzuban, A.V.; Bogdanov, A.G.; Davidovich, G.N.; Shylov, G.V.; Utenishev, A.N.; et al. Assembling the Puzzle of Taxifolin Polymorphism. Molecules 2020, 25, 5437. [Google Scholar] [CrossRef]
- Taldaev, A.; Terekhov, R.P.; Selivanova, I.A.; Pankov, D.I.; Anurova, M.N.; Markovina, I.Y.; Cong, Z.; Ma, S.; Dong, Z.; Yang, F.; et al. Modification of Taxifolin Properties by Spray Drying. Sci. Pharm. 2022, 90, 67. [Google Scholar] [CrossRef]
- Taldaev, A.; Savina, A.D.; Olicheva, V.V.; Ivanov, S.V.; Terekhov, R.P.; Ilyasov, I.R.; Zhevlakova, A.K.; Selivanova, I.A. Protective Properties of Spheroidal Taxifolin Form in Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2023, 24, 11962. [Google Scholar] [CrossRef]
- Kolhir, V.K.; Bykov, V.A.; Baginskaja, A.I.; Sokolov, S.Y.; Glazova, N.G.; Leskova, T.E.; Sakovich, G.S.; Tjukavkina, N.A.; Kolesnik, Y.A.; Rulenko, I.A. Antioxidant activity of a dihydroquercetin isolated from Larix gmelinii (Rupr.) Rupr. wood. Phytother. Res. 1996, 10, 478–482. [Google Scholar] [CrossRef]
- Selivanova, I.A.; Tyukavkina, N.A.; Kolesnik, Y.A.; Nesterov, V.N.; Kuleshova, L.N.; Khutoryanskii, V.A.; Bazhenov, B.N.; Saibotalov, M.Y. Study of the crystalline structure of dihydroquercetin. Pharm. Chem. J. 1999, 33, 222–224. [Google Scholar] [CrossRef]
- The State Pharmacopoeia of the Russian Federation, XV Edition. Available online: https://pharmacopoeia.regmed.ru/pharmacopoeia/izdanie-15/?PAGEN_1=5 (accessed on 2 November 2023).
- European Pharmacopoeia (Ph. Eur.) 11th Edition—European Directorate for the Quality of Medicines & HealthCare. Available online: https://www.edqm.eu/en/european-pharmacopoeia-ph.-eur.-11th-edition (accessed on 2 November 2023).
- Lundgren, L.N.; Theander, O. Cis- and trans-dihydroquercetin glucosides from needles of Pinus sylvestris. Phytochemistry 1988, 27, 829–832. [Google Scholar] [CrossRef]
- Kasai, R.; Hirono, S.; Tanaka, O.; Chou, W.H.; Chen, F.H. Sweet Dihydroflavonol Rhamnoside from Leaves of Engelhardtia chrysolepis, a Chinese Folk Medicine, Hung-qi. Chem. Pharm. Bull. 1988, 36, 4167–4170. [Google Scholar] [CrossRef]
- Inada, A.; Murata, H.; Somekawa, M.; Nakanishi, T. Phytochemical Studies of Seeds of Medicinal Plants. II. A New Dihydroflavonol Glycoside and a New 3-Methyl-1-butanol Glycoside from Seeds of Platycodon grandiflorum A. DE CANDOLLE. Chem. Pharm. Bull. 1992, 40, 3081–3083. [Google Scholar] [CrossRef]
- Sakurai, A.; Okumura, Y. Chemical Studies on the Mistletoe. V. The Structure of Taxillusin, a New Flavonoid Glycoside Isolated from Taxillus kaempferi. Bull. Chem. Soc. Jpn. 2006, 56, 542–544. [Google Scholar] [CrossRef]
- Dzhavakhyan, M.A.; Prozhogina, Y.E.; Pavelieva, O.K.; Kalenikova, E.I. Natural Deep Eutectic Solvents as Alternative Flavonoid Extractants from the Sedative Plant Composition. Drug Dev. Regist. 2022, 11, 75–83. [Google Scholar] [CrossRef]
- Marakhova, A.I.; Sorokina, A.A.; Stanishevskiy, Y.M. Application of through standardization principle in the analysis of flavonoids motherwort (leonurus, l.) herb and it’s preparation. Drug Dev. Regist. 2016, 1, 150–154. [Google Scholar]
- Kalashnikova, O.A.; Ryzhov, V.M.; Kurkin, V.A. Method of Quantitative Determination of the Total Flavonoid Content in Leaves of Giant Cephalaria. Pharm. Chem. J. 2023, 57, 382–387. [Google Scholar] [CrossRef]
- Dimova, G.; Sabotinova, D.; Rosenova, Y.; Zhelev, I. Changes in the content of phenolic compounds in cotinus coggygria scop. leaves collected in different months and Antimicrobial Activity of Extracts Thereof. Certif. J. Rosenovaal. World J. Pharm. Res. 2023, 12, 1125. [Google Scholar] [CrossRef]
- Andonova, T.; Muhovski, Y.; Slavov, I.; Vrancheva, R.; Georgiev, V.; Apostolova, E.; Naimov, S.; Mladenov, R.; Pavlov, A.; Dimitrova-Dyulgerova, I. Phenolic Profile, Antioxidant and DNA-Protective Capacity, and Microscopic Characters of Ailanthus altissima Aerial Substances. Plants 2023, 12, 920. [Google Scholar] [CrossRef] [PubMed]
- Poluyanov, A.M.; Sokolova, A.Y.; Koynova, A.D.; Kulikova, S.D.; Malashenko, E.A.; Bobkova, N.V. Identification and Quantitative Determination of Flavonoids by HPLC-UV Method in the Raw Materials of Some Representatives of the Genus Rumex of Three Vegetation Time. Drug Dev. Regist. 2023, 12, 134–142. [Google Scholar] [CrossRef]
- Gerasimov, M.A.; Perova, I.B.; Eller, K.I.; Akimov, M.Y.; Sukhanova, A.M.; Rodionova, G.M.; Ramenskaya, G.V. Investigation of Polyphenolic Compounds in Different Varieties of Black Chokeberry Aronia melanocarpa. Molecules 2023, 28, 4101. [Google Scholar] [CrossRef] [PubMed]
- Kurkin, V.A.; Zimenkina, N.I. HPLC Determination of Myricitrin in Juglans nigra L. Bark. Pharm. Chem. J. 2021, 55, 881–885. [Google Scholar] [CrossRef]
- Epshtein, N.A. Validation of Chromatographic Methods: Checking the Peak Purity and the Specificity of Methods with Diode Array Detectors (Review). Drug Dev. Regist. 2020, 9, 129–136. [Google Scholar] [CrossRef]
- Voronin, K.S.; Fenin, A.A.; Zhevlakova, A.K.; Zavadskii, S.P.; Selivanova, I.A. Polyphenolic Profile of Larch Knotwood. Pharm. Chem. J. 2021, 55, 781–786. [Google Scholar] [CrossRef]
- Voronin, K.S.; Fenin, A.A.; Zhevlakova, A.K.; Pyzhov, V.S.; Selivanova, I.A. Development and Validation of a Method for Simultaneous Quantification of Dihydroquercetin and Secoisolariciresinol. Pharm. Chem. J. 2023, 57, 740–744. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Wang, Y.; Li, C.; Zhang, M.; Chen, H.; Chen, W.; Zhong, Q.; Pei, J.; Chen, W.; et al. Unraveling the Antioxidant Activity of 2R,3R-dihydroquercetin. Int. J. Mol. Sci. 2023, 24, 14220. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A.G.M.; Branco, A.; Câmara, C.A.; Silva, T.M.S.; Silva, T.M.G.; de Oliveira, A.P.; Santos, A.D.d.C.; Dutra, L.M.; Rolim, L.A.; de Oliveira, G.G.; et al. Identification of flavonoids in Hymenaea martiana Hayne (Fabaceae) by HPLC-DAD-MSn analysis. Nat. Prod. Res. 2021, 35, 2414–2419. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Yang, D.; Ha, S.H.; Lee, S.Y. Biosynthesis of dihydroquercetin in Escherichia coli from glycerol. bioRxiv 2020, 11, 401000. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, W.T.; So, Y.S.; Lim, H.; Bin; Lee, J. Taxifolin and sorghum ethanol extract protect against hepatic insulin resistance via the mir-195/irs1/pi3k/akt and ampk signalling pathways. Antioxidants 2021, 10, 1331. [Google Scholar] [CrossRef] [PubMed]
- Abad-García, B.; Garmón-Lobato, S.; Berrueta, L.A.; Gallo, B.; Vicente, F. A fragmentation study of dihydroquercetin using triple quadrupole mass spectrometry and its application for identification of dihydroflavonols in Citrus juices. Rapid Commun. Mass Spectrom. 2009, 23, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- Kiehlmann, E.; Li, E.P.M. Isomerization of dihydroquercetin. J. Nat. Prod. 1995, 58, 450–455. [Google Scholar] [CrossRef]
- Choonara, I.; Haynes, B.; Cholerton, S.; Breckenridge, A.; Park, B. Enantiomers of warfarin and vitamin K1 metabolism. Br. J. Clin. Pharmacol. 1986, 22, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Boulton, D.W.; Fawcett, J.P. Enantioselective Disposition of Albuterol in Humans. Clin. Rev. Allergy Immunol. 1996, 14, 115–138. [Google Scholar] [CrossRef]
- FDA’s policy statement for the development of new stereoisomeric drugs. Chirality 1992, 4, 338–340. [CrossRef]
- Gaggeri, R.; Rossi, D.; Christodoulou, M.S.; Passarella, D.; Leoni, F.; Azzolina, O.; Collina, S. Chiral Flavanones from Amygdalus lycioides Spach: Structural Elucidation and Identification of TNFalpha Inhibitors by Bioactivity-guided Fractionation. Molecules 2012, 17, 1665–1674. [Google Scholar] [CrossRef]
- Towatari, K.; Yoshida, K.; Mori, N.; Shimizu, K.; Kondo, R.; Sakai, K. Polyphenols from the Heartwood of Cercidiphyllum japonicum and their Effects on Proliferation of Mouse Hair Epithelial Cells. Planta Med. 2002, 68, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, G.I.; Nishioka, I.; Goto, Y.; Kinjo, J.E.; Nohara, T. Tannins and Related Compounds. LII. Studies on the Constituents of the Leaves of Thujopsis dolabrata SIEB. et ZUCC. Chem. Pharm. Bull. 1987, 35, 1105–1108. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).