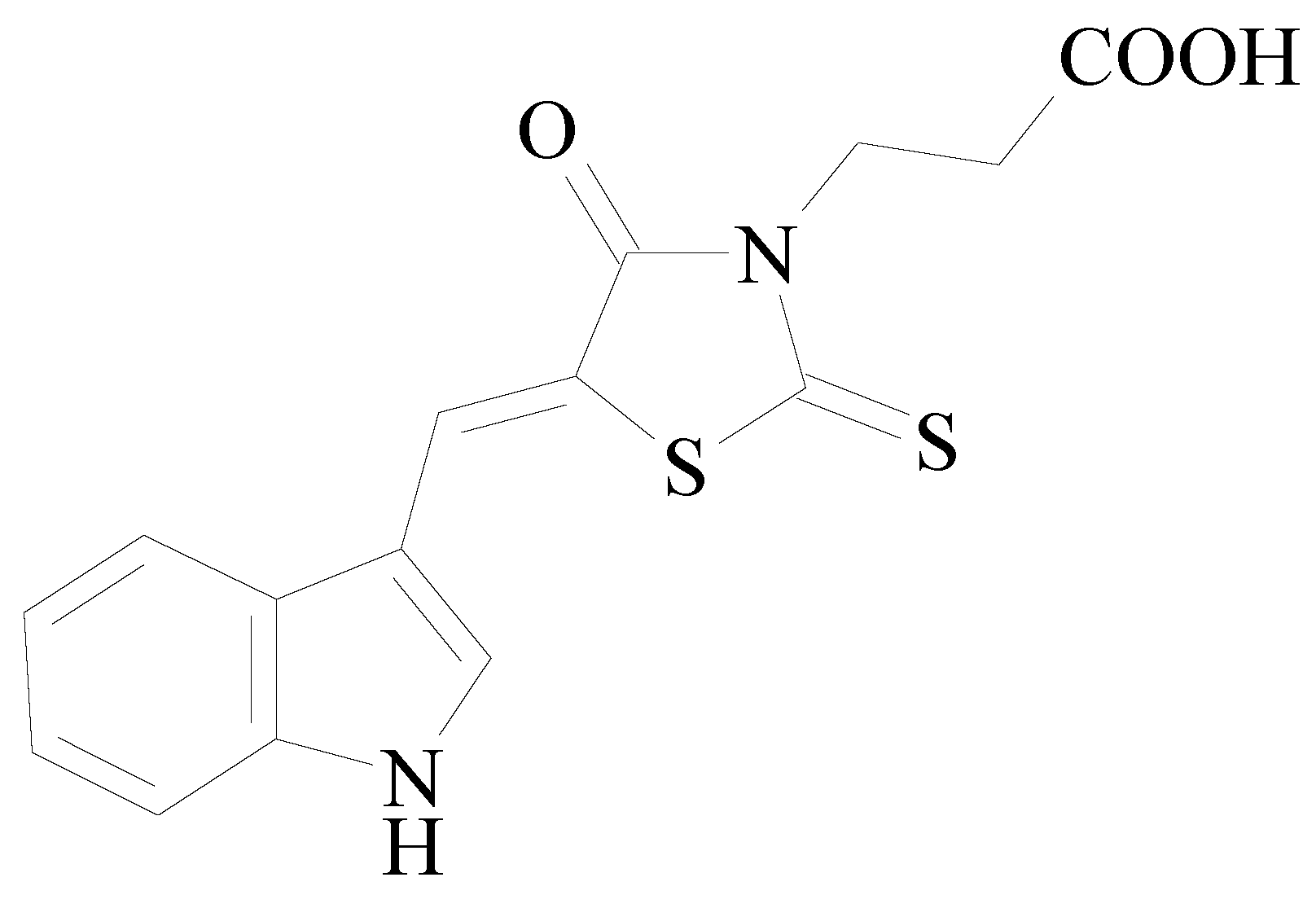
3-[5-(1H-Indol-3-ylmethylene)-4-oxo-2-thioxothiazolidin-3-yl]-propionic Acid as a Potential Polypharmacological Agent
Abstract
1. Introduction
2. Materials and Methods
2.1. Antimicrobial Activity
2.2. Cytotoxicity and Antitumor Effect In Vitro Cell Lines
2.3. Animals and Immunological (ELISA) Studies
2.4. Molecular and Pharmacokinetic Properties
2.5. Molecular Docking
2.6. Statistical Analysis
3. Results
3.1. Antimicrobial Activity
3.2. Cytotoxicity and Antitumor Effect In Vitro
3.3. Immunological (ELISA) Studies
3.4. Molecular and Pharmacokinetic Properties
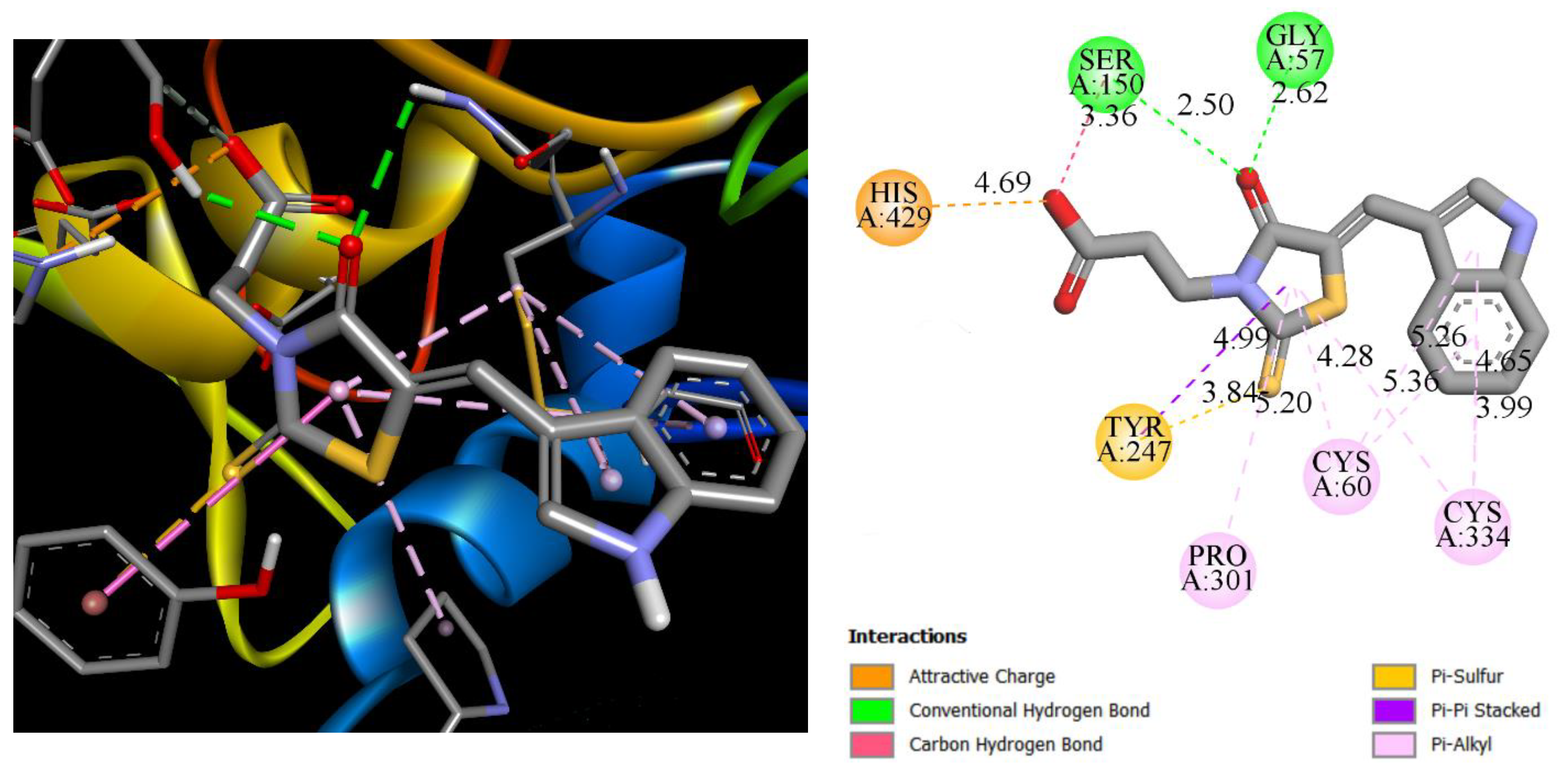
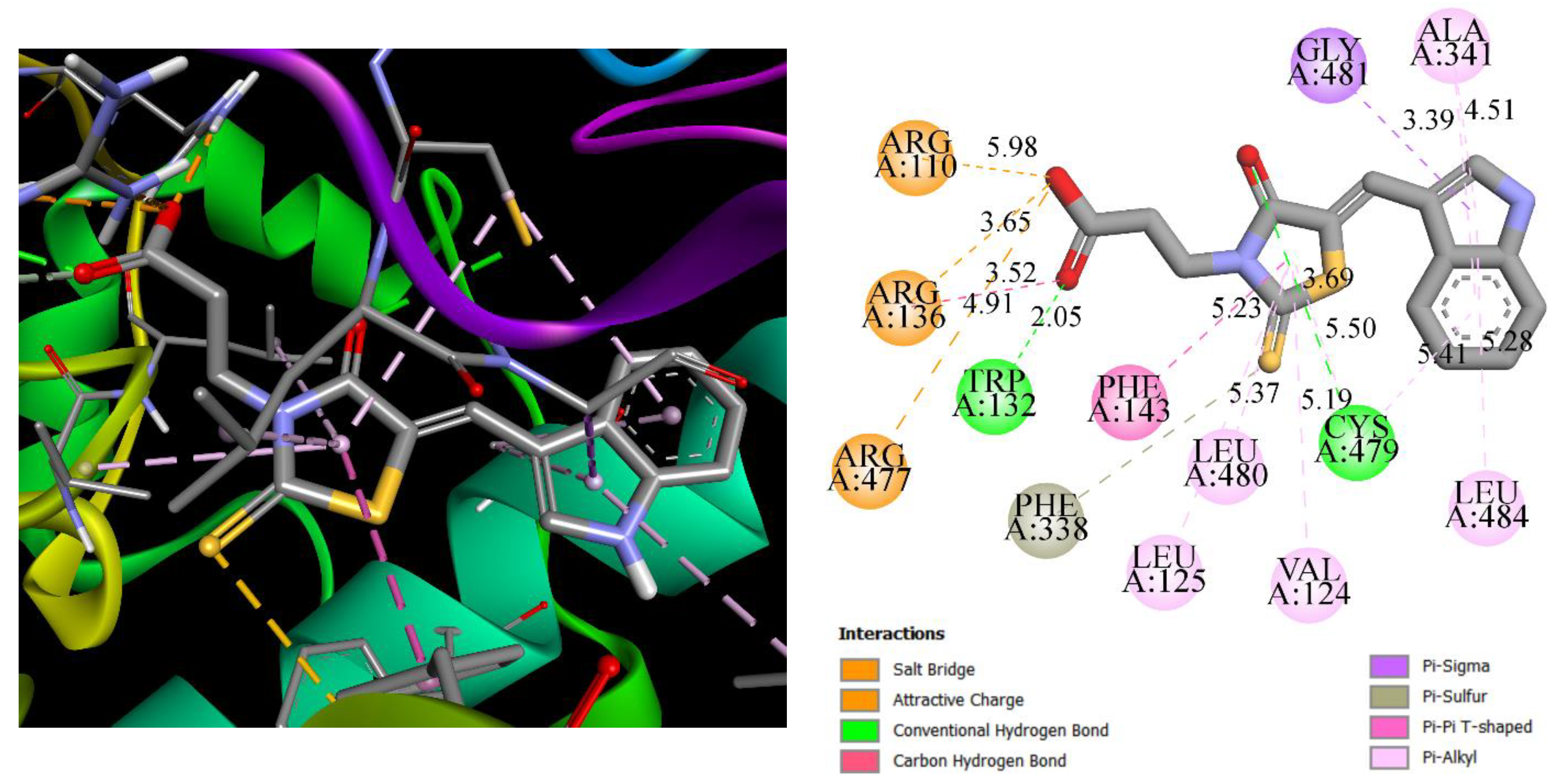
3.5. Molecular Docking
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Yang, B. Polypharmacology; Springer International Publishing: Cham, Switzerland, 2022; ISBN 978-3-031-04997-2. [Google Scholar]
- Anighoro, A.; Bajorath, J.; Rastelli, G. Polypharmacology: Challenges and Opportunities in Drug Discovery. J. Med. Chem. 2014, 57, 7874–7887. [Google Scholar] [CrossRef]
- Hughes, J.; Rees, S.; Kalindjian, S.; Philpott, K. Principles of Early Drug Discovery. Br. J. Pharmacol. 2011, 162, 1239–1249. [Google Scholar] [CrossRef]
- Konechnyi, Y.; Hrushka, O.; Pryzyhley, H.; Konechna, R.; Lozynskyi, A.; Korniychuk, O.; Lesyk, R. Cell Immunity of Laboratory Animals under the Influence of 5-Indolylmethylene Rhodanine-3-Carboxylic/Sulphonic Acid Derivative. Sci. Pharm. Sci. 2021, 1, 76–81. [Google Scholar] [CrossRef]
- Konechnyi, Y.T.; Lozynskyi, A.V.; Horishny, V.Y.; Konechna, R.T.; Vynnytska, R.B.; Korniychuk, O.P.; Lesyk, R.B. Synthesis of Indoline-Thiazolidinone Hybrids with Antibacterial and Antifungal Activities. Biopolym. Cell 2020, 36, 381–391. [Google Scholar] [CrossRef]
- EUCAST. Disk Diffusion—Manual v 10.0 (1 January 2022). Available online: https://www.eucast.org/ast_of_bacteria/disk_diffusion_methodology/ (accessed on 5 October 2022).
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Ivasechko, I.; Yushyn, I.; Roszczenko, P.; Senkiv, J.; Finiuk, N.; Lesyk, D.; Holota, S.; Czarnomysy, R.; Klyuchivska, O.; Khyluk, D.; et al. Development of Novel Pyridine-Thiazole Hybrid Molecules as Potential Anticancer Agents. Molecules 2022, 27, 6219. [Google Scholar] [CrossRef]
- Suckow, M.A.; Stevens, K.A.; Wilson, R.P. (Eds.) The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Fischbach, F.T.; Dunning, M.B. A Manual of Laboratory and Diagnostic Tests; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009. [Google Scholar]
- Alekseyeva, O.; Duyeva, L. Allergiya k Promyshlennym Khimicheskim Soyedineniyam-M.: Medicina. 1978, pp. 106–116. Available online: https://cyberleninka.ru/article/n/formirovanie-giperchuvstvitelnosti-k-toksikantam-alkiliruyuschego-deystviya (accessed on 1 February 2023). (In Russian).
- Keohane, E.M.; Otto, C.N.; Walenga, J. Rodak’s Hematology-E-Book: Clinical Principles and Applications; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Pagana, K.D.; Pagana, T. Mosby’s Manual of Diagnostic and Laboratory Tests-E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Halgren, T.A. Merck Molecular Force Field. I. Basis, Form, Scope, Parameterization, and Performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Schumacher, W.A.; Lucchesi, B.R. Effect of the Thromboxane Synthetase Inhibitor UK-37,248 (Dazoxiben) upon Platelet Aggregation, Coronary Artery Thrombosis and Vascular Reactivity. J. Pharmacol. Exp. Ther. 1983, 227, 790–796. [Google Scholar]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated Docking Using a Lamarckian Genetic Algorithm and an Empirical Binding Free Energy Function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Petrou, A.; Geronikaki, A.; Kartsev, V.; Kousaxidis, A.; Papadimitriou-Tsantarliotou, A.; Kostic, M.; Ivanov, M.; Sokovic, M.; Nicolaou, I.; Vizirianakis, I.S. N-Derivatives of (Z)-Methyl 3-(4-Oxo-2-Thioxothiazolidin-5-Ylidene)Methyl)-1H-Indole-2-Carboxylates as Antimicrobial Agents—In Silico and In Vitro Evaluation. Pharmaceuticals 2023, 16, 131. [Google Scholar] [CrossRef]
- Horishny, V.; Kartsev, V.; Geronikaki, A.; Matiychuk, V.; Petrou, A.; Glamoclija, J.; Ciric, A.; Sokovic, M. 5-(1H-Indol-3-Ylmethylene)-4-Oxo-2-Thioxothiazolidin-3-Yl)Alkancarboxylic Acids as Antimicrobial Agents: Synthesis, Biological Evaluation, and Molecular Docking Studies. Molecules 2020, 25, 1964. [Google Scholar] [CrossRef] [PubMed]
- Zoete, V.; Daina, A.; Bovigny, C.; Michielin, O. SwissSimilarity: A Web Tool for Low to Ultra High Throughput Ligand-Based Virtual Screening. J. Chem. Inf. Model. 2016, 56, 1399–1404. [Google Scholar] [CrossRef]
- Gfeller, D.; Grosdidier, A.; Wirth, M.; Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: A Web Server for Target Prediction of Bioactive Small Molecules. Nucleic Acids Res. 2014, 42, W32–W38. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Mahiddine, F.; Kim, G. Leptin Modulates the Metastasis of Canine Inflammatory Mammary Adenocarcinoma Cells through Downregulation of Lysosomal Protective Protein Cathepsin A (CTSA). Int. J. Mol. Sci. 2020, 21, 8963. [Google Scholar] [CrossRef]
- Toss, M.S.; Miligy, I.M.; Haj-Ahmad, R.; Gorringe, K.L.; AlKawaz, A.; Mittal, K.; Ellis, I.O.; Green, A.R.; Rakha, E.A. The Prognostic Significance of Lysosomal Protective Protein (Cathepsin A) in Breast Ductal Carcinoma in Situ. Histopathology 2019, 74, 1025–1035. [Google Scholar] [CrossRef]
- Southan, C. Caveat Usor: Assessing Differences between Major Chemistry Databases. ChemMedChem 2018, 13, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Khavrutskii, I.V.; Compton, J.R.; Jurkouich, K.M.; Legler, P.M. Paired Carboxylic Acids in Enzymes and Their Role in Selective Substrate Binding, Catalysis, and Unusually Shifted p K a Values. Biochemistry 2019, 58, 5351–5365. [Google Scholar] [CrossRef]
- Amano, H.; Nakamura, M.; Ito, Y.; Kakutani, H.; Eshima, K.; Kitasato, H.; Narumiya, S.; Majima, M. Thromboxane A Synthase Enhances Blood Flow Recovery from Hindlimb Ischemia. J. Surg. Res. 2016, 204, 153–163. [Google Scholar] [CrossRef]
- Mesitskaya, D.F.; Syrkin, A.L.; Aksenova, M.G.; Zhang, Y.; Zamyatnin, A.A., Jr.; Kopylov, P.Y. Thromboxane A Synthase: A New Target for the Treatment of Cardiovascular Diseases. Cardiovasc. Hematol. Agents Med. Chem. 2019, 16, 81–87. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y. Crosstalk Between Peroxisome Proliferator-Activated Receptor Gamma and the Canonical WNT/β-Catenin Pathway in Chronic Inflammation and Oxidative Stress During Carcinogenesis. Front. Immunol. 2018, 9, 745. [Google Scholar] [CrossRef]
- Sawant, R.L.; Wadekar, J.B.; Kharat, S.B.; Makasare, H.S. Targeting PPAR-γ to Design and Synthesize Antidiabetic Thiazolidines. EXCLI J. 2018, 17, 598–607. [Google Scholar] [CrossRef]
- Sauer, S. Ligands for the Nuclear Peroxisome Proliferator-Activated Receptor Gamma. Trends Pharmacol. Sci. 2015, 36, 688–704. [Google Scholar] [CrossRef]
- Furth, P.A. Peroxisome Proliferator-Activated Receptor Gamma and BRCA1. Endocr. Relat. Cancer 2019, 26, R73–R79. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.M.; Coquet, J.M.; Tibbitt, C.A. The Role of PPAR-γ in Allergic Disease. Curr. Allergy Asthma Rep. 2021, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, H.A.; Liesum, A.; Kroll, K.; Böhnisch, B.; Buning, C.; Ruf, S.; Sadowski, T. Crystal Structure of Cathepsin A, a Novel Target for the Treatment of Cardiovascular Diseases. Biochem. Biophys. Res. Commun. 2014, 445, 451–456. [Google Scholar] [CrossRef]
- Auch-Schwelk, W.; Katusic, Z.S.; Vanhoutte, P.M. Thromboxane A2 Receptor Antagonists Inhibit Endothelium-Dependent Contractions. Hypertension 1990, 15, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Kaminskyy, D.; Kryshchyshyn, A.; Lesyk, R. Recent Developments with Rhodanine as a Scaffold for Drug Discovery. Expert Opin. Drug Discov. 2017, 12, 1233–1252. [Google Scholar] [CrossRef] [PubMed]
- Tomasic, T.; Masic, L. Rhodanine as a Privileged Scaffold in Drug Discovery. Curr. Med. Chem. 2009, 16, 1596–1629. [Google Scholar] [CrossRef]
- Tomašić, T.; Peterlin Mašič, L. Rhodanine as a Scaffold in Drug Discovery: A Critical Review of Its Biological Activities and Mechanisms of Target Modulation. Expert Opin. Drug Discov. 2012, 7, 549–560. [Google Scholar] [CrossRef]
| N | Type of Species | Species of Bacteria and Fungi | Zone of Growth Inhibition (mm ± SE) and Some MIC Value (μM) | |||||
|---|---|---|---|---|---|---|---|---|
| Les-6614 | DMSO | Vanco- Mycin | Cipro- Floxacin | Clotri- Mazole | ||||
| 1 | Gram-negative bacteria | Reference strains | Pseudomonas aeruginosa ATCC 10145 | 7.0 ± 0.3 | 7.0 ± 0.3 | - | 35.0 ± 0.3 | - |
| 2 | Raoultella terrigena ATCC 33257 | 6.5 ± 0.25 | 6.5 ± 0.25 | - | 42.0 ± 0.5 | - | ||
| 3 | Raoultella ornithinolytica DSM 7464 | 7.0 ± 0.3 | 7.0 ± 0.3 | - | 40.0 ± 0.5 | - | ||
| 4 | Escherichia coli ATCC 25922 | n/a | n/a | - | 28.0 ± 0.5 | - | ||
| 5 | Clinical strains | Klebsiella pneumoniae 189 | n/a | n/a | - | 20.0 ± 0.2 | - | |
| 6 | Aeromonas hydrophila N196 | 8.0 ± 0.25 | n/a | - | 27.0 ± 0.4 | - | ||
| 7 | Escherichia coli N168 | n/a | n/a | - | 15.3 ± 0.2 | - | ||
| 8 | Morganella morganii N55 | 7.0 ± 0.3 | 7.0 ± 0.3 | - | 21.0 ± 0.4 | - | ||
| 9 | Kocuria marina N133 | 7.0 ± 0.3 | 7.0 ± 0.3 | - | 27.5 ± 0.5 | - | ||
| 10 | Gram-positive bacteria | Reference strains | Streptococcus agalactiae ATCC 13813 | 8.8 ± 0.25 | n/a | 32.0 ± 0.5 | - | - |
| 11 | Staphylococcus aureus subsp. aureus ATCC 25923 | 9.4 ± 0.4 | n/a | 32.0 ± 0.5 | 35.0 ± 0.5 | - | ||
| 12 | Staphylococcus epidermidis ATCC 12228 | 9.5 ± 0.5 | 12.0 ± 0.5 | 25.0 ± 0.5 | 52.5 ± 0.8 | - | ||
| 13 | Baccilus subtilis ATCC 6633 | 9.4 ± 0.2 | 7.4 ± 0.2 | 27.0 ± 0.5 | - | - | ||
| 14 | Clinical strains | Staphylococcus aureus N 23 | 8.2 ± 0.4 | n/a | 11.4 ± 0.3 | 9.0 ± 0.2 | - | |
| 15 | Enterococcus faecalis N26 | n/a | n/a | 11.1 ± 0.3 | 13.3 ± 0.5 | - | ||
| 16 | Enterococcus faecalis N191 | n/a | n/a | 11.0 ± 0.4 | 13.4 ± 0.5 | - | ||
| 17 | Fungi | Reference strains | Candida. albicans (ATCC 885-653) | 9.0 ± 0.4 | n/a | - | - | 18.0 ± 0.5 |
| 18 | Clinical strains | Candida albicans N67 | 9.0 ± 0.3/MIC 3003 µM | n/a | - | - | 11.0 ± 0.3 MIC 2.9 µM | |
| 19 | Candida guilliermondii N83 | n/a | n/a | - | - | 11.0 ± 0.3 | ||
| 20 | Candida utilis N127 | n/a | n/a | - | - | 11.0 ± 0.3 | ||
| 21 | Candida kefyr N92 | n/a | n/a | - | - | 11.0 ± 0.3 | ||
| 22 | Candida lusitaniae N89 | 7.0 ± 0.3 | n/a | - | - | 11.0 ± 0.3 | ||
| 23 | Saccharomyces cerevisiae N62 | 7.0 ± 0.4 | n/a | - | - | 8.0 ± 0.2 | ||
| Comp./Cell Line | HCT-116 | HCT-116 p53-/- | MCF-7 | KB3-1 | K562 | J774.2 | NIH 3T3 | HaCaT |
|---|---|---|---|---|---|---|---|---|
| Les-6614 | ˃100 | ˃100 | ˃100 | ˃100 | 83.20 ± 2.25 | 98.00 ± 2.30 | ˃100 | ˃100 |
| Doxorubicin | 0.57 ± 1.22 | 1.36 ± 0.91 | 1.53 ± 1.40 | 1.20 ± 1.38 | 1.34 ± 0.4 | 1.20 ± 0.53 | 1.56 ± 0.22 | ˃10 |
| Indicator | Control | 25 Per * | 75 Per * | Les-6614 | 25 Per * | 75 Per * | Change % | p |
|---|---|---|---|---|---|---|---|---|
| Ig E, ng/mL | 173.7 | 21.60 | 183.80 | 22.9 | <2.3 | 40.80 | −86.82 | |
| Ig E &, ng/mL | 244.6 | 200.4 | 302.7 | 161.6 | 103.4 | 144.1 | −33.93 | |
| Ig A, g/L | 1.6 ± 0.2 | 1.1 ± 0.1 | −35.29 | p < 0.05 | ||||
| IgM, g/L | 0.55 ± 0.04 | 0.48 ± 0.02 | −12.73 | p > 0.05 | ||||
| Ig G, g/L | 8.5 ± 0.3 | 10.3 ± 0.2 | 21.18 | p < 0.05 | ||||
| IL-2, pg/mL | 115.7 | 100.7 | 144 | 76.9 | 7.1 | 106 | −33.54 | |
| IL-6, pg/mL | 257.6 | 240.8 | 285.2 | 276.11 | 249.5 | 296.8 | 7.19 | |
| TNF-α, pg/mL | 44.5 | 28.6 | 58.2 | 26.2 | 21.1 | 27.6 | −41.12 |
| Physicochemical properties | ||
| 1 | Molecular weight | 332.40 |
| 2 | Num. heavy atoms | 22 |
| 3 | Num. arom. heavy atoms | 9 |
| 4 | Num. rotatable bonds | 4 |
| 5 | Num. H-bond acceptors | 3 |
| 6 | Num. H-bond donors | 2 |
| 7 | Molar Refractivity | 94.48 |
| 8 | TPSA Ų | 130.79 |
| 9 | Consensus log Po/w | 2.29 |
| 10 | Lipinski rule | Yes |
| Pharmacokinetics | ||
| 11 | GI absorption | High |
| 12 | BBB permeant | No |
| 13 | P-gp substrate | No |
| 14 | CYP1A2 inhibitor | Yes |
| 15 | CYP2C19 inhibitor | Yes |
| 16 | CYP2C9 inhibitor | Yes |
| 17 | CYP2D6 inhibitor | No |
| 18 | CYP3A4 inhibitor | Yes |
| 19 | Log Kp (SP) (cm/s) (skin permeation) | −6.48 |
| 20 | Bioavailability score | 0.56 |
| Compound | LPP (PDB 4CIA) | PPARγ (PDB 5Y2O) | VEGFR2 (PDB 4AGD) | TXAS | ||||
|---|---|---|---|---|---|---|---|---|
| Binding Energy | Inhibition Constant, Ki, µM | Binding Energy | Inhibition Constant, Ki, µM | Binding Energy | Inhibition Constant, Ki, µM | Binding Energy | Inhibition Constant, Ki, µM | |
| Compound | −8.47 | 0.62 | −8.49 | 0.60 | −9.43 | 0.121 | −9.07 | 0.225 |
| 6KZ 1 | −9.42 | 0.125 | - | - | - | - | - | - |
| Pioglitazone | - | - | −13.12 | 0.00024 | - | - | - | - |
| Sunitinib | - | - | - | - | −12.61 | 0.00057 | - | - |
| Dazoxiben | - | - | - | - | −10.14 | 0.037 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konechnyi, Y.; Lozynskyi, A.; Ivasechko, I.; Dumych, T.; Paryzhak, S.; Hrushka, O.; Partyka, U.; Pasichnyuk, I.; Khylyuk, D.; Lesyk, R. 3-[5-(1H-Indol-3-ylmethylene)-4-oxo-2-thioxothiazolidin-3-yl]-propionic Acid as a Potential Polypharmacological Agent. Sci. Pharm. 2023, 91, 13. https://doi.org/10.3390/scipharm91010013
Konechnyi Y, Lozynskyi A, Ivasechko I, Dumych T, Paryzhak S, Hrushka O, Partyka U, Pasichnyuk I, Khylyuk D, Lesyk R. 3-[5-(1H-Indol-3-ylmethylene)-4-oxo-2-thioxothiazolidin-3-yl]-propionic Acid as a Potential Polypharmacological Agent. Scientia Pharmaceutica. 2023; 91(1):13. https://doi.org/10.3390/scipharm91010013
Chicago/Turabian StyleKonechnyi, Yulian, Andrii Lozynskyi, Iryna Ivasechko, Tetiana Dumych, Solomiya Paryzhak, Oksana Hrushka, Ulyana Partyka, Iryna Pasichnyuk, Dmytro Khylyuk, and Roman Lesyk. 2023. "3-[5-(1H-Indol-3-ylmethylene)-4-oxo-2-thioxothiazolidin-3-yl]-propionic Acid as a Potential Polypharmacological Agent" Scientia Pharmaceutica 91, no. 1: 13. https://doi.org/10.3390/scipharm91010013
APA StyleKonechnyi, Y., Lozynskyi, A., Ivasechko, I., Dumych, T., Paryzhak, S., Hrushka, O., Partyka, U., Pasichnyuk, I., Khylyuk, D., & Lesyk, R. (2023). 3-[5-(1H-Indol-3-ylmethylene)-4-oxo-2-thioxothiazolidin-3-yl]-propionic Acid as a Potential Polypharmacological Agent. Scientia Pharmaceutica, 91(1), 13. https://doi.org/10.3390/scipharm91010013








