Antiviral Molecular Targets of Essential Oils against SARS-CoV-2: A Systematic Review
Abstract
1. Introduction
2. Research Question
3. Methods
3.1. Search Strategy
3.2. Inclusion and Exclusion Criteria
3.3. Screening and Selection of the Records
3.4. Data Extraction and Presentation
3.5. Quality Appraisal
4. Results
4.1. Results from the Literature Search
4.2. Characteristics of the Included Studies
4.3. Primary Outcome
4.4. Secondary Outcome
4.5. Results from the Quality Appraisal
| Author, Year [Ref.] | Sample a | Major Compound * | In Vitro Assay/In Vivo Model | Outcome | Remarks |
|---|---|---|---|---|---|
| Ak Sakallı et al., 2022, [33] | Essential oils from E. globulus Labill. and E. citriodora Hook. | E. globulus: 1,8-Cineole (30.9%), α-pinene (11.4%), and β-pinene (11.4%) E. citriodora: Citronellal (79.9%) |
|
| Anti-SARS-CoV-2 activity is not determined. |
| Demirci et al., 2021, [30] | Commercial menthol and essential oils from M. arvensis L., M. citrata L., and M. spicata L. | M. arvensis: Menthol (82%) M. citrata: Menthone (22.2%), menthol (menthol) M. spicata: Carvone (88.7%) |
|
| Anti-SARS-CoV-2 activity is not determined. |
| Demirci et al., 2022, [31] | Commercial 1.8-cineole and essential oil from R. officinalis. | R. officinalis: 1,8-cineole (62.7%), α-pinene (12.6%), and camphor (8.3%) |
|
| Anti-SARS-CoV-2 activity is not determined. |
| Biltekin et al., 2022, [32] | L. angustifolia, L. stoechas, and L. heterophylla | L. heterophylla: Linalool (30.6%), linalool acetate (19.6), camphor (15%) and 1,8-cineole (11.3%) L. stoechas: Camphor (54.7%) and α-fenchone (19.2%) L. angustifolia: Camphor (17.9%), 1,8-cineole (12.3%), linalool (22.4%), and linalool acetate (19.2%). |
|
| Anti-SARS-CoV-2 activity is not determined. |
| Asaad et al., 2022, [37] | C. clementine fruits were crushed in ethanol solution and added with water before filtration. The filtrate was partitioned with n-hexane to produce the essential oil. | C. clementine: Limonene (92.28%) |
|
| The binding with SARS-CoV-2 spike protein is only observed by molecular docking. |
| González-Maldonado et al., 2022, [34] | Essential oils: G. sarmientoi, C. aurantium L. var. amara, M. frondosus, A. emarginata, E. globulus, L. alba, C. citratusv Volatile compounds: β-Caryophyllene, Caryophyllene oxide, Linalool, Trans-anethole, S-Limonene, R-Limonene, cis-Verbenol, Guaiol, Macrophominol, Acetylphomolactone, Botryodiplodin, Asperline, Isoasperline | No compound identifications were carried out on the essential oil, but the commercial essential oil constituents (volatile compounds) were assayed directly. |
|
| Required confirmation using the whole SARS-CoV-2 |
| Kumar et al., 2020, [35] | Essential oils: C. bergamia, P. nigrum, M. chamomilla, C. annum, C. winterianus, S. sclarea, C. sempervirens, C. valgare, E. globulus, F. vulgare, Boswellia sp., P. graveolens, Z. officinale, J. communis, K. ambigua, C. limon, L. officinalis, C. aurantifolia, L. cubeba, O. majorana, M. communis, C. aurantium, C. martinii, P. cablin, M. piperita, C. aurantium, C. camphora, R. officinalis, C. reticulata, and M. alternifolia. Volatile compound: Citronellol, geraniol, neryl acetate, and limonene. | C. limon: Citronellol (27.1%), geraniol (21.4%), and neryl acetate (10.5%) P. graveolens: Limonene (73%) |
|
| Anti-SARS-CoV-2 activity is not determined. |
| Mohamed et al., 2022, [36] | Essential oil from A. robusta bark obtained through hydrodistillation | Tricyclene (11.89%), α-pinene (19.49%), d-camphene (7.13%), limonene (9.37%), trans-pinocarveol (4.95%), borneol (2.32%), α-phellandren-8-ol (2.51%), and α-terpineol (9.59%). |
|
| The molecular interactions have not been confirmed in vitro/in vivo |
4.6. Non-Confirmed In Silico Studies
5. Discussion
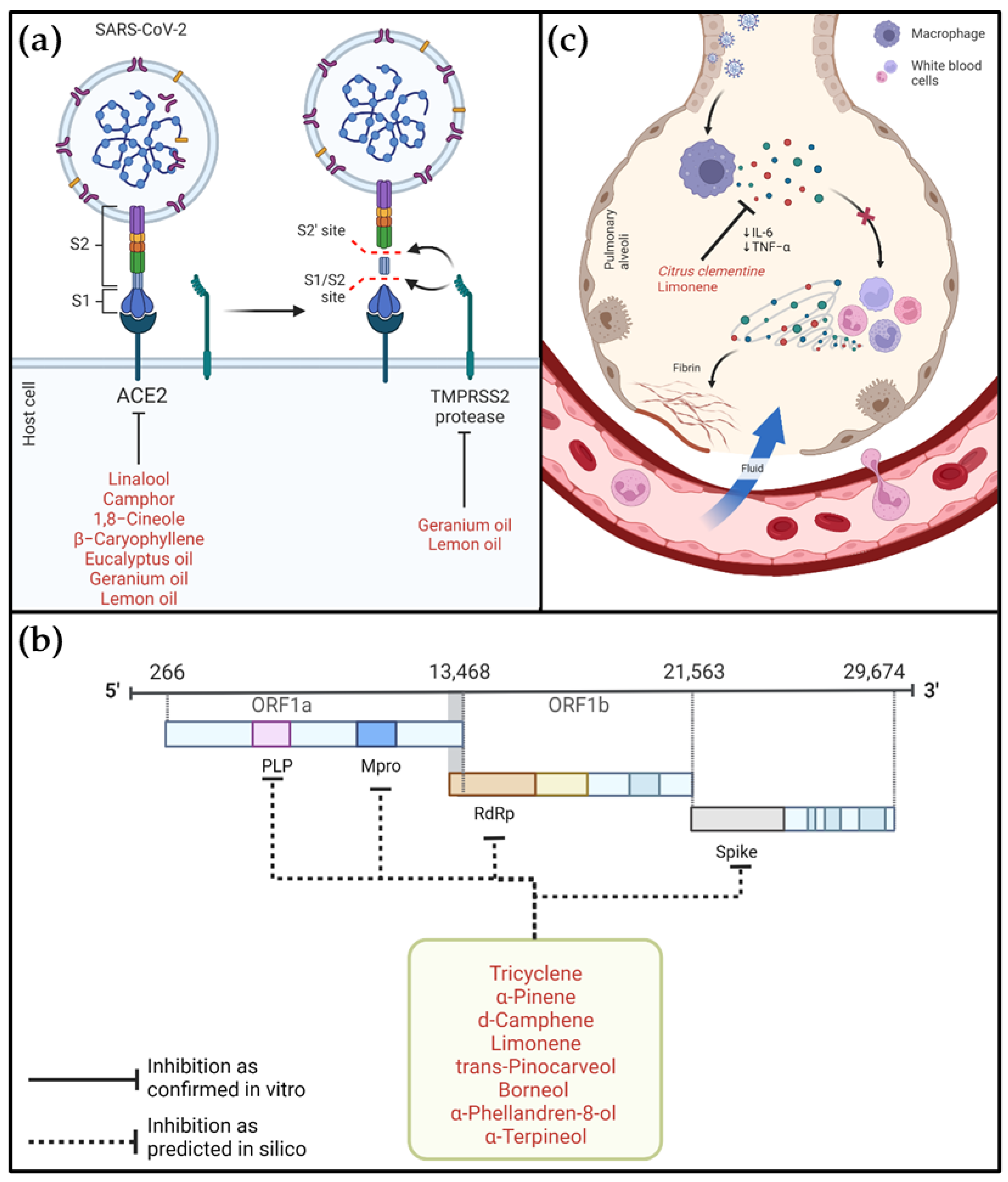
5.1. Targeting SARS-CoV-2-Related Proteins
5.2. Targeting Inflammatory Factors
5.3. Other In Vitro Studies on Anti-SARS-CoV-2 Activity of Essential Oils
5.4. In Vitro Study Design
5.5. Comments on Molecular Docking Studies
6. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 15 December 2022).
- Mulay, A.; Konda, B.; Garcia, G.; Yao, C.; Beil, S.; Villalba, J.M.; Koziol, C.; Sen, C.; Purkayastha, A.; Kolls, J.K.; et al. SARS-CoV-2 infection of primary human lung epithelium for COVID-19 modeling and drug discovery. Cell Rep. 2021, 35, 109055. [Google Scholar] [CrossRef] [PubMed]
- Salamanna, F.; Maglio, M.; Landini, M.P.; Fini, M. Body Localization of ACE-2: On the Trail of the Keyhole of SARS-CoV-2. Front. Med. 2020, 7, 594495. [Google Scholar] [CrossRef]
- Hassan, W.; Kazmi, S.K.; Tahir, M.J.; Ullah, I.; Royan, H.A.; Fahriani, M.; Nainu, F.; Rosa, S.G. Global acceptance and hesitancy of COVID-19 vaccination: A narrative review. Narra J. 2021, 1, e57. [Google Scholar] [CrossRef]
- Tang, B.; Zhang, X.; Li, Q.; Bragazzi, N.L.; Golemi-Kotra, D.; Wu, J. The minimal COVID-19 vaccination coverage and efficacy to compensate for a potential increase of transmission contacts, and increased transmission probability of the emerging strains. BMC Public Health 2022, 22, 1258. [Google Scholar] [CrossRef]
- Sharun, K.; Tiwari, R.; Yatoo, M.I.; Natesan, S.; Megawati, D.; Singh, K.P.; Michalak, I.; Dhama, K. A comprehensive review on pharmacologic agents, immunotherapies and supportive therapeutics for COVID-19. Narra J. 2022, 2, e92. [Google Scholar] [CrossRef]
- Robinson, P.C.; Liew, D.F.; Tanner, H.L.; Grainger, J.R.; Dwek, R.A.; Reisler, R.B.; Steinman, L.; Feldmann, M.; Ho, L.-P.; Hussell, T. COVID-19 therapeutics: Challenges and directions for the future. Proc. Natl. Acad. Sci. USA 2022, 119, e2119893119. [Google Scholar] [CrossRef]
- Dhama, K.; Nainu, F.; Frediansyah, A.; Yatoo, M.I.; Mohapatra, R.K.; Chakraborty, S.; Zhou, H.; Islam, M.R.; Mamada, S.S.; Kusuma, H.I.; et al. Global emerging Omicron variant of SARS-CoV-2: Impacts, challenges and strategies. J. Infect. Public Health 2023, 16, 4–14. [Google Scholar] [CrossRef]
- Ma, L.; Yao, L. Antiviral effects of plant-derived essential oils and their components: An updated review. Molecules 2020, 25, 2627. [Google Scholar] [CrossRef]
- Sandner, G.; Heckmann, M.; Weghuber, J. Immunomodulatory Activities of Selected Essential Oils. Biomolecules 2020, 10, 1139. [Google Scholar] [CrossRef] [PubMed]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef]
- Dutt, J.G.; Suthar, B.; Malek, N.; Shukla, M.; Shukla, B.; Shukla, K.; Pandit, K.; Rachchh, S.; Gokani, M.; Bhalani, R.; et al. A randomized and comparative study to assess safety and efficacy of supplemental treatment of a herbal formulation—Aayudh Advance comprising essential oils in patients with corona virus 2019 (COVID-19). Contemp. Clin. Trials Commun. 2021, 22, 100755. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.; Hires, C.; Keenan, L.; Dunne, E. Aromatherapy blend of thyme, orange, clove bud, and frankincense boosts energy levels in post-COVID-19 female patients: A randomized, double-blinded, placebo controlled clinical trial. Complement. Med. 2022, 67, 102823. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.B.; Sulaiman, N.; Rashid, W.S.W.; Ken, Z.Z.; Ali, W.K.; Othman, U.K.; Samat, S.N.; Kori, M.N.; Periyasamy, N.; Zakaria, P.; et al. Early viral clearance among COVID-19 patients when gargling with povidone-iodine and essential oils—A clinical trial. Int. Med. J. 2020, 27, 651–654. [Google Scholar]
- Wang, Y.; Wu, Y.; Fu, P.; Zhou, H.; Guo, X.; Zhu, C.; Tu, Y.; Wang, J.; Li, H.; Chen, Z. Effect of garlic essential oil in 97 patients hospitalized with COVID-19: A multi-center experience. Pak. J. Pharm. Sci. 2022, 35, 1077–1082. [Google Scholar] [CrossRef]
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, S.; Gou, J.; Wen, Y.; Fan, L.; Zhou, J.; Zhou, G.; Xu, G.; Zhang, Z. Spike-mediated ACE2 down-regulation was involved in the pathogenesis of SARS-CoV-2 infection. J. Infect. 2022, 85, 418–427. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, H.; Duan, Y.; Yang, H. The main protease and RNA-dependent RNA polymerase are two prime targets for SARS-CoV-2. Biochem. Biophys. Res. Commun. 2021, 538, 63–71. [Google Scholar] [CrossRef]
- Pourmand, S.; Zareei, S.; Shahlaei, M.; Moradi, S. Inhibition of SARS-CoV-2 pathogenesis by potent peptides designed by the mutation of ACE2 binding region. Comput. Biol. Med. 2022, 146, 105625. [Google Scholar] [CrossRef]
- Elsebai, M.F.; Albalawi, M.A. Essential Oils and COVID-19. Molecules 2022, 27, 7893. [Google Scholar] [CrossRef]
- Torres Neto, L.; Monteiro, M.L.G.; Galvan, D.; Conte-Junior, C.A. An evaluation of the potential of essential oils against SARS-CoV-2 from in silico studies through the systematic review using a chemometric approach. Pharmaceuticals 2021, 14, 1138. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Tran, L.; Tam, D.N.H.; Elshafay, A.; Dang, T.; Hirayama, K.; Huy, N.T. Quality assessment tools used in systematic reviews of in vitro studies: A systematic review. BMC Med. Res. Methodol. 2021, 21, 101. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Barata, P.; Charmier, A.; Lehmann, I.; Rodrigues, S.; Melosini, M.M.; Pais, P.J.; Sousa, A.P.; Teixeira, C.; Santos, I.; et al. Cannabidiol and Terpene Formulation Reducing SARS-CoV-2 Infectivity Tackling a Therapeutic Strategy. Front. Immunol. 2022, 13, 841459. [Google Scholar] [CrossRef] [PubMed]
- Torres Neto, L.; Monteiro, M.L.G.; Fernández-Romero, J.; Teleshova, N.; Sailer, J.; Conte Junior, C.A. Essential oils block cellular entry of SARS-CoV-2 delta variant. Sci. Rep. 2022, 12, 20639. [Google Scholar] [CrossRef]
- Lionis, C.; Karakasiliotis, I.; Petelos, E.; Linardakis, M.; Diamantakis, A.; Symvoulakis, E.; Panopoulou, M.; Kampa, M.; Pirintsos, S.A.; Sourvinos, G.; et al. A mixture of essential oils from three Cretan Aromatic Plants (thyme, Greek sage and Cretan dittany, CAPeo) inhibits SARS-CoV-2 proliferation: In vitro evidence and a Proof-of-Concept intervention study in mild ambulatory COVID-19-positive patients. MedRxiv 2021, 1–20. [Google Scholar] [CrossRef]
- Esharkawy, E.R.; Almalki, F.; Hadda, T.B. In vitro potential antiviral SARS-CoV-19 activity of natural product thymohydroquinone and dithymoquinone from Nigella sativa. Bioorg. Chem. 2022, 120, 105587. [Google Scholar] [CrossRef] [PubMed]
- Ćavar Zeljković, S.; Schadich, E.; Džubák, P.; Hajdúch, M.; Tarkowski, P.; Hajdúch, M.; Tarkowski, P. Antiviral Activity of Selected Lamiaceae Essential Oils and Their Monoterpenes Against SARS-CoV-2. Front. Pharm. 2022, 13, 893634. [Google Scholar] [CrossRef]
- Demirci, F.; Karadağ, A.E.; Biltekin, S.N.; Demirci, B. In Vitro ACE2 and 5-LOX Enzyme Inhibition by Menthol and Three Different Mint Essential Oils. Nat. Prod. Commun. 2021, 16, 1–5. [Google Scholar] [CrossRef]
- Demirci, F.; Karadağ, A.E.; Biltekin, S.N.; Demirci, B. In vitro ACE2 and 5-LOX Inhibition of Rosmarinus officinalis L. Essential Oil and its Major Component 1,8-Cineole. Rec. Nat. Prod. 2022, 16, 194–199. [Google Scholar] [CrossRef]
- Biltekin, S.N.; Karadağ, A.E.; Demirci, B.; Demirci, F. ACE2 and LOX Enzyme Inhibitions of Different Lavender Essential Oils and Major Components Linalool and Camphor. ACS Omega 2022, 7, 36561–36566. [Google Scholar] [CrossRef] [PubMed]
- Sakallı, E.A.; Teralı, K.; Karadağ, A.E.; Biltekin, S.N.; Koşar, M.; Demirci, B.; Başer, K.H.C.; Demirci, F. In vitro and in silico Evaluation of ACE2 and LOX Inhibitory Activity of Eucalyptus Essential Oils, 1,8-Cineole, and Citronellal. Nat. Prod. Commun. 2022, 17, 1–8. [Google Scholar] [CrossRef]
- González-Maldonado, P.; Alvarenga, N.; Burgos-Edwards, A.; Flores-Giubi, M.E.; Barúa, J.E.; Romero-Rodríguez, M.C.; Soto-Rifo, R.; Valiente-Echeverría, F.; Langjahr, P.; Cantero-González, G.; et al. Screening of Natural Products Inhibitors of SARS-CoV-2 Entry. Molecules 2022, 27, 1743. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.J.S.; Vani, M.G.; Wang, C.-S.; Chen, C.-C.; Chen, Y.-C.; Lu, L.-P.; Huang, C.-H.; Lai, C.-S.; Wang, S.-Y. Geranium and lemon essential oils and their active compounds downregulate angiotensin-converting enzyme 2 (ACE2), a SARS-CoV-2 spike receptor-binding domain, in epithelial cells. Plants 2020, 9, 770. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.E.; Tawfeek, N.; Elbaramawi, S.S.; Fikry, E. Agathis robusta Bark Essential Oil Effectiveness against COVID-19: Chemical Composition, In Silico and In Vitro Approaches. Plants 2022, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- Asaad, G.F.; Abdelhameed, M.F.; Elraey, M.; Roshdy, W.H.; Elgamal, A.; Moamen, Y. Citrus clementine peels essential oil exhibited anti-SARS-CoV-2 and its modulatory effect against cytokine storm: Evidence from in vitro and in silico studies. Egypt. J. Chem. 2022, 65, 419–427. [Google Scholar] [CrossRef]
- Abdelli, I.; Hassani, F.; Brikci, S.B.; Ghalem, S. In silico study the inhibition of angiotensin converting enzyme 2 receptor of COVID-19 by Ammoides verticillata components harvested from Western Algeria. J. Biomol. Struct. Dyn. 2021, 39, 3263–3276. [Google Scholar] [CrossRef]
- Costa, R.; Martins, R.; de Lima, G.; Stamford, T.; Tadei, W.; Maciel, M.A.; Rêgo, A.D.; Xavier-Júnior, F. Molecular docking in silico analysis of Brazilian essential oils against host targets and SARS-CoV-2 proteins. J. Braz. Chem. Soc. 2022, 33, 1219–1235. [Google Scholar] [CrossRef]
- Da Silva, J.K.R.; Figueiredo, P.L.B.; Byler, K.G.; Setzer, W.N. Essential oils as antiviral agents. Potential of essential oils to treat SARS-CoV-2 infection: An in−silico investigation. Int. J. Mol. Sci. 2020, 21, 3426. [Google Scholar] [CrossRef]
- Habibzadeh, S.; Zohalinezhad, M.E. Evaluation of the inhibitory activities of ferula gummosa bioactive compounds against the druggable targets of SARS-CoV-2: Molecular docking simulation. Biointerface Res. Appl. Chem. 2022, 12, 6382–6392. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Nagarajan, S.K.; Ramesh, V.; Palaniyandi, V.; Selvam, S.P.; Madhavan, T. Computational evaluation of major components from plant essential oils as potent inhibitors of SARS-CoV-2 spike protein. J. Mol. Struct. 2020, 1221, 128823. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Jugreet, B.S.; Zengin, G.; Lesetja, L.J.; Abdallah, H.H.; Ezzat, M.O.; Gallo, M.; Montesano, D. Seven Compounds from Turmeric Essential Oil Inhibit Three Key Proteins Involved in SARS-CoV-2 Cell Entry and Replication in silico. J. Comput. Biophys. Chem. 2021, 20, 785–795. [Google Scholar] [CrossRef]
- My, T.T.A.; Loan, H.T.P.; Hai, N.T.T.; Hieu, L.T.; Hoa, T.T.; Thuy, B.T.P.; Quang, D.T.; Triet, N.T.; Van Anh, T.T.; Dieu, N.T.X.; et al. Evaluation of the Inhibitory Activities of COVID-19 of Melaleuca cajuputi Oil Using Docking Simulation. ChemistrySelect 2020, 5, 6312–6320. [Google Scholar] [CrossRef]
- Panikar, S.; Shoba, G.; Arun, M.; Sahayarayan, J.J.; Nanthini, A.U.R.; Chinnathambi, A.; Alharbi, S.A.; Nasif, O.; Kim, H.-J. Essential oils as an effective alternative for the treatment of COVID-19: Molecular interaction analysis of protease (Mpro) with pharmacokinetics and toxicological properties. J. Infect. Public Health 2021, 14, 601–610. [Google Scholar] [CrossRef]
- Sharma, A.D.; Kaur, I. Jensenone from eucalyptus essential oil as a potential inhibitor of COVID-19 corona virus infection. Res. Rev. Biotechnol. Biosci. 2020, 7, 60–67. [Google Scholar] [CrossRef]
- Sharma, A.D.; Kaur, I. Molecular docking and pharmacokinetic screening of eucalyptol (1,8 cineole) from eucalyptus essential oil against SARS-CoV-2. Not. Sci. Biol. 2020, 12, 536–545. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, A.; Bhattacharyya, D.; Chauhan, R.S. Computational identification of potential inhibitory compounds in Indian medicinal and aromatic plant species against major pathogenicity determinants of SARS-CoV-2. J. Biomol. Struct. Dyn. 2021, 40, 14096–14114. [Google Scholar] [CrossRef]
- Sharma, A.D.; Kaur, I. GC-FID based aromatic profiling and molecular docking studies of lemon grass (Cymbopogon citratus L.) essential oil as novel therapeutic for SARS-CoV-2 spike protein. Arab. J. Med. Aromat. Plants 2022, 8, 1–20. [Google Scholar] [CrossRef]
- Siddiqui, S.; Ahmad, R.; Alaidarous, M.; Zia, Q.; Mir, S.A.; Alshehri, B.; Srivastava, A.; Trivedi, A. Phytoconstituents from Moringa oleifera fruits target ACE2 and open spike glycoprotein to combat SARS-CoV-2: An integrative phytochemical and computational approach. J. Food Biochem. 2022, 46, e14062. [Google Scholar] [CrossRef]
- Quy, P.T.; My, T.T.A.; Hai, N.T.T.; Bui, T.Q.; Quang, D.T.; Triet, N.T.; Hien, P.P.; Nhung, N.T.A. A Computational Screening on Inhibitability of Piper Betle Essential Oil Chemical Structures against Spike Proteins of Mutated SARS-CoV-2-variants D614G, N501Y, and S477N. Smart Sci. 2022, 10, 246–263. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sharma, A.K.; Sharma, M.; Sharma, M. Medicinal and aromatic plants: A potential source of novel bioactive compounds showing antiviral efficacy against coronavirus (SARS-CoV-2). Indian J. Ecol. 2021, 48, 7–16. [Google Scholar] [CrossRef]
- Wysocki, J.; Schulze, A.; Batlle, D. Novel variants of angiotensin converting enzyme-2 of shorter molecular size to target the kidney renin angiotensin system. Biomolecules 2019, 9, 886. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Lee, M.-S.; Lucht, A.; Chou, F.-P.; Huang, W.; Havighurst, T.C.; Kim, K.; Wang, J.-K.; Antalis, T.M.; Johnson, M.D. TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells. Am. J. Pathol. 2010, 176, 2986–2996. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Chen, Q. Insight into 2019 novel coronavirus—An updated interim review and lessons from SARS-CoV and MERS-CoV. Int. J. Infect. Dis. 2020, 94, 119–124. [Google Scholar] [CrossRef]
- Henderson, R.; Edwards, R.J.; Mansouri, K.; Janowska, K.; Stalls, V.; Gobeil, S.; Kopp, M.; Li, D.; Parks, R.; Hsu, A.L. Controlling the SARS-CoV-2 spike glycoprotein conformation. Nat. Struct. Mol. Biol. 2020, 27, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef] [PubMed]
- e Silva, A.C.S.; Teixeira, M.M. ACE inhibition, ACE2 and angiotensin-(1 7) axis in kidney and cardiac inflammation and fibrosis. Pharmacol. Res. 2016, 107, 154–162. [Google Scholar] [CrossRef]
- Hoffmann, M.; Schroeder, S.; Kleine-Weber, H.; Müller, M.A.; Drosten, C.; Pöhlmann, S. Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19. Antimicrob. Agents Chemother. 2020, 64, e00754-20. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, L.; Peng, Z.; Chen, L.-L.; Meng, X.; Zhang, C.; Ip, J.D.; Chan, W.-M.; Chu, A.W.-H.; Chan, K.-H.; et al. SARS-CoV-2 Omicron variant shows less efficient replication and fusion activity when compared with Delta variant in TMPRSS2-expressed cells. Emerg. Microbes Infect. 2022, 11, 277–283. [Google Scholar] [CrossRef]
- Meyer, B.; Chiaravalli, J.; Gellenoncourt, S.; Brownridge, P.; Bryne, D.P.; Daly, L.A.; Grauslys, A.; Walter, M.; Agou, F.; Chakrabarti, L.A.; et al. Characterising proteolysis during SARS-CoV-2 infection identifies viral cleavage sites and cellular targets with therapeutic potential. Nat. Commun. 2021, 12, 5553. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef]
- Romano, M.; Ruggiero, A.; Squeglia, F.; Maga, G.; Berisio, R. A structural view of SARS-CoV-2 RNA replication machinery: RNA synthesis, proofreading and final capping. Cells 2020, 9, 1267. [Google Scholar] [CrossRef]
- Masyeni, S.; Iqhrammullah, M.; Frediansyah, A.; Nainu, F.; Tallei, T.; Emran, T.B.; Ophinni, Y.; Dhama, K.; Harapan, H. Molnupiravir: A lethal mutagenic drug against rapidly mutating severe acute respiratory syndrome coronavirus 2—A narrative review. J. Med. Virol. 2022, 94, 3006–3016. [Google Scholar] [CrossRef]
- Gordon, C.J.; Tchesnokov, E.P.; Feng, J.Y.; Porter, D.P.; Götte, M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020, 295, 4773–4779. [Google Scholar] [CrossRef]
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020, 40, 37. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Sharma, K.; Silakari, O. The interplay between inflammatory pathways and COVID-19: A critical review on pathogenesis and therapeutic options. Microb. Pathog. 2021, 150, 104673. [Google Scholar] [CrossRef]
- Damiescu, R.; Lee, D.Y.W.; Efferth, T. Can Essential Oils Provide an Alternative Adjuvant Therapy for COVID-19 Infections and Pain Management at the Same Time? Pharmaceuticals 2022, 15, 1387. [Google Scholar] [CrossRef]
- Asif, M.; Saleem, M.; Saadullah, M.; Yaseen, H.S.; Al Zarzour, R. COVID-19 and therapy with essential oils having antiviral, anti-inflammatory, and immunomodulatory properties. Inflammopharmacology 2020, 28, 1153–1161. [Google Scholar] [CrossRef]
- Ayola-Serrano, N.C.; Roy, N.; Fathah, Z.; Anwar, M.M.; Singh, B.; Ammar, N.; Sah, R.; Elba, A.; Utt, R.S.; Pecho-Silva, S. The role of 5-lipoxygenase in the pathophysiology of COVID-19 and its therapeutic implications. Inflamm. Res. 2021, 70, 877–889. [Google Scholar] [CrossRef]
- Powell, W.S.; Rokach, J. Biochemistry, biology and chemistry of the 5-lipoxygenase product 5-oxo-ETE. Prog. Lipid Res. 2005, 44, 154–183. [Google Scholar] [CrossRef]
- Kuhn, H.; Banthiya, S.; Van Leyen, K. Mammalian lipoxygenases and their biological relevance. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2015, 1851, 308–330. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Fischer, A. A Transcriptome analysis identifies potential preventive and therapeutic approaches towards COVID-19. Preprint 2020, 2020040399. [Google Scholar] [CrossRef]
- Lu, Q.; Zhu, Z.; Tan, C.; Zhou, H.; Hu, Y.; Shen, G.; Zhu, P.; Yang, G.; Xie, X. Changes of serum IL-10, IL-1β, IL-6, MCP-1, TNF-α, IP-10 and IL-4 in COVID-19 patients. Int. J. Clin. Pract. 2021, 75, e14462. [Google Scholar] [CrossRef] [PubMed]
- Schultheiß, C.; Willscher, E.; Paschold, L.; Gottschick, C.; Klee, B.; Henkes, S.-S.; Bosurgi, L.; Dutzmann, J.; Sedding, D.; Frese, T.; et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep. Med. 2022, 3, 100663. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, M.; Zhou, Z.; Guan, X.; Xiang, Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020, 111, 102452. [Google Scholar] [CrossRef]
- Meeran, M.N.; Seenipandi, A.; Javed, H.; Sharma, C.; Hashiesh, H.M.; Goyal, S.N.; Jha, N.K.; Ojha, S. Can limonene be a possible candidate for evaluation as an agent or adjuvant against infection, immunity, and inflammation in COVID-19? Heliyon 2021, 7, e05703. [Google Scholar] [CrossRef]
- Ku, C.-M.; Lin, J.-Y. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013, 141, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Lappas, C.M.; Lappas, N.T. D-Limonene modulates T lymphocyte activity and viability. Cell. Immunol. 2012, 279, 30–41. [Google Scholar] [CrossRef]
- Sadlon, A.E.; Lamson, D.W. Immune-modifying and antimicrobial effects of Eucalyptus oil and simple inhalation devices. Altern. Med. Rev. 2010, 15, 33–43. [Google Scholar]
- Asrani, P.; Hussain, A.; Nasreen, K.; AlAjmi, M.F.; Amir, S.; Sohal, S.S.; Hassan, M.I. Guidelines and safety considerations in the laboratory diagnosis of SARS-CoV-2 infection: A prerequisite study for health professionals. Risk Manag. Healthc. Policy 2021, 14, 379. [Google Scholar] [CrossRef] [PubMed]
- Tito, A.; Colantuono, A.; Pirone, L.; Pedone, E.; Intartaglia, D.; Giamundo, G.; Conte, I.; Vitaglione, P.; Apone, F. Pomegranate Peel Extract as an Inhibitor of SARS-CoV-2 Spike Binding to Human ACE2 Receptor (in vitro): A Promising Source of Novel Antiviral Drugs. Front. Chem. 2021, 9, 638187. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, I.-M.; Padera, R.F.; Solomon, I.H.; Kanjilal, S.; Hammer, M.M.; Hornick, J.L.; Sholl, L.M. In situ detection of SARS-CoV-2 in lungs and airways of patients with COVID-19. Mod. Pathol. 2020, 33, 2104–2114. [Google Scholar] [CrossRef]
- Cagno, V. SARS-CoV-2 cellular tropism. Lancet Microbe 2020, 1, e2. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Chan, J.F.-W.; Yuen, T.T.-T.; Shuai, H.; Yuan, S.; Wang, Y.; Hu, B.; Yip, C.C.-Y.; Tsang, J.O.-L.; Huang, X. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: An observational study. Lancet Microbe 2020, 1, e14–e23. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Chung, Y.-S.; Jo, H.J.; Lee, N.-J.; Kim, M.S.; Woo, S.H.; Park, S.; Kim, J.W.; Kim, H.M.; Han, M.-G. Identification of coronavirus isolated from a patient in Korea with COVID-19. Osong Public Health Res. Perspect. 2020, 11, 3. [Google Scholar] [CrossRef]
- Wurtz, N.; Penant, G.; Jardot, P.; Duclos, N.; La Scola, B. Culture of SARS-CoV-2 in a panel of laboratory cell lines, permissivity, and differences in growth profile. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 477–484. [Google Scholar] [CrossRef]
- Chan, P.K.; To, K.F.; Lo, A.W.; Cheung, J.L.; Chu, I.; Au, F.W.; Tong, J.H.; Tam, J.S.; Sung, J.J.; Ng, H.K. Persistent infection of SARS coronavirus in colonic cells in vitro. J. Med. Virol. 2004, 74, 1–7. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Zhang, M.; Hu, X.; Hu, T.; Liu, Y.; Hu, Q.; Wu, S.; Yue, J. High expression of ACE2 and TMPRSS2 and clinical characteristics of COVID-19 in colorectal cancer patients. NPJ Precis. Oncol. 2021, 5, 1. [Google Scholar] [CrossRef]
- Martínez-Maqueda, D.; Miralles, B.; Recio, I. HT29 Cell Line. In The Impact of Food Bioactives on Health: In vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 113–124. [Google Scholar]
- Houston, D.R.; Walkinshaw, M.D. Consensus docking: Improving the reliability of docking in a virtual screening context. J. Chem. Inf. Model. 2013, 53, 384–390. [Google Scholar] [CrossRef]
- Andalia, N.; Salim, M.N.; Saidi, N.; Ridhwan, M.; Iqhrammullah, M.; Balqis, U. Molecular docking reveals phytoconstituents of the methanol extract from Muntingia calabura as promising α-glucosidase jnhibitors. Karbala Int. J. Mod. Sci. 2022, 8, 330–338. [Google Scholar] [CrossRef]
- Purnama, A.; Rizki, D.R.; Qanita, I.; Iqhrammullah, M.; Ahmad, K.; Mardina, V.; Puspita, K.; Hasballah, K. Molecular docking investigation of calotropone as a potential natural therapeutic agent against pancreatic cancer. J. Adv. Pharm. Technol. Res. 2022, 13, 44. [Google Scholar] [PubMed]
- Lopes, A.J.O.; Calado, G.P.; Fróes, Y.N.; Araújo, S.A.d.; França, L.M.; Paes, A.M.d.A.; Morais, S.V.d.; Rocha, C.Q.d.; Vasconcelos, C.C. Plant Metabolites as SARS-CoV-2 Inhibitors Candidates: In Silico and In Vitro Studies. Pharmaceuticals 2022, 15, 1045. [Google Scholar] [CrossRef] [PubMed]

| Criteria | Description |
|---|---|
| Study design rationale | Study design is in line with the research question |
| Reproducibility | Methods are clear Materials and samples are presented in detail. |
| Replication | Performed in triplicate or more |
| Negative/positive control | Results compared with positive/negative control |
| Anti-SARS-CoV-2 activity | Investigation on the viral entry or replication, regardless the types of cell culture used. |
| Study adequacy | Study design is sufficient to reveal the molecular mechanism of essential oils as anti-SARS-CoV-2 |
| Author, Year, Ref | Study Design Rationale | Reproducibility | Replication | Negative Control | Positive Control | Anti-SARS-CoV-2 Activity | Study Adequacy |
|---|---|---|---|---|---|---|---|
| Ak Sakallı et al., 2022, [33] | Yes | Yes | Yes | Yes | Yes/No a | No | No |
| Asaad et al., 2022, [37] | Yes | Yes | Yes | Yes | No | Yes | No |
| Demirci et al., 2021, [30] | Yes | Yes | Yes | Yes | Yes/No a | No | No |
| Demirci et al., 2022, [31] | Yes | Yes | Yes | Yes | Yes/No a | No | No |
| Biltekin et al., 2022, [32] | Yes | Yes | Yes | Yes | Yes/No a | No | No |
| González-Maldonado et al., 2022, [34] | Yes | Yes | Yes | Yes | No | Yes | No |
| Kumar et al., 2020, [35] | Yes | Yes | Yes | Yes | No | No | No |
| Mohamed et al., 2022, [36] | Yes | Yes | Yes | Yes | No | Yes | No |
| Author, Year, [Ref.] | Plant | Compound of Interest | Molecular Target |
|---|---|---|---|
| Abdelli et al., 2021, [38] | Ammoides verticillate (Desf.) Briq | Isothymol | ACE2 |
| Costa et al., 2022, [39] | Stylosanthes guianensis Copaifera langsdorffii | γ-Eudesmol, β-selinene | Mpro |
| da Silva et al., 2020, [40] | Matricaria recutita L. | (E,E)-α-Farnesene, €-β-farnesene, (E,E)-farnesol | Mpro, endoribonuclease, ADP-ribose phosphatase, RdRp, spike RBD. ACE2 |
| Habibzadeh et al., 2022, [41] | Ferula gummosa | Δ-Cadinene, β-eudesmol, bulnesol | 3CLpro, Spike RBD, PLpro, RdRp |
| Kulkarni et al., 2020, [42] | Family Lamiaceae and Geraniaceae | Thymol, pulegone | Spike RBD |
| Mahomoodally et al., 2021, [43] | Cucurma longa L. | β-sesquiphellandrene, α-zingiberene | COVID-19 crystal structure |
| My et al., 2020, [44] | Melaleuca cajuputi | Guaiol and linanool | ACE2 |
| Panikar et al., 2021, [45] | Eucalyptus globulus Corymbia citrodora | 1.8-cineole | Mpro |
| Sharma et al., 2020 [47] | Eucalyptus sp. | 1.8-cineole | Mpro 3CLpro |
| Sharma et al., 2020 [46] | Eucalyptus sp. | Jensenone | 3CLpro, Mpro |
| Sharma et al., 2021 [52] | Eucalyptus sp. | Torquatone | Spike protein |
| Sharma et al., 2022 [49] | Cymbopogon citratus L. | Citral | Spike protein |
| Siddiqui et al., 2022, [50] | Moringa oleifera | 2-pyrrolidinone | Spike protein, ACE2 |
| Tu Quy PTA, 2022, [51] | Piper betle | Chavicol acetate, trans-Isoeugenol, Eugenol acetate | Spike protein |
| Author, Year [Ref.] | Sample | Major Compound * | In Vitro Assay | Outcome |
|---|---|---|---|---|
| Zeljković et al., 2022 [29] | Essential oils: Mentha sp., Micromeria thymifolia (Scop.) Fritsch, and Ziziphora clinopodioides Lam | p-Cymene; thymol; carvacrol; limonene; 1,8-cineol; linalool; menthone; menthofuran; menthol; terpinene-4-ol; α-terpineol; pulegone; and carvone | SARS-CoV-2-infected Vero 76 cells | M. pulegium, M. microphylla, M. vilosa, and M. thymifolia essential oils have SI => 13.47, 7.81, 9.27, and 6.73, respectively, against SARS-CoV-2 |
| Esharkawy et al., 2022 [28] | Nigella satvia | Thymoquinone 2,5-dihydroxy-para-cymene | SARS-CoV-2-infected Vero 76 cells | N. sativa essential oil has SI = 1.4 against SARS-CoV-2 |
| Lionis et al., 2021 [27] | Thymbra capitata (L.) Cav., Salvia fruticosa Mill., and Origanum dictamnus L. | Not reported | SARS-CoV-2-infected Vero 76 cells | Essential oils combination reduces the viral release up to >80% |
| Neto et al., 2022 [26] | Syzygium aromaticum, Cymbopogon citratus, Citrus limon, Pelargonium graveolens, Origanum vulgare, Illicium verum, and Matricaria recutita | (E)-Anetole, limonene, β-pinene, citronellol, and eugenol | SARS-CoV-2 delta pseudovirus infected to ACE2-expressing HeLa cells | I. verum, S. aromaticum, C. limon, and P. graveolens essential oils have SI > 4 (60, 4.4, 8.7, and 8.5, respectively) |
| Method [Ref.] | Advantages | Disadvantages |
|---|---|---|
| Commercial assay kit [30,31,33] |
|
|
| ACE2 expressing cancer cells [35] |
|
|
| Pseudotype virus [34] |
|
|
| SARS-CoV-2 virus [36,37] |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqhrammullah, M.; Rizki, D.R.; Purnama, A.; Duta, T.F.; Harapan, H.; Idroes, R.; Ginting, B. Antiviral Molecular Targets of Essential Oils against SARS-CoV-2: A Systematic Review. Sci. Pharm. 2023, 91, 15. https://doi.org/10.3390/scipharm91010015
Iqhrammullah M, Rizki DR, Purnama A, Duta TF, Harapan H, Idroes R, Ginting B. Antiviral Molecular Targets of Essential Oils against SARS-CoV-2: A Systematic Review. Scientia Pharmaceutica. 2023; 91(1):15. https://doi.org/10.3390/scipharm91010015
Chicago/Turabian StyleIqhrammullah, Muhammad, Diva Rayyan Rizki, Agnia Purnama, Teuku Fais Duta, Harapan Harapan, Rinaldi Idroes, and Binawati Ginting. 2023. "Antiviral Molecular Targets of Essential Oils against SARS-CoV-2: A Systematic Review" Scientia Pharmaceutica 91, no. 1: 15. https://doi.org/10.3390/scipharm91010015
APA StyleIqhrammullah, M., Rizki, D. R., Purnama, A., Duta, T. F., Harapan, H., Idroes, R., & Ginting, B. (2023). Antiviral Molecular Targets of Essential Oils against SARS-CoV-2: A Systematic Review. Scientia Pharmaceutica, 91(1), 15. https://doi.org/10.3390/scipharm91010015









