Carthamus tinctorius Suppresses LPS-Induced Anti-Inflammatory Responses by Inhibiting the MAPKs/NF-κB Signaling Pathway in HaCaT Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Plant Sample
2.2. Preparation of Ethanol Extract
2.3. Determination of Total Phenolic Content
2.4. Cell Culture
2.5. Cell Viability Assay
2.6. Nitric Oxide Production Assay
2.7. Isolation of Total RNA and Real Time-Polymerase Chain Reaction (RT-PCR)
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Western Blot Analysis
2.10. Phytochemical Analysis of C. tinctorius by UPLC-QTOF-MS/MS
2.11. Statistical Analysis
3. Results
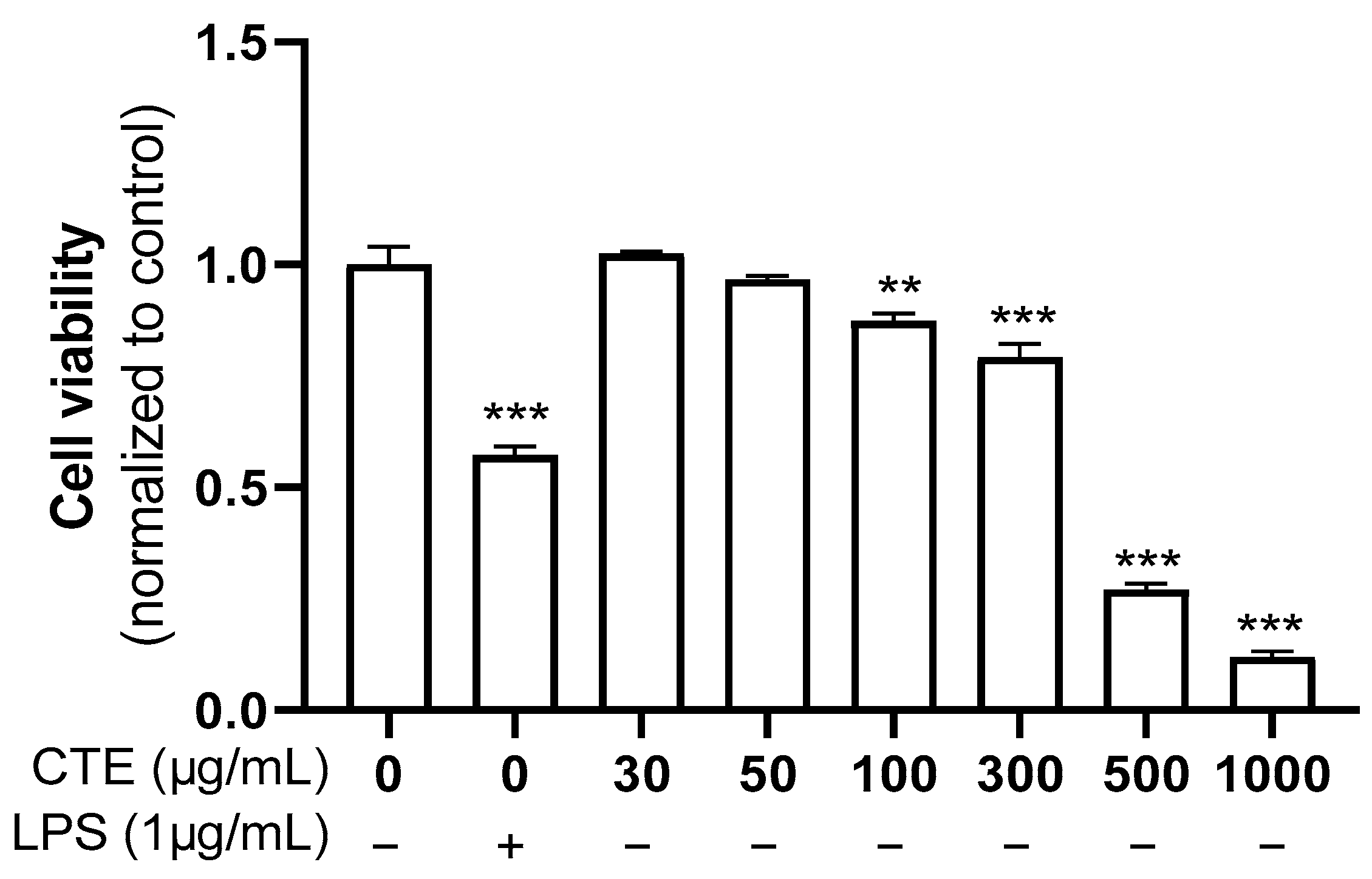
3.1. The Effect of Ethanol Extract of C. tinctorius on the Viability of HaCaT Cells
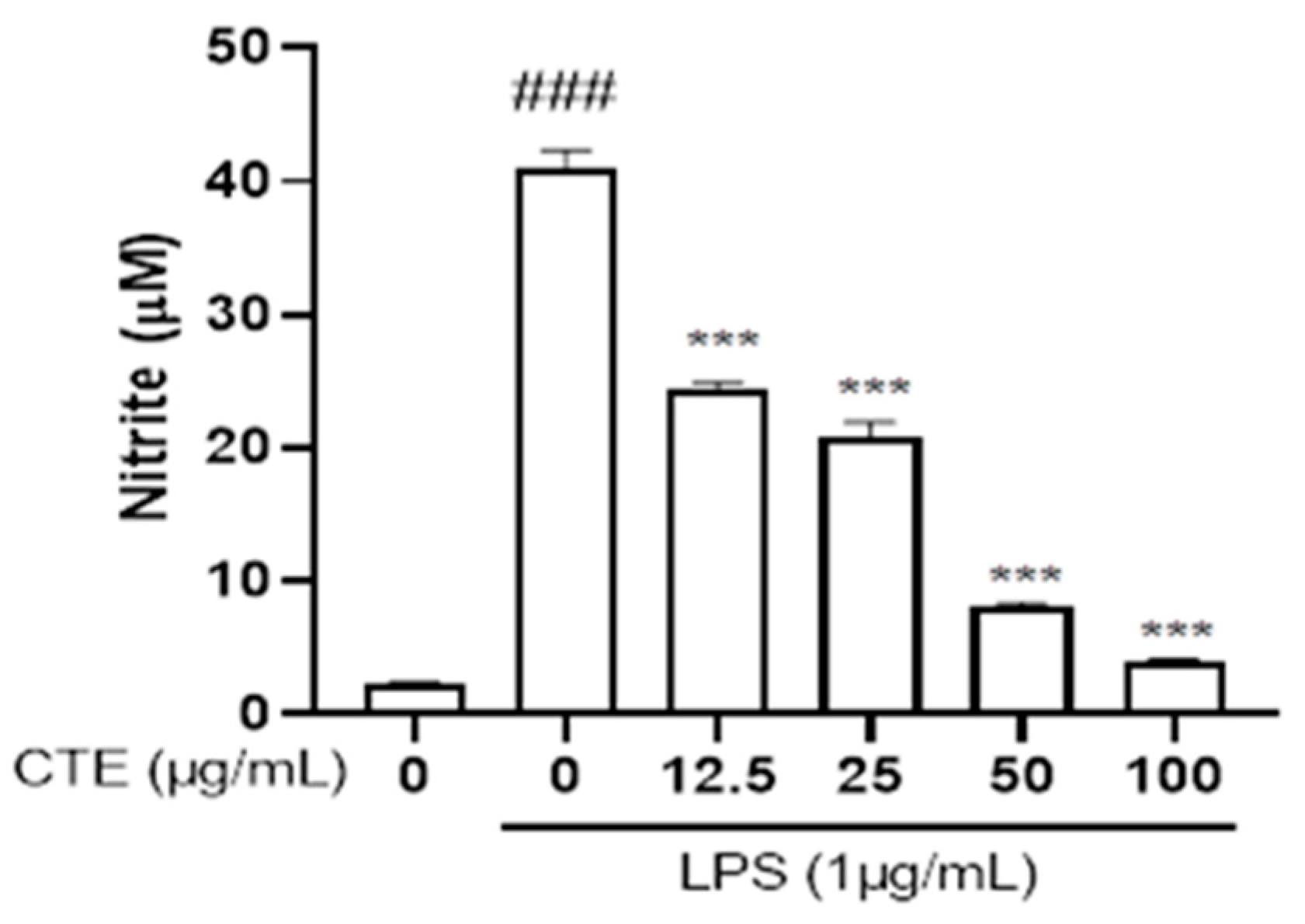
3.2. Effect of C. tinctorius Ethanol Extract on Nitric Oxide Production in LPS-Stimulated HaCaT Cells
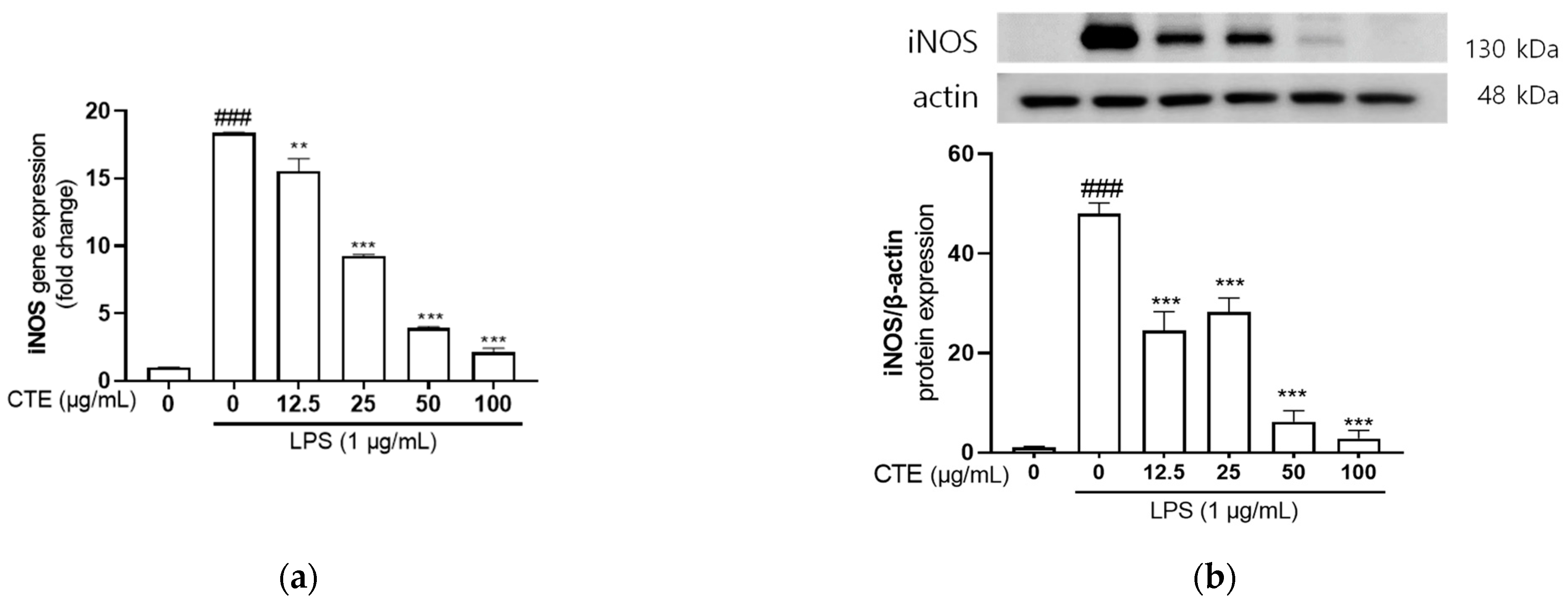
3.3. The Effect of Ethanol Extract of C. tinctorius on mRNA and Protein Expressions of iNOS in LPS-Stimulated HaCaT Cells
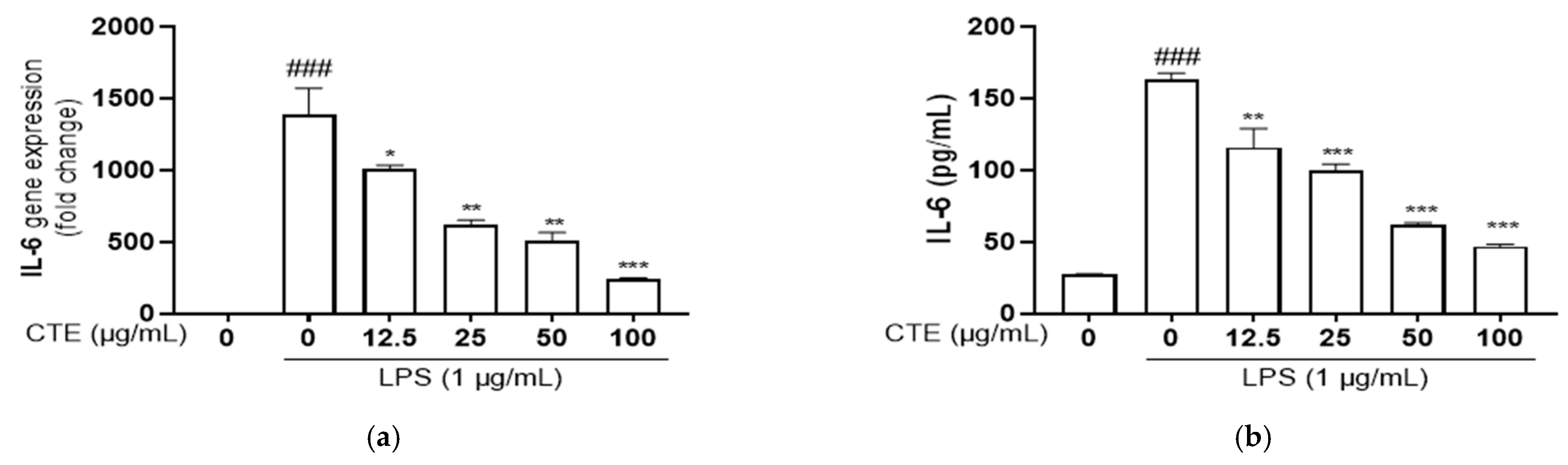
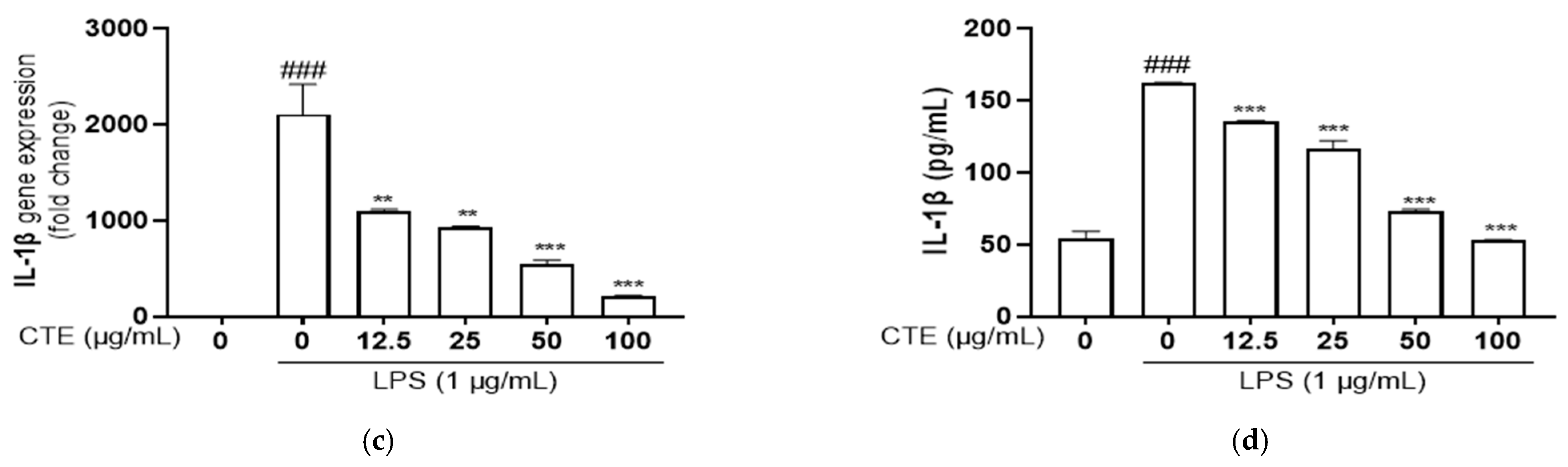
3.4. The Effect of Ethanol Extract of C. tinctorius on Pro-Inflammatory Cytokines in LPS-Stimulated HaCaT Cells
3.5. The Effect of C. tinctorius Ethanol Extract on MAPKs/NF-κB Activation in LPS-Stimulated HaCaT Cells
3.6. UPLC-QTOF-MS/MS Analysis of C. tinctorius Leaves
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Stuehr, D.J.; Vasquez-Vivar, J. Nitric oxide synthases-from genes to function. Nitric Oxide 2017, 63, 29. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Kanwar, R.K.; Burrow, H.; Baratchi, S. Recent advances on the roles of NO in cancer and chronic inflammatory disorders. Curr. Med. Chem. 2009, 16, 2373–2394. [Google Scholar] [CrossRef]
- Minghetti, L.; Pocchiari, M. Cyclooxygenase-2, prostaglandin E2, and microglial activation in prion diseases. Int. Rev. Neurobiol. 2007, 82, 265–275. [Google Scholar] [PubMed]
- Zheng, L.; Cao, H.; Qiu, J.; Chi, C. Inhibitory Effect of FMRFamide on NO Production During Immune Defense in Sepiella japonica. Front. Immunol. 2022, 13, 825634. [Google Scholar] [CrossRef]
- Jeong, J.W.; Lee, H.H.; Han, M.H.; Kim, G.Y.; Hong, S.H.; Park, C.; Choi, Y.H. Ethanol extract of Poria cocos reduces the production of inflammatory mediators by suppressing the NF-kappaB signaling pathway in lipopolysaccharide-stimulated RAW 264.7 macrophages. BMC Complement. Altern. Med. 2014, 14, 101. [Google Scholar] [CrossRef]
- Dinarello, C.A. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef]
- Mert, T.; Sahin, M.; Sahin, E.; Yaman, S. Anti-inflammatory properties of Liposome-encapsulated clodronate or Anti-Ly6G can be modulated by peripheral or central inflammatory markers in carrageenan-induced inflammation model. Inflammopharmacology 2019, 27, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.P.; Carmody, R.J. NF-κB and the Transcriptional Control of Inflammation. Int. Rev. Cell Mol. Biol. 2018, 335, 41–84. [Google Scholar]
- Barnabei, L.; Laplantine, E.; Mbongo, W.; Rieux-Laucat, F.; Weil, R. NF-κB: At the Borders of Autoimmunity and Inflammation. Front. Immunol. 2021, 12, 716469. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. Phytochemicals suppress nuclear factor-κB signaling: Impact on health span and the aging process. Curr. Opin. Clin. Nutr. Metab. Care. 2012, 15, 23–28. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Cui, X.; Lee, D.S.; Sohn, J.H.; Yim, J.H.; Kim, Y.C.; Oh, H. Anti-inflammatory effect of neoechinulin a from the marine fungus Eurotium sp. SF-5989 through the suppression of NF-κB and p38 MAPK pathways in lipopolysaccharide-stimulated RAW264.7 macrophages. Molecules 2013, 18, 13245–13259. [Google Scholar] [CrossRef]
- Lu, Y.; Yeh, W.; Ohashi, P. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Li, Y.; Wang, Z.; Xiao, M.; Yin, P.; Lu, Y.; Qian, X.; Xu, Y.; Liu, J. A novel naphthalimide derivative, exhibited anti-inflammatory effects via targeted inhibiting TAK1 following down-regulation of ERK1/2- and p38 MAPKmediated activation of NF-κB in LPS-stimulated RAW264.7 macrophages. Int. Immunopharmacol. 2013, 17, 216–228. [Google Scholar] [CrossRef]
- Ren, Q.; Guo, F.; Tao, S.; Huang, R.; Ma, L.; Fu, P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways in septic AKI mice. Biomed. Pharmacother. 2020, 122, 109772. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhu, G.; Zhu, M.; Song, J.; Cai, H.; Song, Y.; Wang, J.; Jin, M. Edaravone Attenuated Particulate Matter-Induced Lung Inflammation by Inhibiting ROS-NF-κB Signaling Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 6908884. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Fu, Y.; Bo, L.; Liu, Z.; Li, D.; Liang, D.; Wen, Z.; Cao, Y.; Zhang, N.; Zhang, X. Stevioside suppressed inflammatory cytokine secretion by Downregulation of NF-κB and MAPK signaling pathways in LPS-stimulated RAW264.7 cells. Inflammation 2012, 35, 1669–1675. [Google Scholar]
- Carter, A.B.; Knudtson, K.L.; Monick, M.M.; Hunninghake, G.W. The p38 mitogenactivated protein kinase is required for NF-kappaB-dependent gene expression. The role of TATA-binding protein (TBP). J. Biol. Chem. 1999, 274, 30858–30863. [Google Scholar] [CrossRef]
- Dong, J.; Li, J.; Cui, L.; Wang, Y.; Lin, J.; Qu, Y.; Wang, H. Cortisol modulates inflammatory responses in LPS-stimulated RAW264.7 cells via the NF-κB and MAPK pathways. BMC Vet. Res. 2018, 14, 30. [Google Scholar] [CrossRef]
- Asgary, S.; Rahimi, P.; Mahzouni, P.; Madani, H. Antidiabetic effect of hydroalcoholic extract of Carthamus tinctorius L. in alloxan-induced diabetic rats. J. Res. Med. Sci. 2012, 17, 386–392. [Google Scholar]
- Chang, Y.; Moore, P.S.; Talbot, S.J.; Boshoff, C.H.; Zarkowska, T.; Godden, K.; Paterson, H.; Weiss, R.A.; Mittnacht, S. Cyclin encoded by KS herpesvirus. Nature 1996, 382, 410. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Uhm, C.; Bae, C. Effects of safflower (Carthamus tinctorius L.) seed powder on fracture healing in rats. Korean J. Electron. Microsc. 2001, 31, 307–314. [Google Scholar]
- Zhou, X.; Tang, L.; Xu, Y.; Zhou, G.; Wang, Z. Towards a better understanding of medicinal uses of Carthamus tinctorius L. in traditional Chinese medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2014, 151, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, M.; Takahashi, T.; Komatsu, M.; Kido, T.; Kasahara, Y. Antioxidant and neuroprotective activities of Mogami-benibana (safflower, Carthamus tinctorius Linne). Neurochem. Res. 2009, 34, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Li, X.R.; Liu, J.; Peng, C.; Zhou, Q.M.; Liu, F.; Guo, L.; Xiong, L. Polyacetylene glucosides from the florets of Carthamus tinctorius and their anti-inflammatory activity. Phytochemistry 2021, 187, 112770. [Google Scholar] [CrossRef]
- Aarland, R.C.; Bañuelos-Hernández, A.E.; Fragoso-Serrano, M.; Sierra-Palacios, E.D.; Díaz de León-Sánchez, F.; Pérez-Flores, L.J.; Rivera-Cabrera, F.; Mendoza-Espinoza, J.A. Studies on phytochemical, antioxidant, anti-inflammatory, hypoglycaemic and antiproliferative activities of Echinacea purpurea and Echinacea angustifolia extracts. Pharm. Biol. 2017, 55, 649–656. [Google Scholar] [CrossRef]
- Yang, Y.I.; Woo, J.H.; Seo, Y.J.; Lee, K.T.; Lim, Y.; Choi, J.H. Protective effect of brown alga phlorotannins against hyperinflammatory responses in lipopolysaccharide-induced sepsis models. J. Agric. Food Chem. 2016, 64, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Hong, M.; Kim, T.H.; Lee, K.Y.; Park, S.J.; Hong, S.H.; Sowndhararajan, K.; Kim, S. Anti-inflammatory effect of liverwort (Marchantia polymorpha L.) and racomitrium moss (Racomitrium canescens (Hedw.) Brid.) growing in Korea. Plants 2021, 10, 2075. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.I.; Shin, H.C.; Kim, S.H.; Park, W.Y.; Lee, K.T.; Choi, J.H. 6,6′-Bieckol, isolated from marine alga Ecklonia cava, suppressed LPS-induced nitric oxide and PGE2 production and inflammatory cytokine expression in macrophages: The inhibition of NF-κB. Int. Immunopharmacol. 2012, 12, 510–517. [Google Scholar] [CrossRef]
- Sapkota, A.; Gair, B.P.; Kang, M.G.; Choi, J.W. S1P2 contributes to microglial activation and M1 polarization following cerebral ischemia through ERK1/2 and JNK. Sci. Rep. 2019, 9, 12106. [Google Scholar] [CrossRef]
- Baek, H.S.; Min, H.J.; Hong, V.S.; Kwon, T.K.; Park, J.W.; Lee, J.; Kim, S. Anti-Inflammatory Effects of the Novel PIM Kinase Inhibitor KMU-470 in RAW 264.7 Cells through the TLR4-NF-kappaB-NLRP3 Pathway. Int. J. Mol. Sci. 2020, 21, 5138. [Google Scholar] [CrossRef] [PubMed]
- Hirai, S.; Horii, S.; Matsuzaki, Y.; Ono, S.; Shimmura, Y.; Sato, K.; Egashira, Y. Anti-inflammatory effect of pyroglutamyl-leucine on lipopolysaccharide-stimulated RAW 264.7 macrophages. Life Sci. 2014, 117, 1–6. [Google Scholar] [CrossRef]
- Shin, S.; Saravanakumar, K.; Sathiyaseelan, A.; Mariadoss, A.V.A.; Park, S.; Park, S.; Han, K.; Wang, M.-H. Phytochemical profile and antidiabetic effect of the bioactive fraction of Cirsium setidens in streptozotocin-induced type 2 diabetic mice. Process Biochem. 2022, 116, 60–71. [Google Scholar] [CrossRef]
- Wang, H.; Peters, T.; Sindrilaru, A.; Kochanek, K.S. Key role of macrophages in the pathogenesis of CD18 hypomorphic murine model of psoriasis. J. Investg. Dermatol. 2009, 129, 1100–1114. [Google Scholar] [CrossRef]
- Kasraie, S.; Werfel, T. Role of macrophages in the pathogenesis of atopic dermatitis. Mediat. Inflamm. 2013, 2013, 942375. [Google Scholar] [CrossRef] [PubMed]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D.; et al. HaCaT cells as a reliable in vitro differentiation model to dissect the inflammatory/repair response of human keratinocytes. Mediat. Inflamm. 2017, 2017, 7435621. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Chiou, Y.S.; Pan, M.H.; Shahidi, F. Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages. Food Chem. 2012, 134, 742–748. [Google Scholar] [CrossRef]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Giampieri, F.; Afrin, S.; Mezzetti, B.; Quiles, J.L.; Bompadre, S.; Battino, M. Protective effect of strawberry extract against inflammatory stress induced in human dermal fibroblasts. Molecules 2017, 22, 164. [Google Scholar] [CrossRef] [PubMed]
- Rea Martinez, J.; Montserrat-de la Paz, S.; De la Puerta, R.; Garcia-Gimenez, M.D.; Fernandez-Arche, A. Characterization of bioactive compounds in defatted hempseed (Cannabis sativa L.) by UHPLC-HRMS/MS and anti-inflammatory activity in primary human monocytes. Food Funct. 2020, 11, 4057–4066. [Google Scholar] [CrossRef]
- Ciucure, C.T.; Geana, E.-I.; Sandru, C.; Tita, O.; Botu, M. Phytochemical and Nutritional Profile Composition in Fruits of Different Sweet Chestnut (Castanea sativa Mill.) Cultivars Grown in Romania. Separations 2022, 9, 66. [Google Scholar] [CrossRef]
- Liu, M.; He, M.; Gao, H.; Guo, S.; Jia, J.; Ouyang, H.; Feng, Y.; Yang, S. Strategy for rapid screening of antioxidant and anti-inflammatory active ingredients in Gynura procumbens (Lour.) Merr. based on UHPLC-Q-TOF-MS/MS and characteristic ion filtration. Biomed. Chromatogr. 2019, 33, e4635. [Google Scholar] [PubMed]
- Wang, C.; Zhang, N.; Wang, Z.; Qi, Z.; Zhu, H.; Zheng, B.; Li, P.; Liu, J. Nontargeted Metabolomic Analysis of Four Different Parts of Platycodon grandiflorum Grown in Northeast China. Molecules 2017, 22, 1280. [Google Scholar] [CrossRef]
- Liao, Y.; Liang, F.; Liu, H.; Zheng, Y.; Li, P.; Peng, W.; Su, W. Safflower yellow extract inhibits thrombus formation in mouse brain arteriole and exerts protective effects against hemorheology disorders in a rat model of blood stasis syndrome. Biotechnol. Biotechnol. Equip. 2018, 32, 487–497. [Google Scholar] [CrossRef]
- Suleimanov, T.A. Phenolic Compounds from Carthamus tinctorius. Chem. Nat. Compd. 2004, 40, 13–15. [Google Scholar] [CrossRef]
- Razgonova, M.; Zakharenko, A.; Pikula, K.; Manakov, Y.; Ercisli, S.; Derbush, I.; Kislin, E.; Seryodkin, I.; Sabitov, A.; Kalenik, T.; et al. LC-MS/MS Screening of Phenolic Compounds in Wild and Cultivated Grapes Vitis amurensis Rupr. Molecules 2021, 26, 3650. [Google Scholar] [CrossRef]
- Zhou, Y.Z.; Ma, H.Y.; Chen, H.; Qiao, L.; Yao, Y.; Cao, J.Q.; Pei, Y.H. New acetylenic glucosides from Carthamus tinctorius. Chem. Pharm. Bull. 2006, 54, 1455–1456. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar]
- Oh, Y.C.; Cho, W.K.; Jeong, Y.H.; Im, G.Y.; Yang, M.C.; Hwang, Y.H.; Ma, J.Y. Anti-inflammatory effect of citrus unshiu peel in LPS-stimulated RAW 264.7 macrophage cells. Am. J. Chin. Med. 2012, 40, 611–629. [Google Scholar] [CrossRef]
- Zong, Y.; Sun, L.; Liu, B.; Deng, Y.S.; Zhan, D.; Chen, Y.L.; He, Y.; Liu, J.; Zhang, Z.J.; Sun, J. Resveratrol inhibits LPS-induced MAPKs activation via activation of the phosphatidylinositol 3-kinase pathway in murine RAW 264.7 macrophage cells. PloS ONE 2012, 7, e44107. [Google Scholar] [CrossRef]
- Porcherie, A.; Cunha, P.; Trotereau, A.; Roussel, P.; Gilbert, F.B.; Rainard, P.; Germon, P. Repertoire of Escherichia coli agonists sensed by innate immunity receptors of the bovine udder and mammary epithelial cells. Vet. Res. 2012, 43, 14. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Li, R.; Li, L.; Zhang, Y.; Deng, S.; Zhu, L. Multi-target regulation of pro-inflammatory cytokine production by transcription factor Blimp-1. Inflamm. Res. 2023, 72, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Scheibel, M.; Klein, B.; Merkle, H.; Schulz, M.; Fritsch, R.; Greten, F.R.; Arkan, M.C.; Schneider, G.; Schmid, R.M. IκBβ is an essential co-activator for LPS-induced IL-1β transcription in vivo. J. Exp. Med. 2010, 207, 2621. [Google Scholar] [CrossRef]
- Liao, H.; Li, Y.; Zhai, X.; Zheng, B.; Banbury, L.; Zhao, X.; Li, R. Comparison of inhibitory effects of safflower decoction and safflower injection on protein and mRNA expressions of iNOS and IL-1β in LPS-activated RAW264.7 cells. J. Immunol. Res. 2019, 2019, 1018274. [Google Scholar] [CrossRef]
- Hoffmann, A.; Baltimore, D. Circuitry of nuclear factor kappaB signaling. Immunol. Rev. 2006, 210, 171–186. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, L.; Jin, M.; Zang, B.X. Hydroxysafflor yellow alleviates early inflammatory response of bleomycin-induced mice lung injury. Biol. Pharm. Bull. 2012, 35, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Jun, M.S.; Ha, Y.M.; Kim, H.S.; Jang, H.J.; Kim, Y.M.; Lee, Y.S.; Kim, H.J.; Seo, H.G.; Lee, J.H.; Lee, S.H.; et al. Anti-inflammatory action of methanol extract of Carthamus tinctorius involves in heme oxygenase-1 induction. J. Ethanopharm. 2011, 133, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.P.; Shi, F.F.; Zhang, W.W.; Zhang, Z.H.; Wang, K. Antioxidant and anti-inflammatory activities of Safflower (Carthamus tinctorius L.) honey extract. Foods 2020, 9, 1039. [Google Scholar] [CrossRef]
- Jo, A.R.; Han, H.S.; Seo, S.; Shin, J.S.; Lee, J.Y.; Kim, H.J.; Lee, K.T. Inhibitory effect of moschamine isolated from Carthamus tinctorius on LPS-induced inflammatory mediators via AP-1 and STAT1/3 inactivation in RAW 264.7 macrophages. Bioorg. Med. Chem. Lett. 2017, 27, 5245–5251. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Y.; Zhang, Z.; Chi, Q.; Liu, Y.; Yang, L.; Xu, K. Safflower yellow alleviates osteoarthritis and prevents inflammation by inhibiting PGE2 release and regulating NF-κB/SIRT1/AMPK signaling pathways. Phytomedicine 2020, 78, 153305. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Choy, C.S.; Liu, Y.H.; Cheah, K.P.; Li, J.S.; Wang, J.T.; Yu, W.Y.; Lin, C.W.; Cheng, H.W.; Hu, C.M. Protective effect of dried safflower petal aqueous extract and its main constituent, carthamus yellow, against lipopolysaccharide-induced inflammation in RAW264.7 macrophages. J. Sci. Food Agric. 2011, 91, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Chen, J.; Zhang, G.; Li, C.; Zhu, J.; Xue, H.; Li, J.; Guan, T.; Zheng, H.; Liu, Y.; et al. Hydroxysafflor Yellow A exerts anti-inflammatory effects mediated by SIRT1 in lipopolysaccharide-induced microglia activation. Front. Pharmacol. 2020, 11, 1315. [Google Scholar] [CrossRef] [PubMed]
- Jalil, S.; Mikhova, B.; Taskova, R.; Mitova, M.; Duddeck, H.; Choudhary, M.I.; Atta-ur-Rahman. In vitro anti-inflammatory effect of Carthamus lanatus L. Z. Nat. C J. Biosci. 2003, 58, 830–832. [Google Scholar] [CrossRef]
- Pratheeshkumar, P.; Kuttan, G. Modulation of immune response by L. inhibits the proinflammatory cytokine profile, iNOS, and COX-2 expression in LPS-stimulated macrophages. Immunopharmacol. Immunotoxicol. 2011, 33, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Berghe, W.V.; Plaisance, S.; Boone, E.; Bosscher, K.D.; Schmitz, M.L.; Fiers, W.; Haegeman, G. p38 and extracellular signal-regulated kinase mitogen activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor. J. Biol. Chem. 1998, 273, 3285–3290. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Zhang, H.; Wang, C.; Jiao, F.; Xu, H.; Wang, X.; Luan, W.; Ma, F.; Ni, L.; Tang, X.; et al. Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. FASEB J. 2019, 33, 12515–12527. [Google Scholar] [CrossRef]
- Li, S.; Xie, R.; Jiang, C.; Liu, M. Schizandrin A alleviates LPS-induced injury in human keratinocyte cell HaCaT through a microRNA-127-dependent regulation. Cell Physiol. Biochem. 2018, 49, 2229–2239. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, C.; Ma, Q.; Li, Y. Sinomenine retards LPS-elicited inflammation via down-regulating CCAT1 in HaCaT cells. Life Sci. 2019, 233, 116703. [Google Scholar] [CrossRef]
- Wang, H.; Wei, S. Tanshinol relieves lipopolysaccharide-induced inflammatory injury of HaCaT cells via down-regulation of microRNA-122. Phytother. Res. 2019, 33, 910–918. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007, 2007, 45673. [Google Scholar]
- Zeng, K.; Thompson, K.E.; Yates, C.R.; Miller, D.D. Synthesis and biological evaluation of quinic acid derivatives as anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2009, 19, 5458–5460. [Google Scholar] [CrossRef]
- Pragasam, S.J.; Venkatesan, V.; Rasool, M. Immunomodulatory and anti-inflammatory effect of p-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation 2013, 36, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Sreedhar, R.; Giridharan, V.V.; Watanabe, K. Molecular targets of quercetin with anti-inflammatory properties in atopic dermatitis. Drug Discov. Today 2016, 21, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A. Anti-hypertensive effect of cereal antioxidant ferulic acid and its mechanism of action. Front. Nutr. 2019, 6, 121. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Liu, C.; Zeng, X.; Li, X.; Zhao, J. Anti-Inflammatory Effects of p-coumaric Acid in LPS-Stimulated RAW264.7 Cells: Involvement of NF-κB and MAPKs Pathways. Med. Chem. 2016, 6, 327–330. [Google Scholar] [CrossRef]
- Pluemsamran, T.; Onkoksoong, T.; Panich, U. Caffeic acid and ferulic acid inhibit UVA-induced matrix metalloproteinase-1 through regulation of antioxidant defense system in keratinocyte HaCaT cells. Photochem. Photobiol. 2012, 88, 961–968. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, P.; Tang, C.; Wang, Y.; Li, Y.; Zhang, H. Antinociceptive and anti-inflammatory activities of extract and two isolated flavonoids of Carthamus tinctorius L. J. Ethnopharmacol. 2014, 151, 944–950. [Google Scholar] [CrossRef]



| Target Gene | Primer Sequence | Accession Number | |
|---|---|---|---|
| iNOS | Forward | 5′-CATGCTACTGGAGGTGGGTG-3′ | NM_010927 |
| Reverse | 5′-CATTGATCTCCGTGACAGCC-3′ | ||
| IL-6 | Forward | 5′-GAGGATACCACTCCCAACAGACC-3′ | NM_031168 |
| Reverse | 5′-AAGTGCATCATCGTTGTTCATACA-3′ | ||
| IL-1β | Forward | 5′-ACCTGCTGGTGTGTGACGTT-3′ | NM_008361 |
| Reverse | 5′-TCGTTGCTTGGTTCTCCTTG-3′ | ||
| β-actin | Forward | 5′-ATCACTATTGGCAACGAGCG-3′ | NM_007393 |
| Reverse | 5′-TCAGCAATGCCTGGGTACAT-3′ | ||
| S. NO. | RT (min) | Tentative Identification | Formula | Chemical Class | m/z [M-H]- | Mass Error (ppm) | Response | Fragmentation (m/z) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.18 | Protocatechuic acid | C7H6O4 | Phenolic acid | 153.0192 | –0.9 | 259240 | 109.0297 | [40] |
| 2 | 1.31 | Dihydrocaffeic acid | C9H10O4 | Phenylpropanoid | 181.0503 | –1.6 | 148736 | 135.0448 | [41] |
| 3 | 1.60 | Salicylic acid | C7H6O3 | Beta hydroxy acid | 137.0245 | 0.4 | 604343 | 93.0354 | [40] |
| 4 | 1.76 | Esculetin | C9H6O4 | Hydroxycoumarin | 177.0191 | –1.1 | 150029 | 133.0295 | [42] |
| 5 | 1.78 | Caffeic acid | C9H8O4 | Hydroxycinnamic acid | 179.0347 | –1.4 | 415615 | 135.045 | [42] |
| 6 | 1.89 | Coumaroylquinic acid | C16H18O8 | Hydroxycinnamic acid | 337.0930 | 0.3 | 86393 | 191.0563 | [42,43] |
| 7 | 2.15 | Benzoic acid | C7H6O2 | Carboxylic acid | 121.0298 | 2.6 | 226386 | 93.0344 | [42] |
| 8 | 2.30 | 6-Hydroxykaempferol hexoside | C21H20O12 | Flavonoid | 463.0883 | 0.3 | 299116 | 301.0359 | [44] |
| 9 | 2.38 | Coumaric acid | C9H8O3 | Hydroxycinnamic acid | 163.0399 | –1.1 | 1097613 | 119.0504 | [44] |
| 10 | 2.50 | 3-(β-D-Glucopyranosyloxy)-5-octenoic acid | C14H24O8 | Fatty acid | 319.1394 | –1.3 | 27071 | 157.0862 | Pubmed |
| 11 | 2.61 | Quercetin hexoside | C21H20O12 | Flavonoid | 463.0884 | 0.4 | 127814 | 300.0288 | [44] |
| 12 | 2.69 | Ferulic acid | C10H10O4 | Hydroxycinnamic acid | 193.0503 | –1.6 | 106615 | 134.0371, 149.060 | [40] |
| 13 | 2.83 | Kaempferol-3-O-β-rutinoside | C27H30O15 | Flavonoid | 593.1518 | 1.1 | 334736 | 285.0413 | [44] |
| 14 | 2.99 | Kaempferol hexoside | C21H20O11 | Flavonoid | 447.0936 | 0.7 | 985035 | 285.0402 | [44] |
| 15 | 3.40 | Azelaic acid | C9H16O4 | Dicarboxylic acid | 187.0977 | 0.5 | 2328525 | 143.1076 | [40,41,42] |
| 16 | 3.41 | Carthamine | C43H42O22 | Hydroxycinnamic acid | 449.1093 | 0.8 | 371603 | 287.0562 | [45,46] |
| 17 | 4.90 | 4,6-Decadiyne-1-O-β-D-glucopyranoside | C16H24O6 | Polyacetylene glycoside | 311.1503 | 0.9 | 878235 | - | [47] |
| 18 | 5.81 | Trihydroxyoctadecenoic acid | C18H34O5 | Fatty acid | 329.2333 | –0.2 | 273521 | 211.1338 | [41,43] |
| 19 | 7.67 | Hydroperoxyoctadecadienoic acid | C18H32O4 | Fatty acid | 311.2225 | –0.7 | 281517 | 223.1670 | [41] |
| 20 | 7.75 | 9-Octadecenedioic acid | C18H32O4 | Fatty acid | 311.2226 | –0.6 | 144857 | - | [41] |
| 21 | 10.01 | Hydroxyoctadecadienoic acid | C18H32O3 | Fatty acid | 295.2274 | –1.4 | 376589 | 277.2175 | [41] |
| 22 | 10.09 | Oxylipin | C18H32O3 | Fatty acid | 295.2275 | –1.1 | 249495 | 277.2176 | [41] |
| 23 | 10.33 | Oxooctadecadienoic acid | C18H30O3 | Fatty acid | 293.2115 | –2.4 | 227547 | 277.2175 | [41] |
| 24 | 12.58 | Linolenic acid | C18H30O2 | Fatty acid | 277.2175 | 0.7 | 1114693 | - | [41,42] |
| 25 | 12.73 | n-Pentadecanal | C15H30O | Fatty aldehyde | 225.2221 | 0 | 149217 | 197.1912 | [43] |
| 26 | 13.48 | Linoleic acid | C18H32O2 | Fatty acid | 279.2328 | –0.5 | 583977 | - | [42] |
| 27 | 14.29 | Palmitic acid | C16H32O2 | Fatty acid | 255.2325 | –1.8 | 182918 | - | [41,42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-Y.; Hong, M.; Deepa, P.; Sowndhararajan, K.; Park, S.J.; Park, S.; Kim, S. Carthamus tinctorius Suppresses LPS-Induced Anti-Inflammatory Responses by Inhibiting the MAPKs/NF-κB Signaling Pathway in HaCaT Cells. Sci. Pharm. 2023, 91, 14. https://doi.org/10.3390/scipharm91010014
Kim S-Y, Hong M, Deepa P, Sowndhararajan K, Park SJ, Park S, Kim S. Carthamus tinctorius Suppresses LPS-Induced Anti-Inflammatory Responses by Inhibiting the MAPKs/NF-κB Signaling Pathway in HaCaT Cells. Scientia Pharmaceutica. 2023; 91(1):14. https://doi.org/10.3390/scipharm91010014
Chicago/Turabian StyleKim, So-Yeon, Minji Hong, Ponnuvel Deepa, Kandhasamy Sowndhararajan, Se Jin Park, SeonJu Park, and Songmun Kim. 2023. "Carthamus tinctorius Suppresses LPS-Induced Anti-Inflammatory Responses by Inhibiting the MAPKs/NF-κB Signaling Pathway in HaCaT Cells" Scientia Pharmaceutica 91, no. 1: 14. https://doi.org/10.3390/scipharm91010014
APA StyleKim, S.-Y., Hong, M., Deepa, P., Sowndhararajan, K., Park, S. J., Park, S., & Kim, S. (2023). Carthamus tinctorius Suppresses LPS-Induced Anti-Inflammatory Responses by Inhibiting the MAPKs/NF-κB Signaling Pathway in HaCaT Cells. Scientia Pharmaceutica, 91(1), 14. https://doi.org/10.3390/scipharm91010014






