Abstract
This study introduces a novel approach for enhancing the transdermal permeability of ibuprofen through the skin by utilising a rotating magnetic field (RMF). The core objective is to systematically evaluate the influence of a 50 Hz RMF on ibuprofen’s skin permeability across various formulation types, each employing distinct physical forms and excipients. The experimental setup involved Franz cells with skin as the membrane, exposed to a 50 Hz RMF in conjunction with specific formulations. Subsequent comprehensive analysis revealed a notable increase in the transdermal transport of ibuprofen, irrespective of the formulation employed. Notably, the differences in the initial 30 min of permeation were particularly pronounced. Crucially, this investigation establishes that the application of a 50 Hz RMF resulted in a remarkable over-sevenfold increase in ibuprofen permeability compared to the control group without RMF exposure. It is noteworthy that in all semi-solid pharmaceutical formulations tested, RMF effectively reduced the delay time to zero, underscoring the efficiency of RMF in overcoming barriers to transdermal drug delivery. This research positions the application of RMF as a highly promising and innovative technology, significantly enhancing the transdermal penetration of anti-inflammatory and analgesic drugs through the skin. The demonstrated effectiveness of RMF across diverse formulations suggests its potential in transdermal drug delivery, offering a novel and efficient strategy for improving therapeutic outcomes in the administration of ibuprofen and potentially other pharmaceutical agents.
1. Introduction
The delivery of pharmaceutical agents through biological barriers, including the skin, is an intriguing facet of active substance transport. This non-invasive approach offers numerous advantages, such as bypassing hepatic metabolism, reducing degradation risks in the gastrointestinal tract, and minimising unwanted interactions with food components or other medications. It is particularly valuable in managing chronic diseases by reducing the dosing frequency for drugs with short biological half-lives. However, the skin’s barrier properties pose inherent challenges, restricting substance permeability [1,2,3,4,5,6,7,8].
Various strategies have been devised to enhance the transport of therapeutic substances through the skin, including molecular modifications, formulation selection, and barrier property alterations. Non-invasive techniques utilising magnetic fields have also been explored, offering promise for efficient drug delivery without the need for invasive procedures [9,10,11].
Traditional methods in this field often employ constant magnetic fields, necessitating the use of strong magnets near the biological barrier to create a magnetic field gradient. While effective, they require separating active substances from magnetic carriers, which can be challenging. In contrast, a pulsed magnetic field, exemplified by the dermaportation system, surrounds the biological membrane with a magnetic coil. This approach limits the magnetic field’s influence on the biological barrier, but it does not affect active substances, limiting its effectiveness [12].
It is noteworthy that there is a conspicuous gap in the existing body of research concerning the utilisation of a rotating magnetic field in drug delivery. This unconventional method harnesses electric fields induced by the magnetic field, propelling active substances and facilitating their movement across biological barriers, such as the skin. The potential of the rotating magnetic field to augment drug transport introduces a novel dimension to pharmaceutical science, holding the promise of overcoming prevailing limitations and enhancing therapeutic outcomes.
The impetus behind our exploration of this novel approach lies in its apparent novelty and the perceived unexplored potential it presents. The unique ability of the rotating magnetic field to induce electric fields led us to hypothesise that this method could offer a distinct and efficient mechanism for propelling active substances across biological barriers. This anticipation of transformative benefits prompted our investigation into the potential advantages of the rotating magnetic field, adding a layer of scientific curiosity to its exploration.
In this study, we thoroughly examine the impact of the rotating magnetic field on the permeability of ibuprofen from various pharmaceutical formulations. This investigation highlights the method’s potential as a non-invasive drug delivery approach with broad clinical applications. The novelty of our research lies in the application of a rotating magnetic field to enhance drug permeability through the skin. Specifically, we employed a 50 Hz rotating magnetic field, and commercially available preparations containing ibuprofen served as our model drug. The rotating magnetic field method offers distinct advantages over traditional permeability-enhancing techniques, particularly in its non-invasiveness, unique mechanism of action, and practical considerations.
2. Results
Owing to its numerous advantages, transdermal drug delivery stands as an intriguing alternative to the oral route. Regrettably, this method of drug administration is not without its share of challenges, primarily linked to the restricted passage of active pharmaceutical ingredients (API) through the protective skin barrier. This study presents research findings that explore the application of a rotating magnetic field in enhancing the permeability of ibuprofen through the skin. Four different commercially available formulations containing ibuprofen were employed in the investigation. The study examined the permeability of ibuprofen through pigskin both in the presence (MF—magnetic field) and absence (C—control) of exposure to a rotating magnetic field. A comparative analysis of the permeability results between the magnetic field-assisted and control conditions is illustrated in Figure 1.
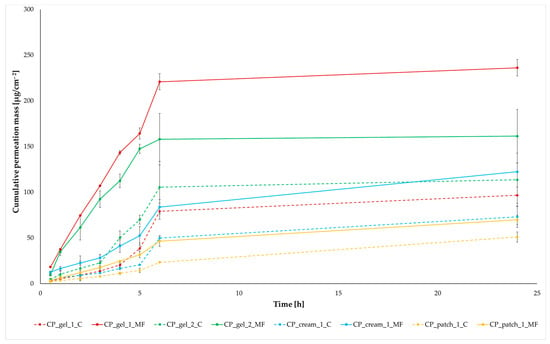
Figure 1.
IBU permeation profiles from different formulations with (MF) and without (C) rotating magnetic field exposure. The values are the means with standard deviation; n = 3.
The permeability of the active substance in tests using a rotating magnetic field is significantly higher, regardless of the formulation used. It is clear that the permeability itself depends on the type and composition of the formulation. The highest permeability, regardless of the tested time point, was obtained for CP_gel_1_MF; after 24 h of testing, 236.099 µg/cm2 IBU permeated from this preparation (Figure 1 and Figure 2). In the case of this preparation, the largest difference between the control sample and the test in the presence of a magnetic field is visible—an increase of approximately 144 to 695%, depending on the measurement point. The lowest permeability was obtained for the control permeation from the patch (CP_patch_1_C), 50.993 µg/cm2 IBU after 24 h of testing, an increase compared to the control sample of approximately 37 to 127%. Analysing all time points at which the acceptor fluid was collected for analysis, the highest penetration was observed for CP_gel_1_PM and CP_gel_2_PM. These compounds form a separate group that differs significantly from the others (Figure 3).
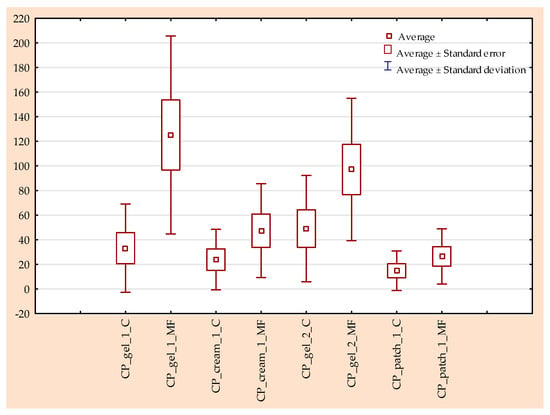
Figure 2.
Box-plot of cumulative mass of IBU throughout the entire 24 h study with and without rotating magnetic field exposure.
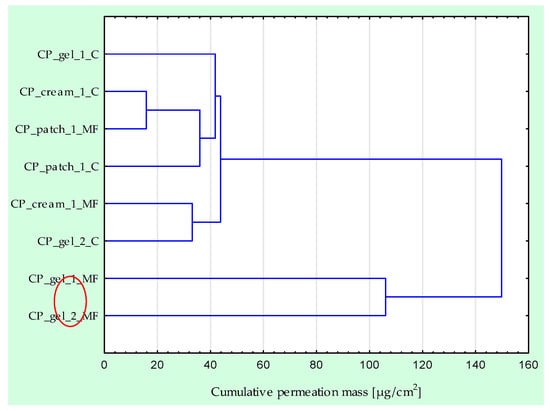
Figure 3.
Cluster analysis graph for the mean accumulated mass of IBU throughout the 24 h study with and without rotating magnetic field exposure. The compounds that penetrate best throughout the 24 h study form a separate cluster (red circle).
In the case of ibuprofen and other pain-relief medications, expeditious skin permeation is desirable to expedite the therapeutic response. This urgency arises from the fact that swift and enhanced permeation leads to a more rapid reduction in inflammation within the underlying tissues [13]. Figure 4 displays the permeation rate calculated for each time interval. Typically, the highest permeation rate into the acceptor fluid was observed in samples obtained during the initial phase of the experiments, particularly within the first hour. Consequently, this suggests that the pain-relief effect will manifest promptly under such conditions. The highest permeation rate into the acceptor fluid for semi-solid formulations, when subjected to a magnetic field, was consistently observed within the initial hour of the experiments. Consequently, this implies a notably rapid onset of the pain-relief effect. In contrast, the control trial, which reached its maximum penetration rate between 3 and 6 h, would entail a prolonged waiting period for patients, and there is even the possibility of the drug being inadvertently rubbed off the skin before it fully penetrates. Interestingly, even after the same time frame, the test utilising a magnetic field maintained a slightly higher permeation rate than the control test. Comparatively, peak permeation rates were achieved over an extended testing period when examining solid formulations. In this instance, akin to the semi-solid variant, testing in the presence of a magnetic field led to an increase in permeation rate, albeit with the highest rate being reached after a more extended period, specifically after 6 h. Importantly, when employing a medical patch, there is no risk of unintentionally removing the medication from the skin surface until it has been intentionally taken off.
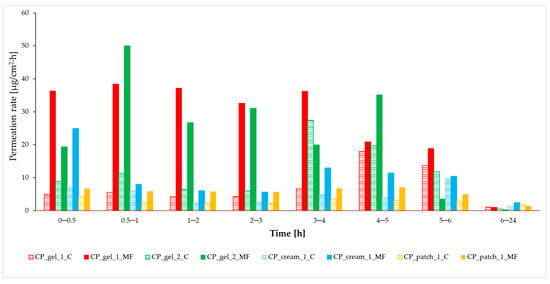
Figure 4.
Permeation rate of IBU from different formulations with and without rotating magnetic field exposure during the 24 h permeation, α = 0.05 (mean ± SD, n = 3).
The ibuprofen content in the acceptor fluid collected at 0.5 h and 24 h of permeation is summarised in Table 1. When we analyse the permeation of IBU separately under the influence of a rotating magnetic field (RMF) and without RMF, it becomes evident that in the presence of RMF, the cumulative mass determined after 0.5 h of permeation exhibited the following order: CP_gel_1_MF > CP_cream_1_MF > CP_gel_2_MF > CP_patch_1_MF. Notably, CP_gel_1_MF had the highest concentration, with a cumulative mass of IBU measuring 18.163 ± 2.564 µg/cm2. A similar trend persisted for the cumulative mass determined after 24 h of testing in the presence of RMF, where CP_gel_1_MF also demonstrated the most significant result, specifically 236.099 ± 26.206 µg/cm2. Conversely, a slightly different pattern emerged in the absence of RMF. Without RMF, the highest result after 0.5 and 24 h, seen for CP_gel_2_C, amounted to 4.446 ± 1.601 and 113.618 ± 6.159 µg/cm2, respectively.

Table 1.
Cumulated mass of IBU from different formulations after 0.5 h and 24 h of the examination with and without rotating magnetic field exposure; different letters indicate significant differences between the results obtained using RMF and the control sample; p < 0.05, mean ± SD, n = 3. The statistically significant difference was estimated by ANOVA using Tukey’s test.
The results of ex vivo permeation experiments evaluating ibuprofen efficiency are summarised in Table 2. Permeation parameters were calculated, including flux, apparent permeability coefficient, lag time, diffusion coefficient in the skin, skin partition coefficient, percent drug permeated after 24 h, and enhancement factor. There was a noticeable contrast in the steady-state flux depending on exposure to the magnetic field applied during the research. Ibuprofen exhibited the least penetration (3.01 ± 0.38 µg/cm2) when the rotating magnetic field was not utilised (CP_patch_1_C). This observation is consistent across all diffusion parameters. Conversely, all the tested semi-solid formulations showed higher percutaneous flux, with steady-state flux measurements of 4.43 ± 0.36 µg/cm2 for CP_cream_1_C, 9.26 ± 1.80 µg/cm2 for CP_gel_1_C, and 18.74 ± 4.45 µg/cm2 for CP_gel_2_C, respectively. This parameter exhibited significant increases when RMF was employed, particularly in the case of CP_gel_1_MF, yielding the best results (30.54 ± 3.42 µg/cm2). In the case of CP_gel 1, a significant difference was also demonstrated between the penetration with and without a magnetic field, analysing all time points during the 24 h study (Table 3).

Table 2.
Permeation parameters of IBU from different formulations using RMF (f = 50 Hz, Bmax ≈ 34 mT) or without RMF.

Table 3.
Significant differences in the cumulative mass of IBU, taking into account all time points during the entire 24 h permeation, were estimated by the Mann–Whitney test.
Furthermore, the permeability coefficient (KP), a quantitative measure of the rate at which a molecule can traverse the skin barrier, was also determined. KP takes into account factors related to both the drug and the barrier, considering their interactions and eliminating the impact of compound concentration. For the tested formulations, KP values ranged from 0.88 ± 0.07 × 103 cm/h for CP_cream_1_C to 3.75 ± 0.89 × 103 cm/h for CP_gel_2_C when RMF exposure was applied. In contrast, they ranged from 1.85 ± 0.60 × 103 cm/h for CP_cream_1_MF to 6.11 ± 0.68 × 103 cm/h for CP_gel_1_MF when magnetic fields were used.
Significantly, a profound effect of the rotating magnetic field on the lag time was observed. All semi-solid samples exhibited no lag time, indicating that the substance commenced penetrating the skin almost immediately. In practical terms, this suggests that the substance can permeate the barrier almost instantly upon application, which can be pivotal in achieving a rapid drug response. This contrasts with the control sample, where the release time ranged from 0.32 to 1.37 h. The lag times for the patch were similar, approximately 0.2 h. Additionally, the percentage of the permeated applied dose was determined, revealing the highest enhancement in ibuprofen penetration when using a magnetic field for the CP_gel_1 formulation and the lowest enhancement for CP_patch_1.
3. Discussion
The study presented explores the potential of a rotating magnetic field (RMF) in enhancing the permeability of ibuprofen through the skin. The results indicate a significant impact of RMF on the permeation of ibuprofen, with promising implications for transdermal drug delivery.
As is known, transdermal drug delivery is a valuable alternative to oral administration due to its numerous advantages, including controlled release, reduced side effects, and improved patient compliance. However, the skin barrier presents a challenge, limiting the passage of active pharmaceutical ingredients (API) into the bloodstream. The use of a rotating magnetic field (RMF) significantly improved the permeability of ibuprofen, regardless of the formulation used. The highest permeability was observed for CP_gel_1_MF, indicating that the specific formulation played a role in the extent of permeation. In the case of ibuprofen and similar pain-relief medications, rapid skin permeation is desirable for achieving a swift therapeutic response. The study demonstrated that the highest permeation rates occurred within the initial hours, particularly within the first hour, suggesting a prompt onset of pain relief. In contrast, the control group, without the assistance of RMF, exhibited a delayed onset of permeation, reaching the maximum penetration rate between 3 and 6 h. This could lead to a longer waiting time for patients and a risk of the drug being rubbed off before full penetration. The study considered various permeation parameters, which offered insights into the mechanisms involved in the permeation process. One significant observation was the complete elimination of lag time in all semi-solid samples when RMF was employed. This implies that the substance began permeating the skin almost immediately, which could be critical for achieving a rapid therapeutic effect. In contrast, control samples exhibited lag times ranging from 0.32 to 1.37 h.
The results suggest that the choice of formulation plays a crucial role in drug permeation. CP_gel_1_MF demonstrated the highest concentration and permeability, while CP_patch_1_C showed the lowest permeation. The results of the experiments, particularly the differences in permeability observed between the various formulations and the impact of a rotating magnetic field (RMF), can be analysed in the context of the ingredients used in the different preparations. All semi-solid products used in this research are available in the active version of 50 mg/g. The presence of isopropyl alcohol and poloxamer 407 in CP_gel_1, which are known for their solubilising and penetration-enhancing properties, may contribute to the enhanced permeability when exposed to RMF. Similar to CP_gel_1, CP_gel_2 also contains isopropyl alcohol and another alcohol, ethanol. The inclusion of ethanol and isopropyl alcohol could contribute to the better solubility of ibuprofen, possibly leading to higher permeation rates when using RMF. The greater permeability enhancement using gel formulations is most likely related to the lower viscosity of these systems. In the case of CP_cream_1, the presence of glycerol monostearate and xanthan gum might affect the formulation’s texture and, subsequently, its permeability properties. In contrast to the semi-solid forms, this formulation is in solid patch form and contains 200 mg of the active substance per patch. The excipients include various polymers and materials to form the adhesive, coating, and protective layers. The solid form of this preparation naturally has different permeation characteristics compared to the semi-solid formulations. The use of RMF played a significant role in enhancing the permeability of all formulations. However, the specific composition of each formulation, including the excipients and their solubilising properties, has contributed to the observed differences in permeability. For example, CP_gel_1 exhibited the highest permeability when RMF was applied, and this might be attributed to the presence of isopropyl alcohol and poloxamer 407, which are known for their permeation-enhancing properties.
In our investigation, the composition of the formulation containing isopropyl alcohol and poloxamer 407 was found to significantly impact the skin permeability of ibuprofen. To provide a comprehensive understanding of this phenomenon, we delve into the mechanistic details of how the application of the rotating magnetic field (RMF) contributes to the controlled modulation of skin permeability. The RMF, operating at a frequency of 50 Hz, induces electric fields that interact with the formulation components, resulting in a series of dynamic changes. Firstly, the electric fields influence the solubility of ibuprofen within the formulation, affecting its overall availability for transdermal transport. Additionally, the RMF alters the diffusion kinetics of the drug through the formulation, impacting the rate at which ibuprofen permeates the skin. Moreover, the interaction between the RMF and the formulation components plays a crucial role in the drug’s interaction with the skin barrier. This intricate interplay affects the skin’s permeability characteristics, providing a controlled and modulated environment for drug transport. These insights contribute to a more robust understanding of the observed changes in skin permeability induced by the combination of the active pharmaceutical ingredient and the formulation base in the presence of the rotating magnetic field.
In summary, the composition of the formulations, including the choice of excipients, played a crucial role in determining the permeability of ibuprofen through the skin. The use of a rotating magnetic field further enhanced this permeability, making it a potential method for improving transdermal drug delivery. Additional studies and clinical trials may be needed to explore the safety and efficacy of these formulations and the impact of RMF in clinical applications.
The findings have practical implications for transdermal drug delivery, especially for pain-relief medications where rapid onset is critical. The implications of our findings bear direct relevance to transdermal drug delivery, particularly in the context of pain-relief medications where achieving a rapid onset is of paramount importance. The application of a rotating magnetic field (RMF) emerges as a potential strategy to enhance the efficiency of drug delivery through the skin. In practical terms, this study underscores the promising role of RMF in improving the permeability of ibuprofen, thereby offering potential advancements in the efficacy of transdermal drug delivery. RMF can potentially be utilised to enhance the efficiency of drug delivery through the skin. In conclusion, this study showcases the potential of a rotating magnetic field in improving the permeability of ibuprofen, which is promising for enhancing the efficacy of transdermal drug delivery. The choice of formulation and the application of RMF are crucial factors that impact the extent and speed of drug permeation. Further research and clinical trials may be needed to fully evaluate the safety and effectiveness of this approach in clinical settings.
4. Materials and Methods
4.1. Materials
All the reagents and solvents were obtained from commercial sources and utilised without further purification. AmBeed (Arlington Hts, IL, USA) supplied (R,S)-ibuprofen (IBU) (2-(4-isobutylphenyl)propanoic acid) (IBU). Baker (Radnor, PA, USA) supplied acetonitrile. POCH (Gliwice, Poland) delivered the methanol and the procurement of potassium dihydrogen phosphate (p.a.) from Merck (Darmstadt, Germany). Sigma-Aldrich (Steinheim am Albuch, Germany) was the supplier of the PBS tablets. A local abattoir supplied porcine hide.
Four commercial preparations (CP) containing ibuprofen were selected for experimentation, each available in different formulations, either semi-solid or solid, and distinguished by the presence of various excipients: (1) CP_gel_1 (semi-solid form)—active substance content: 50 mg/g. Excipients: isopropyl alcohol, solketal (2,2-dimethyl-4-hydroxymethyl-1,3-dioxalate), poloxamer 407, medium-chain triglycerides of saturated fatty acids (Miglyol 812), lavender oil, orange oil, purified water. (2) CP_gel_2 (semi-solid form)—active substance content: 50 mg/g. Excipients: ethanol (96%), isopropyl alcohol, hydroxyethylcellulose, levomenthol, Reflex 12122 flavour (methyl salicylate), diethylene glycol monoethyl ether, macrogolglycerides caprycaproates (Makrogol 400), glycerol, sodium hydroxide (10% aqueous solution), purified water. (3) CP_cream_1 (semi-solid form)—active substance content: 50 mg/g. Excipients: triglycerides of medium-chain saturated fatty acids, glycerol monostearate, macrogol-30-glycerol monostearate, macrogol-100-glycerol monostearate, propylene glycol, sodium methyl parahydroxybenzoate, xanthan gum, lavender oil, orange oil, purified water. (4) CP_patch (solid form)—active substance content: 200 mg/patch. Excipients: adhesive layer: macrogol 400, macrogol 20000, levomenthol, block copolymer Styrene-Isoprene-Styrene 22, polyisobutylene (PIB) 55k, polyisobutylene (PIB) 1100k, ester of hydrogenated rosin with glycerol, liquid paraffin. Coating layer: ethylene terephthalate, woven. Protective layer: siliconised ethylene polyterephthalate.
4.2. Exposure to Rotating Magnetic Field (RMF)
Experiments were conducted using a set of reactors equipped with an external electromagnetic field generator. The current study system was composed of two identical reactors (one with the exposure of a rotating magnetic field and the second without the control process) connected to the same heat source, which prevented any temperature fluctuation impact. The utilised system of reactors is presented in Figure 5.
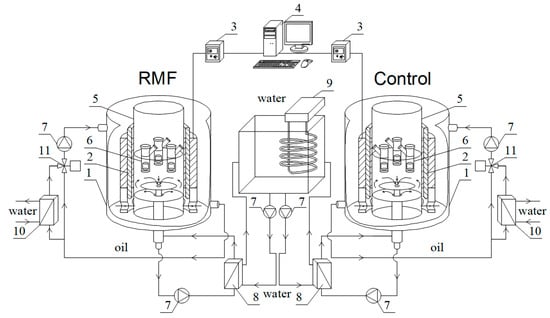
Figure 5.
Schematic of experimental reactor setup: (1) tank; (2) 3-phase coils; (3) phase inverter; (4) PC; (5) process chamber; (6) Franz diffusion cells; (7) pump; (8) plate heat exchanger; (9) precise thermostat; (10) oil heat exchanger; (11) 3-way valve.
The experimental setup was composed of two identical reactors. Each reactor consists of a stainless steel tank (1), inside which a set of 3-phase coils was placed, powered by the AC through the phase inverter (3) connected to PC (4), allowing control of the current–voltage and other properties. In the current studies, we utilised currents of 100 V and 50 Hz. The rotating magnetic field (RMF), a specific form of electromagnetic field, was created by powering those coils with maximal magnetic induction at Bmax = 34 mT. A transparent process chamber (5) is placed axially in the centre of the coils. A holder with Franz diffusion cells (6) is placed inside the process chamber. This holder is rinsed in the process water circulated by the pump (7) through the plate heat exchanger (8). On the other side, this heat exchanger is supplied by hot water from the precise thermostat, which maintains the temperature at a constant level with only slight changes of 0.1 Celsius. It should be highlighted that the single thermostat is a heat source for both reactors. According to Figure 5, therefore, any temperature fluctuations will not affect differences in results comparing these two reactors. It should be noted that the powered coils are generating additional heat because of the electric resistance of the wires. Therefore, we rinsed those coils in a non-toxic silicon oil with electric insulator properties. The silicon oil is circulated through an external loop equipped with another heat exchanger (10). Therefore, the tap water can cool down part of the oil stream to remove excessive heat from the system. The oil flow in this external loop is controlled by a 3-way valve (11) with an electric engine connected to the temperature sensor and controller, which allows constant oil temperature.
Permeability studies were conducted using Franz diffusion cells (Phoenix DB-6, ABL&E-JASCO, Wien, Austria) equipped with diffusion areas of 1 cm2. The acceptor chamber, filled with a PBS solution (pH 7.4), maintained a constant temperature of 37.0 ± 0.1 °C. As the permeable membrane, pig skin with a thickness of 0.05 cm and suitable impedance was employed. The fresh porcine skin was washed in PBS buffer pH 7.4 and then dermatomed to the needed thickness. The skin samples were stored in aluminium foil in a freezer at −20 °C for three months to preserve their skin barrier properties. Before use, the skin samples were thawed slowly at room temperature for 30 min. Next, the skin was mounted on the donor chamber. The integrity of the skin has been examined by checking its impedance, which was measured using an LCRmeter4080 (Conradelectronic, Wernberg-Köblitz, Germany). Each donor chamber contained 1 g of a semi-solid formulation or a patch segment. The experiments extended over 24 h, and samples were collected and analysed at specific time intervals: 0.5 h, 1 h, 2 h, 3 h, 4 h, 5 h, 6 h, and 24 h. The API content in the samples was quantified using HPLC via the standard curve method. The HPLC system (Knauer, Berlin, Germany) consisted of a 2600 UV detector, a Smartline model 1050 pump, and a Smartline model 3950 autosampler operated with ClarityChrom 2009 software. A 125 × 4 mm chromatographic column packed with Hyperisil ODS (C18) of 5 µm particle size was used. The mobile phase was a mixture of 0.02 mol/dm3 potassium dihydrogen phosphate, acetonitrile, and methanol (45/45/10, v/v/v) adjusted to pH 2.5 with orthophosphoric acid. The spectrophotometric detector was set at 220 nm, with a 1 cm3/min flow rate. The column temperature was maintained at 25 °C, and the injection volume was 20 μL.
The kinetic profiling of ex vivo infinite-dose steady-state percutaneous absorption was characterised using Fick’s laws of diffusion, as is common practice [14,15,16]. Key permeation parameters, including flux (JSS), diffusion coefficient (KP), lag time (LT), diffusion coefficient (D), and skin partition coefficient (Km), were determined.
4.3. Statistical Analysis
The results are expressed as the mean ± standard deviation (SD). We employed a one-way analysis of variance (ANOVA) for the analysis. Specifically, to assess the significance of differences between individual groups regarding the cumulative mass after 24 h of permeation and the cumulative mass in the skin, the Tukey test (α < 0.05) was utilised. A cluster analysis was carried out to determine similarities between all preparations tested, i.e., those exposed to a magnetic field and those not exposed, considering all time points of acceptor fluid uptake. On this basis, groups of compounds with similar permeation were presented. Furthermore, the Mann–Whitney test was used to determine significant differences in cumulative mass between compounds subjected to magnetic field exposure and those without exposure, considering all time points of acceptor fluid uptake. All statistical calculations were performed using Statistica 13 PL software 13.3 (StatSoft, Kraków, Poland).
Author Contributions
Conceptualisation, P.O.-R. and R.R.; investigation, P.O.-R.; methodology, A.N. and M.K.; formal analysis, A.N., M.K., A.M.-S. and Ł.K.; writing—original draft preparation, A.N. and P.O.-R.; writing—review and editing, P.O.-R. and R.R.; visualisation, A.N. and M.K.; supervision, P.O.-R. and R.R.; project administration, P.O.-R.; funding acquisition P.O.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Centre for Research and Development, grant number LIDER/53/0225/L-11/19/NCBR/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Morrow, D.I.J.; McCarron, P.A.; Woolfson, A.D.; Donnelly, R.F. Innovative Strategies for Enhancing Topical and Transdermal Drug Delivery. Open Drug Deliv. J. 2007, 1, 36–59. [Google Scholar] [CrossRef]
- Benson, H.A.E. Skin Structure, Function, and Permeation. In Topical and Transdermal Drug Delivery; Benson, H.A.E., Watkinson, A.C., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 1–22. ISBN 978-1-118-14050-5. [Google Scholar]
- Cal, K. Across Skin Barrier: Known Methods, New Performances. In Frontiers in Drug Design and Discovery (Volume 4); Atta-ur-Rahman, W., Caldwell, G., Iqbal Choudhary, M., Yan, Z., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012; pp. 162–188. ISBN 978-1-60805-202-8. [Google Scholar]
- Pandey, A.; Gupta, S. Evaluation of Formulated Transdermal Patches. J. Popul. Ther. Clin. Pharmacol. 2023, 30, 793–798. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent Advances in Transdermal Drug Delivery Systems: A Review. Biomater. Res. 2021, 25, 24. [Google Scholar] [CrossRef] [PubMed]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement Strategies for Transdermal Drug Delivery Systems: Current Trends and Applications. Drug Deliv. Transl. Res. 2022, 12, 758–791. [Google Scholar] [CrossRef] [PubMed]
- Higo, N. The Recent Trend of Transdermal Drug Delivery System Development. Yakugaku Zasshi 2007, 127, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.G.; Ece, E.; Erdem, Ö.; Eş, I.; Inci, F. A Sustainable Solution to Skin Diseases: Ecofriendly Transdermal Patches. Pharmaceutics 2023, 15, 579. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Ahmed, A.B. Natural Permeation Enhancer for Transdermal Drug Delivery System and Permeation Evaluation: A Review. Asian J. Pharm. Clin. Res. 2017, 10, 5. [Google Scholar] [CrossRef]
- Parhi, R.; Mandru, A. Enhancement of Skin Permeability with Thermal Ablation Techniques: Concept to Commercial Products. Drug Deliv. Transl. Res. 2021, 11, 817–841. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C.; Barry, B.W. Penetration Enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Namjoshi, S.; Chen, Y.; Edwards, J.; Benson, H.A.E. Enhanced Transdermal Delivery of a Dipeptide by Dermaportation. Biopolymers 2008, 90, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Savoy, A.W. Ionic Liquids Synthesis and Applications: An Overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Fick, A. On Liquid Diffusion. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1855, 10, 30–39. [Google Scholar] [CrossRef]
- Higuchi, T. Physical Chemical Analysis of Percutaneous Absorption Process from Creams and Ointments. J. Soc. Cosmet. Chem. 1960, 11, 85–97. [Google Scholar]
- Scheuplein, R.J.; Blank, I.H. Permeability of the Skin. Physiol. Rev. 1971, 51, 702–747. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).