Phytochemistry and Evidence-Based Traditional Uses of the Genus Achillea L.: An Update (2011–2021)
Abstract
1. Introduction
2. Materials and Methods
- A.
- Phytochemicals: For the chemical compounds, all the publications that could be accessed were included with any information on the isolation or/and identification methods, excluding articles solely on essential oil and volatile constituents.
- B.
- Ethnopharmacology: For the traditional uses all the publications that could be accessed were included with any information on local medicinal uses of the genus Achillea.
- C.
- Pharmacological activities: For this section, only the literature data which associate the traditional knowledge of the genus Achillea with the pharmacological activities were included.
3. Chemical Constituents
3.1. Phytochemicals
3.1.1. Polyphenols
3.1.2. Flavonoids
| Species | Compound | Ref |
|---|---|---|
| A. abrotanoides Vis. * | Apigenin (2) Catechin (71) Chrysin (1) Galangin (36) Hesperetin (69) Kaempferol (37) Morin (65) Naringenin (66) Naringin (67) Pinocembrin (68) Quercitin (56) Rutin (58) | [16] |
| A. alpina L. | Artemitin (54) Eupatorin (21) Isoorientin (27) Luteolin 7-O-β-d-glucoside (24) | [17] |
| A. alpina L. | Axillarin (47) Chrysosplenol B (50) Isoschaftoside (17) Isovitexin (11) Jaceidin (52) Penduletin (40) Quercetin 3-O-vicianoside (63) Schaftoside (16) 5,7,4′-Τrihydroxy-3,6-dimethoxyflavanone (39) | [18] |
| A. alpina L. | Apigenin 7-O-β-d-glucoside (8) Acacetin 7-O-rutinoside (29) Apigenin (2) Chrysoeriol 7-O-β-d-glucoside (31) Chrysoeriol 7-O-rutinoside (32) Isorhamnetin (45) Isoquercitrin (55) Isorhamnetin 3-O-rutinoside (61) Luteolin 4′-O-β-d-glucoside (25) Rutin (58) Saponaretin (isovitexin) (11) Tricin 7-O-β-d-glucoside (33) | [19] |
| A. asiatica Serg. | Apigenin (2) Apigenin 7-O-β-d-glucoside (8) Luteolin (19) Quercetin 3-O-arabinosyl(1→6)glucoside (63) Schaftoside (16) | [20] |
| A. atrata L. * | Apigenin (2) Apigenin-O-hexoside # Centaureidin (49) Dimethoxy-myricetin-hexoside # Isorhamnetin-O-hexoside # Kaempferol 3-O-rutinoside (43) Luteolin (19) Luteolin 7-O-β-d-glucoside (24) Mearnsetin-hexoside #Nevadensin (7) Schaftoside (16) /isoschaftoside (17) Syringetin 3-O-glucoside (62) | [21] |
| A. atrata L. * | Apigenin (2) Apigenin 7-O-glucoside (8) Isorhamnetin-O-hexoside # Kaempferol 3-O-rutinoside # Luteolin (19) Luteolin-hexoside # Mearnsetin-hexoside # Nevadensin (7) Quercetin-O-hexoside # Quercetin 3-O-rutinoside (58) | [22] |
| A. aucheri Boiss. * | Apigenin (2) Luteolin (19) Luteolin 7-O-β-d-glucoside (24) Quercetin (44) Rutin (58) | [23] |
| A. biebersteinii Afan. | 5,7-Dihydroxy-3,3′,4′-trimethoxy flavone (46) Santin (41) Quercetagetin 3,6,3′-trimethyl ether (52) Quercetagetin 3,6-dimethyl ether (47) | [24] |
| A. biebersteinii Afan. * | Apigenin (2) Luteolin (19) Quercetin (44) Rutin (58) | [25] |
| A. biebersteinii Afan. * | Axillarin (47) 3,8-Dimethylherbacetin (38) Jaceidin (52) Kaempferol (37) | [26] |
| A. coarctata Poir. * | Apigenin (2) Kaempferol (37) Luteolin (19) Naringenin (66) | [27] |
| A. coarctata Poir. | Casticin (53) Centaureidin (49) Luteolin (19) | [7] |
| A. cretica L. * | Apigenin-C-pentoside-C-glucoside # Apigenin–C-glucoside-C-pentoside # Apigenin 6,8-di-C-glucoside (vicenin-2) (14) Apigenin 7-O-β-D-glucoside (8) Isorhamnetin 3-O-glucoside (60) Kaempferol 3-O-hexosylpentoside # Quercetin 3-O-glucoside (Isoquercitrin) (55) | [28] |
| A. distans Waldst. & Kit. subsp. distans * | Apigenin (2) Luteolin (19) Quercetin (44) | [29] |
| A. distans Waldst. & Kit. subsp. alpine * | Apigenin (2) Luteolin (19) Quercetin (44) Rutin (58) | [29] |
| A. filipendulina Lam. * | Apigenin (2) Luteolin (19) Luteolin 7-O-β-d-glucoside (24) Quercetin (44) Rutin (58) | [23] |
| A. fragrantissima (Forssk.) Sch. Bip. | Chrysosplenol D (51) Cirsiliol (22) Cirsimaritin (4) Eupatilin 7-methyl ether (23) | [30] |
| A. fragrantissima (Forssk.) Sch. Bip. | Acacetin 6-C-(6′′acetyl-β-d-glucopyranoside)-8-C-α-L-arabinopyranoside (18) Isovitexin (11) Isovitexin 4′-methyl ether (12) Quercetin 3,6,7-trimethyl ether (51) | [31] |
| A. fragrantissima (Forssk.) Sch. Bip. | Chrysosplenetine (50a) Chrysosplenol D (51) | [32] |
| A. fragrantissima (Forssk.) Sch. Bip. | Apigenin 6-C-glucoside (11) Cosmosiin (8) Jaceidin (52) Luteolin (19) Quercetin 3-O-galactoside (59) | [33] |
| A. grandifolia Friv. * | Luteolin (19) Luteolin 7-O-β-d-glucoside (24) Quercetagetin 3,6-dimethyl ether (47) Quercetin (44) Rutin (58) | [34] |
| A. kotschyi Boiss. subsp. kotschyi * | Apigenin (2) Hesperidin (29) Hyperoside (59) Kaempferol (37) Luteolin (19) Naringenin (66) Quercetin (44) Rutin (58) | [27] |
| A. lingulata Waldst. | Apigenin (2) Hesperetin (69) Morin (65) Rutin (58) | [16] |
| A. ligustica All. | 7-O-methyl apigenin (3) Apigenin (2) Apigenin 7-O-glucuronide (9) Galetin 3,6-dimethyl ether (39) Luteolin (19) Quercetin (44) Quercetin 3-O-glucuronide (57) Santin (41) | [35] |
| A. lycaonica Boiss. & Heldr. * | Apigenin (2) 8-Hydroxysalvigenin (6) Luteolin (19) Naringenin (66) Quercetin (44) Rutin (58) | [36] |
| A. lycaonica Boiss. & Heldr. * | Apigenin (2) Fisetin (35) Hesperidin (29) Hyperoside (59) Kaempferol (37) Luteolin (19) Naringenin (66)Quercetin (44) Rutin (58) | [27] |
| A. magnifica Hiemerl ex Hub.-Mor. * | Luteolin (isolated) (19) Apigenin 6,8-di C-hexoside (vicenin-2 isomer) # Apigenin 6-C-pentoside-8-C-hexosidec # Diosmetin 8-C-glucoside (orientin 4′-methyl ether) (28) Eupatilin (isolated) (23a) Vitexin (10) | [37] |
| A. millefolium L. | 6-OH-Luteolin-7-O-β-d-glucoside (30) Apigenin 7-O-β-d-glucoside (8) Luteolin (19) Luteolin 7-O-β-d-glucoside (24) | [38] |
| A. millefolium L. | 8,8′-bi-3-O-methylquercetin (70) Apigenin (2) Artemitin (54) Casticin (vitexicarpin) (53) Centaureidin (49) Chrysoeriol (20) Jaceidin (52) Luteolin (19) Quercetagetin 3,3′-dimethyl ether (43) | [39] |
| A. millefolium L. * | Apigenin 6-C-glucoside (11) Apigenin 7-O-glucoside (8) Apigenin C-glucose-C-pentoside # Apigenin C-hexoside-C-hexoside # Apigenin O-acetylhexoside # Apigenin O-dihexoside # Apigenin O-pentosyl hexoside # Isorhamnetin O-acetylhexoside # Isorhamnetin O-hexoside # Kaempferol 3-O-glucoside (42) Kaempferol 3-O-rutinoside (43) Kaempferol O-pentosyl-hexoside # Luteolin 6-C-glucoside (Isoorientin) (27) Luteolin 7-O-β-d-glucoside (24) Luteolin O-acetylhexoside # Quercetin 3-O-glucoside (55) Quercetin 3-O-rutinoside (58) Quercetin O-acetylhexoside # Quercetin O-hexoside # Quercetin O-malonylhexosyl-rhamnoside # Quercetin O-pentosyl-hexoside # | [40] |
| A. millefolium L. | Apigenin 7-O-β-d-glucoside (8) Dihydroquercetin (64) Luteolin 7-O-β-d-glucoside (24) Quercetin (44) Salvigenin (5) | [41] |
| A. millefolium L. * | Apigenin (2) Luteolin (19) Luteolin 7-O-β-d-glucoside (24) Rutin (58) | [23] |
| A. millefolium L. * | Axillarin (47) Jaceidin (52) Kaempferol (37) | [26] |
| A. millefolium L. * | Apigenin (2) Apigenin-O-hexoside # Centaureidin (49) Isorhamnetin-O-hexoside # Luteolin (19) Luteolin 3′,7-di-O-glucoside (34) Nevadensin (7) Quercetin 3-O-glucoside (55) Quercetin-O-pentosyl-hexoside # Schaftoside (16)/isoschaftoside (17) Syringetin 3-O-glucoside (62) | [21] |
| A. millefolium L. * | Apigenin (2) Apigenin 7-O-glucoside (8) Apigenin-6,8-di-C-hexoside #C entaureidin (49) Kaempferol 3-O-rutinoside # Kaempferol 3-O-rutinoside # Luteolin (19) Luteolin-hexoside # Nevadensin (7) Quercetin 3-O-rutinoside (58) Quercetin-O-hexoside Quercetin-O-hexoside # | [22] |
| A. monocephala Boiss. & Balansa | 8-Hydroxysalvigenin (6) Luteolin (19) Naringenin (66) | [42] |
| A. moschata W. * | Apigenin (2) Apigenin 7-O-β-d-glucoside (8) Isorhamnetin 3-O-glucosyl # Isorhamnetin 3-O-rutinosyl # Kaempferol 3-O-glucoside (42) Luteolin (19) Luteolin 7-O-β-d-glucoside (24) | [43] |
| A. moschata W. * | Apigenin (2) Apigenin 7-O- glucoside (8) Isorhamnetin 3-O-glucoside (60) Isorhamnetin 3-O-rutinoside (61) Kaempferol 3-O-glucoside (42) Luteolin (19) Luteolin 7-O-glucoside (24) | [44] |
| A. moschata W. * | Apigenin (2) Apigenin-O-hexoside # Centaureidin (49) Dimethoxy-myricetin-hexoside # Isorhamnetin-O-hexoside # Luteolin (19) Mearnsetin-hexoside # Nevadensin (7)Syringetin 3-O-glucoside (62) | [21] |
| A. nobilis L. * | Apigenin (2) Luteolin (19) Luteolin 7-O-β-d-glucoside (24) Quercetin (44) Rutin (58) | [23] |
| A. nobilis L. * | Apigenin-6,8-di-C-glucoside (14) 5-Demethylsinensetin (23) Isoorientin (27) Isoschaftoside (17) Luteolin (19) Luteolin 7-O-β-d-glucoside (24) Orientin (26) Quercetin (44) Vitexin (10) | [45] |
| A. pachycephala Rech. * | Apigenin (2) Luteolin (19) Luteolin 7-O-β-d-glucoside (24) Quercetin (44) Rutin (58) | [23] |
| A. pachycephala Rech. * | Apigenin (2) Apigenin 7-O-β-d-glucoside (9)Kaempferol (37)Luteolin (19) Luteolin 7-O-β-d-glucoside (24) Rutin (58) | [46] |
| A. santolina L. * | Apigenin (2) Luteolin (19) Luteolin 7-O-β-d-glucoside (24) Rutin (58) | [23] |
| A. schurii Sch.-Bip. * | Apigenin (2) Isoquercetin (55) Luteolin (19) Quercetin (44) Rutin (58) | [47] |
| A. setacea Waldst. & Kit. * | Apigenin (2)Luteolin (19)Quercetin (44)Rutin (58) | [25] |
| A. tenuifolia Lam. | 5-Demethylsinensetin (23) 3′,5-Dihydroxy-4′,6,7-trimethoxy flavone (eupatorin) (21) | [48] |
| A. vermicularis Trin. * | 8-Hydroxysalvigenin (6) Naringenin (66) Quercetagetin 3,6-dimethyl ether (47) Rutin (58) | [49] |
| A. wilhelmsii K. Koch * | 5-Demethylsinensetin (23) (isolated)2-(3,4-Dimethoxyphenyl)-5-hydroxy-6,7-dimethoxychromen-4-one # Isoorientin (27) Isoschaftoside (17) Isovitexin (11) Salvigenin (5) (isolated)Schaftoside (16) Swertisin (13)Vicenin-2 (14)Vicenin-3 (15) | [50] |
| A. wilhelmsii K. Koch * | Apigenin (2) Luteolin (19) Quercetin (44) Rutin (58) | [25] |
| A. wilhelmsii K. Koch. | Artemitin (54) isoschaftoside (17) Isovitexin (11) Penduletin (40) Salvigenin (5) Santoflavone (23) Vitexin (10) | [51] |
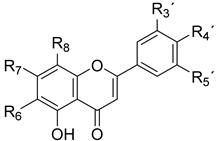 | R6 | R7 | R8 | R3 | R4 | R5 |
|---|---|---|---|---|---|---|
| Chrysin (1) | H | OH | H | H | H | H |
| Apigenin (2) | H | OH | H | H | OH | H |
| 7-O-methyl apigenin (genkwanin) (3) | H | OCH3 | H | H | OH | H |
| Cirsimaritin (4) | OCH3 | OCH3 | H | H | OH | H |
| Salvigenin (5) | OCH3 | OCH3 | H | H | OCH3 | H |
| 8-Hydroxysalvigenin (6) | OCH3 | OCH3 | OH | H | OCH3 | H |
| Nevadensin (7) | OCH3 | OH | OCH3 | H | OCH3 | H |
| Apigenin 7-O-glucoside (cosmosiin)(8) | H | O-Glc | H | H | OH | H |
| Apigenin 7-O-glucuronide (9) | H | O-Gluc | H | H | OH | H |
| Apigenin 8-C-glucoside (vitexin) (10) | H | OH | C-Glc | H | OH | H |
| Apigenin 6-C-glucoside (isovitexin, saponaretin) (11) | C-Glc | OH | H | H | OH | H |
| Isovitexin 4′-methyl ether (12) | C-Glc | OH | H | H | OCH3 | H |
| Swertisin (13) | C-Glc | OCH3 | H | H | OH | H |
| Apigenin 6,8-C-diglucoside (vicenin-2) (14) | C-Glc | OH | C-Glc | H | OH | H |
| Vicenin-3 (15) | C-Glc | OH | C-Xyl | H | OH | H |
| Apigenin 6-C-glucoside-8-C-arabinoside (schaftoside) (16) | C-Glc | OH | C-Ara | H | OH | H |
| Apigenin 6-C-arabinoside-8-C-glucoside (isoschaftoside) (17) | C-Ara | OH | C-Glc | H | OH | H |
| Acacetin 6-C-(6″-acetyl-β-D-(glucopyranoside)-8-C-α-L-arabinopyranoside (18) | C-[6″-acetyl]-Glc | OH | C-Ara | H | OCH3 | H |
| Luteolin (19) | H | OH | H | OH | OH | H |
| Chrysoeriol (20) | H | OH | H | OCH3 | OH | H |
| Eupatorin (21) | OCH3 | OCH3 | H | OH | OCH3 | H |
| Cirsiliol (22) | OCH3 | OCH3 | H | OH | OH | H |
| Eupatilin (23a) | OCH3 | OH | H | OCH3 | OCH3 | H |
| Eupatilin 7-methyl ether (5-demethylsinensetin; santoflavone) (23) | OCH3 | OCH3 | H | OCH3 | OCH3 | H |
| Luteolin 7-O-β-D-glucoside (24) | H | O-Glc | H | OH | OH | H |
| Luteolin 4′-O-β-D-glucoside (25) | H | OH | H | OH | O-Glc | H |
| Luteolin 8-C-glucoside (orientin) (26) | H | OH | C-Glc | OH | OH | H |
| Luteolin 6-C-glucoside (isoorientin) (27) | C-Glc | OH | H | OH | OH | H |
| Orientin 4′-methyl ether (28) | C-Glc | OH | H | OH | OCH3 | H |
| Acacetin 7-O-rutinoside (hesperidin) (29) | H | O-Rut | H | OH | OCH3 | H |
| 6-OH-luteolin 7-O-β-D-glucoside (30) | OH | O-Glc | H | OH | OH | H |
| Chrysoeriol 7-O-β-D-glucoside (31) | OH | O-Glc | H | OCH3 | OH | H |
| Chrysoeriol 7-O-rutinoside (32) | OH | O-Rut | H | OCH3 | OH | H |
| Tricin 7-O-β-D-glucoside (33) | H | O-Gluc | H | OCH3 | OH | OCH3 |
| Luteolin 3′,7-di-O-glucoside (34) | H | O-Glc | H | O-Glc | OH | H |
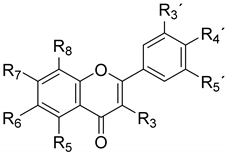 | R3 | R5 | R6 | R7 | R8 | R3’ | R4’ | R5’ |
|---|---|---|---|---|---|---|---|---|
| Fisetin (35) | OH | H | H | OH | H | H | OH | OH |
| Galangin (36) | OH | OH | H | OH | H | H | H | H |
| Kaempferol (37) | OH | OH | H | OH | H | H | OH | H |
| 3,8-Dimethylherbacetin (38) | OCH3 | OH | H | OH | OCH3 | H | OH | H |
| Galetin 3,6-dimethyl ether (5,7,4′-trihydroxy-3,6-dimethoxyflavanone) (39) | OCH3 | OH | OCH3 | OH | H | H | OH | H |
| Penduletin (40) | OCH3 | OH | OCH3 | OCH3 | H | H | OH | H |
| Santin (41) | OCH3 | OH | OCH3 | OH | H | H | OCH3 | H |
| Kaempferol 3-O-glucoside (42) | O-Glc | OH | H | OH | H | H | OH | H |
| Kaempferol 3-O-rutinoside (43) | O-Rut | OH | H | OH | H | H | OH | H |
| Quercetin (44) | OH | OH | H | OH | H | OH | OH | H |
| Isorhamnetin (45) | OH | OH | H | OH | H | OCH3 | OH | H |
| 5,7-dihydroxy-3,3′,4′-trimethoxy flavone (46) | OCH3 | OH | H | OH | H | OCH3 | OCH3 | H |
| Quercetagetin 3,6-dimethyl ether (Axillarin) (47) | OCH3 | OH | OCH3 | OH | H | OH | OH | H |
| Quercetagetin 3,3′-dimethyl ether (48) | OCH3 | OH | OH | OH | H | OCH3 | OH | H |
| Centaureidin (49) | OCH3 | OH | OCH3 | OH | H | OH | OCH3 | H |
| Chrysosplenol B (50) | OCH3 | OH | OCH3 | OCH3 | H | OH | OCH3 | H |
| Chrysosplenetin (50a) | OCH3 | OH | OCH3 | OCH3 | H | OCH3 | OH | H |
| Quercetin 3,6,7-trimethyl ether (Chrysosplenol D) (51) | OCH3 | OH | OCH3 | OCH3 | H | OH | OH | H |
| Jaceidin (Quercetagetin 3,6,3′-trimethyl ether) (52) | OCH3 | OH | OCH3 | OH | H | OCH3 | OH | H |
| Casticin (53) | OCH3 | OH | OCH3 | OCH3 | H | OH | OCH3 | H |
| Artemitin (54) | OCH3 | OH | OCH3 | OCH3 | H | OCH3 | OCH3 | H |
| Quercetin 3-O-glucoside (isoquercetin) (55) | O-Glc | OH | H | OH | H | OH | OH | H |
| Quercitin (56) | O-Rha | OH | H | OH | H | OH | OH | H |
| Quercetin 3-O-glucuronide (57) | O-Glc | OH | H | OH | H | OH | OH | H |
| Rutin (quercetin 3-O-rutinoside) (58) | O-Rut | OH | H | OH | H | OH | OH | H |
| Hyperoside (quercetin 3-O-galactoside) (59) | O-Gal | OH | H | OH | H | OH | OH | H |
| Isorhamnetin 3-O-glucoside (60) | O-Glc | OH | H | OH | H | OCH3 | OH | H |
| Isorhamnetin 3-O-rutinoside (61) | O-Rut | OH | H | OH | H | OCH3 | OH | H |
| Syringetin 3-O-glucoside (62) | O-Glc | OH | H | OH | H | OCH3 | OH | OCH3 |
| Quercetin 3-O-arabinosyl(1→6)glucoside (Quercetin 3-O-vicianoside) (63) | O-Ara 1→6 Glc | OH | H | OH | H | OH | OH | H |
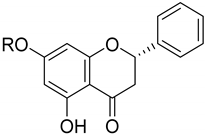
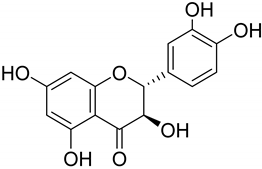 | 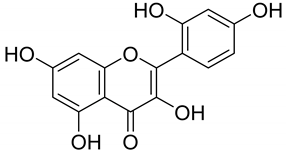 | |||||||
| Dihydroquercetin (64) | Morin (65) | |||||||
 |  | |||||||
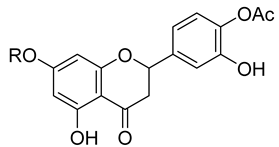
| Naringenin (66) R = H | Pinocembrin (68) | |||||||
| Naringin (67) R = Neohesperidoside | ||||||||
 | 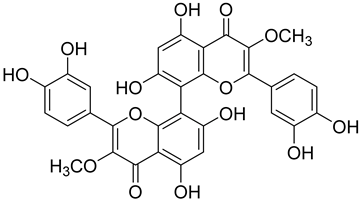 | |||||||
| Hesperetin (69) | 8,8′-bi-3-O-Methylquercetin (70) | |||||||
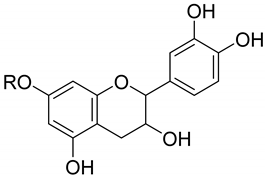 | ||||||||
| Catechin (71) | ||||||||
3.1.3. Phenolic and Quinic Acids Derivatives
| Species | Compound | Ref |
|---|---|---|
| A. alpina L. | Isochlorogenic acid A (86) Isochlorogenic acid B (87) | [57] |
| A. alpina L. | 5-Coumaroyl quinic acid (89) 5-O-Coumaroyl quinic acid methyl ester (92) Chlorogenic acid (81) Chlorogenic acid methyl ester (90) 3,5-Dicaffeoylquinic acid (86) Methyl 3,5-dicaffeoyl quinic acid (91) | [18] |
| A. alpina L. | 1-[4-(β-D-Glucopyranosyloxy)-3,5-dimethoxyphenyl]-propanone (96) 4-(4′-O-β-D-Glucopyranosyl-3′,5′dimethoxyphenyl)-2-butanone (97) | [19] |
| A. asiatica Serg. | Chlorogenic acid (81) | [20] |
| A. atrata L. * | p-Coumaric acid derivative # Chlorogenic acid (81) Dicaffeoylquinic acid # | [21] |
| A. atrata L. * | Caffeoyl-feruloylquinic acid # p-Coumaroyl acid derivative # Chlorogenic acid (81) Dicaffeoylquinic acid # | [22] |
| A. aucheri Boiss * | Caffeic acid (94) p-Coumaric acid (93) Chlorogenic acid (81) 1,3-Dicaffeoylquinic acid (85) Ferulic acid (95) | [23] |
| A. biebersteinii Afan. * | Caffeic acid (94) 3-Caffeoylquinic acid (81) 5-Caffeoylquinic acid (83) 4-Caffeoylquinic acid (82) Coumaroyl-quinic acid isomers # Cynarin (84) Quinic acid (72) | [26] |
| A. biebersteinii Afan. * | Caffeic acid (94) Chlorogenic acid (81) | [25] |
| A. coarctata Poir. * | Caffeic acid (94) Chlorogenic acid (81) 4-Hydroxybenzoic acid (73) Quinic acid (80) Salicylic acid (97a) Vanillin (76) | [27] |
| A. coarctata Poir. * | 3,5-Caffeoylquinic acid | [7] |
| A. cretica L. * | 5-Caffeoylquinic acid (Neochlorogenic acid) (83) 4,5-Dicaffeoylquinic acid (Isochlorogenic acid C) (88) | [28] |
| A. distans Waldst. & Kit. subsp. distans * | Caffeic acid (94) Chlorogenic acid (81) | [29] |
| A. distans Waldst. & Kit. subsp. alpine * | Caffeic acid (94) Chlorogenic acid (81) | [29] |
| A. filipendulina Lam. * | Chlorogenic acid (81) Caffeic acid (94) p-Coumaric acid (93) 1,3-Dicaffeoylquinic acid (85) Ferulic acid (95) | [23] |
| A. fragrantissima (Forssk.) Sch. Bip. | Piceol (77) Veratric acid (74) | [30] |
| A. grandifolia Friv. * | Caffeic acid (94) Chlorogenic acid (81) Dicaffeoylquinic acid # | [34] |
| A. kotschyi Boiss. subsp. kotschyi * | Caffeic acid (94) Chlorogenic acid (81) p-Coumaric acid (93) 4-Hydroxybenzoic acid (73) Protocatechuic acid (75) Quinic acid (80) Salicylic acid (97a) Vanillin (76) | [27] |
| A. ligustica All. | Caffeic acid (94) Chlorogenic acid (81) | [35] |
| A. lycaonica Boiss. & Heldr. * | 4-Hydroxybenzoic acid (73) Caffeic acid (94) Chlorogenic acid (81) Protocatechuic acid (75) Quinic acid (80) Salicylic acid (97a) Vanillin (76) | [27] |
| A. millefolium L. | Chlorogenic acid (81) | [41] |
| A. millefolium L. * | Caffeic acid (94) Caffeic acid hexoside # 5-Caffeoylquinic acid (83) 3-Caffeoylquinic acid (81) 4-Caffeoylquinic acid (82) 3,4-Dicaffeoylquinic acid (87) cis 3,5-Dicaffeoylquinic acid # trans 3,5-Dicaffeoylquinic acid # 4,5-Dicaffeoylquinic acid (88) | [40] |
| A. millefolium L. * | Quinic acid (80) 3-Caffeoylquinic acid (81) 5-Caffeoylquinic acid (83) 4-Caffeoylquinic acid (82) Coumaroyl-quinic acid isomers # Cynarin (84) | [26] |
| A. millefolium L. * | Caffeic acid (94) Chlorogenic acid (81) p-Coumaric acid (93) 1,3-Dicaffeoylquinic acid (85) Ferulic acid (95) | [23] |
| A. millefolium L. * | Chlorogenic acid (81) p-Coumaric acid derivative # Dicaffeoylquinic acid # | [21] |
| A. millefolium L. * | Chlorogenic acid (81) p-Coumaroyl acid derivative # Dicaffeoylquinic acid # | [22] |
| A. moschata W. * | 5-Caffeoylquinic acid (83) 4,5-Dicaffeoylquinic acid (88) | [43] |
| A. moschata W. * | Chlorogenic acid (81) p-Coumaric acid derivative # Dicaffeoylquinic acid # | [21] |
| A. moschata W. * | 5-Caffeoylquinic acid (83) 4,5-Dicaffeoylquinic acid (88) Disuccinylcaffeoylquinic acid # | [44] |
| A. multifidi (D.C.) Boiss. * | Chlorogenic acid (81) Dicaffeoylquinic acid # | [58] |
| A. nobilis L. * | Caffeic acid (94) Chlorogenic acid (81) p-Coumaric acid (93) 1,3-Dicaffeoylquinic acid (85) Ferulic acid (95) | [23] |
| A. nobilis L. * | Chlorogenic acid (81) | [45] |
| A. pachycephala Rech. * | Caffeic acid (94) Chlorogenic acid (81) 1,3-Dicaffeoylquinic acid (85) | [46] |
| A. santolina L. * | Caffeic acid (94) Chlorogenic acid (81) p-Coumaric acid (93) 1,3-Dicaffeoylquinic acid (85) Ferulic acid (95) | [23] |
| A. schurii Sch.-Bip. * | Caffeic acid (94) Genistic acid (78) Chlorogenic acid (81) p-Coumaric acid (93) | [47] |
| A. setacea Waldst. & Kit. * | Caffeic acid (94) Chlorogenic acid (73) | [25] |
| A. tenuifolia Lam. | 2,4-Dihydroxy-methyl benzoate (79) | [48] |
| A. vermicularis Trin. | Caffeic acid (94) Chlorogenic acid (81) Dicaffeoylquinic acid # | [49] |
| A. wilhelmsii K. Koch. * | Caffeic acid (94) Ferulic acid (95) | [50] |
| A. wilhelmsii K. Koch * | Caffeic acid (94) Chlorogenic acid (81) | [25] |
| A. wilhelmsii K. Koch. | Chlorogenic acid (81) | [51] |
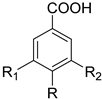 | Benzoic acid R = R1 = R2 = H (72) 4-Hydroxybenzoic acid R = OH, R1 = H, R2 = H (73) | ||
| Veratric acid R = R1 = OCH3, R2 = H (74) Protocatechuic acid R1 = H, R = R2 = OH (75) | |||
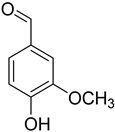 | 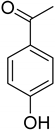 | 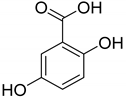 | 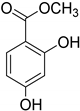 |
| Vanillin (76) | Piceol (77) | Genistic acid (78) | 2,4-Dihydroxy-methyl benzoate (79) |
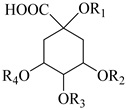 | Quinic acid R1 = R2 = R3 = R4 = H (80) | ||
| 3-Caffeoylquinic acid (chlorogenic acid) R1 = H, R2 = caffeoyl-, R3 = R4 = H (81) | |||
| 4-Caffeoylquinic acid (cryptochlorogenic acid) R1 = R2 = H, R3 = caffeoyl-, R4 = H (82) | |||
| 5-Caffeoylquinic acid (neohlorogenic acid) R1 = R2 = R3 = H, R4 = caffeoyl- (83) Cynarin (1,5-Dicaffeoylquinic acid) R1= R4 = caffeoyl-, R3 = R2 = H (84) 1,3-Dicaffeoylquinic acid R1 = R2 = caffeoyl-, R3 = R4 = H (85) Isochlorogenic acid A (3,5-dicaffeoylquinic acid) R1 = R3 = H, R2 = R4 = caffeoyl-(86) Isochlorogenic acid B (3,4-dicaffeoylquinic acid) R1 = R4 = H, R2 = R3 = caffeoyl-(87) 4,5-Dicaffeoylquinic acid (isochlorogenic acid C) R1 = R2 = H, R3 = R4 = caffeoyl- (88) 5-Coumaroyl quinic acid R1 = R2 = R3 = HR4 = coumaroyl- (89) | |||
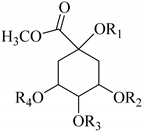 | Chlorogenic acid methyl ester R1 = H, R2 = caffeoyl-, R3 = R4 = H (90) Methyl 3,5-dicaffeoyl quinic acid R1 = R3 = H, R2 = R4 = caffeoyl- (91) 5-O-Coumaroyl quinic acid methyl ester R1 = R2 = R3 = H, R4 = coumaroyl- (92) | ||
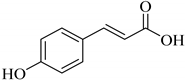 | 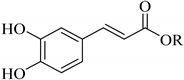 | 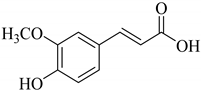 | |
| p-Coumaric acid (93) | Caffeic acid R = H (94) | Ferulic acid (95) | |
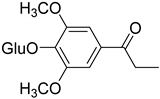 |  | 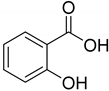 | |
| 1-[4-(β-D-Glucopyranosyloxy)-3,5-dimethoxyphenyl]-propanone (96) | 4-(4′-O-β-D-Glucopyranosyl-3′,5′dimethoxyphenyl)-2-butanone (97) | Salicylic acid (97a) | |
3.1.4. Sesquiterpenes Lactones (SLs)
| Species | Compound | Ref |
|---|---|---|
| A. alpina L. | Achillin (103) | [18] |
| A. biebersteinii Afan. | 4β,10α-Dihydroxy-5β,7β,8βH-guaia-1,11(13)dien-12,8α-olide (116) Micranthin(139) Sintenin (136) | [24] |
| A. biebersteinii Afan. | Rupicolin A (98) Rupicolin B (99) | [59] |
| A. clavennae L. | Apressin (106) 9α-Acetoxycanin (111) 3-β-Methoxy-iso-seco-tanapartholide (124) 3β,9β-Diacetoxy-1β-hydroperoxy-6β,7αH-germacra-4,10(14),11(13)-trien-12,6α-olide (137) 3,9-Diacetoxy-1-hydroxy-6β,7α,11H-germacra-4,10(14)-dien-12,6α-olide (138) Sintenin (136) | [60] |
| A. coarctata Poir. | 3-Acetylridentin (141) Artecalin (140) Arteludovicinolide A (122) 11,13- Dihydrodesacetylmatricarin (100) Rupicolin A (98) Rupicolin B (99) | [7] |
| A. cretica L. | 2β,3β-Epoxy-1α,10α-dihydroxy-4-chloroguaian-6α,12-olide (115) Τanaphillin (2α epimer) (129) Τanaphillin (2β epimer) (130) | [61] |
| A. falcata L. | 3-β-Methoxy-iso-seco-tanapartholide (124) | [62] |
| A. falcata L. | Chrysartemin B (109) 1β,2β-Epoxy-3β,4α,10α-trihydroxyguaian- 6α,12-olide (105) 8-Hydroxy-3-methoxy-iso-seco-tanapartholide (132) 3-β-Methoxy-iso-seco-tanapartholide (124) Iso-seco-tanapartholide (123) Rupin A (110) Tanaphillin (128) | [63] |
| A. ligustica All. | 3β-Chloro-4α,10α-dihydroxy-1α,2α-epoxy-5α,7αH-guaia-11(13)-en-12,6α-olide (113) Iso-seco-tanapartholide (123) Matricarin (101) | [35] |
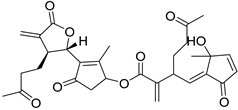
| A. millefolium L. | 3-Acetyl-iso-seco-tanapartholide (125) Arteludovicinolide A (122) 5-Epi-seco-tanapartholide A (131) Iso-seco-tanapartholide (123) 3-Methoxy-tanapartholide (124) Millifolide A (120) Millifolide B (121) Millifolide C (117) Seco-tanapartholide A (126) Seco-tanapartholide B (127) | [64] |
| A. millefolium L. | Achillinin A (104) | [65] |
| A. millefolium L. | Achillinin B (118) Achillinin C (119) | [66] |
| A. millefolium L. | Achillin (103) Leucodin (102) | [67] |
| A. wilhelmsii K. Koch. | 1β,10β-Epoxydesacetoxymatricarin (107) Leucodin (102) | [50] |
| A. wilhelmsii K. Koch. | Artecanin (109) Artecaninhydrate (108) Artemargyinolide B (112) Chloroklotzchin (114) Deacetylmatricarin-8-O-b-glucopyranoside (104) Hanphyllin (134) Leucodin (102) Wilhelmsin (133) Wilhelmsolide (135) | [51] |
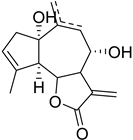 | 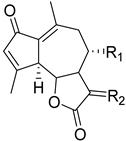 | R1 | R2 |
|---|---|---|---|
| Rupicolin A Δ9(10) (98) | 11,13-Dihydrodesacetylmatricarin (100) | αOH βH | CH2 |
| Rupicolin B Δ10(14) (99) | Matricarin (101) | αOAc βH | αCH3 βH |
| Leucodin (102) | H, H | αCH3 βH | |
| Achillin (103) | H, H | αH βCH3 | |
| Deacetylmatricarin-8-O-b-glucopyranoside (104) | O-Glc | αCH3 βH |
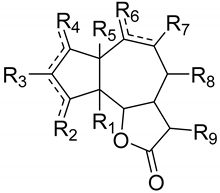 | epoxy | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1β,2β-Epoxy-3β,4α,10α-trihydroxyguaian-6α,12-olide (105) | 1β,2β | αH | αOH βCH3 | αH βOH | αH | - | αOH βCH3 | H | H | CH2 |
| Apressin (106) | 1α,4α | αH | βCH3 | Δ2(3) H | H | - | αOH βCH3 | αOAc βH | H | CH2 |
| 1β,10β-Epoxydesacetoxymatricarin (107) | 1β,10β | αH | αCH3 | Δ3(4) H | O | - | αCH3 | H | H | αCH3 βH |
| Artecaninhydrate (108) | 1α,10α | αH | αCH3 βOH | αOH | βH | - | αOH βCH3 | H | H | CH2 |
| Artecanin (Chrysartemin B) (109) | 1β,2β 3β,4β | αH | αCH3 | α H | αH | - | αOH βCH3 | H | H | CH2 |
| Rupin A (110) | 1β,2β 3β,4β | αH | αCH3 | α H | αH | - | αOH βCH3 | H | αOH βH | CH2 |
| 9α-Acetoxycanin (111) | 1α,2α 3α,4α | αH | βCH3 | β H | βH | - | αOH βCH3 | αOAc βH | H | CH2 |
| Artemargyinolide B (112) | - | H | αCH3 βOH | Δ2(3) H | H | OH | αOH βCH3 | H | H | CH2 |
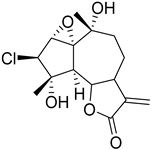 | 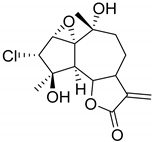 | 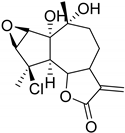 | ||||||||
| 3β-Chloro-4α,10α-dihydroxy-1α,2αa-epoxy-5α,7αH-guaia-11(13)-en-12,6α-olide (113) | Chloroklotzchin (114) | 2β,3β-Epoxy-1α,10α -dihydroxy-4-chloroguaian-6α,12-olide (115) | ||||||||
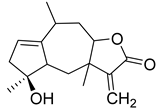 | 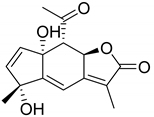 | |||||||||
| 4β,10α-Dihydroxy-5β,7β,8βH-guaia-1,11(13)dien-12,8α-olide (116) | Millifolide C (117) | |||||||||
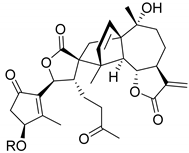 | 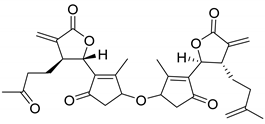 |  |
|---|---|---|
| Achillinin B (118) R = CH3 Achillinin C (119) R = H | Millifolide A (120) | Millifolide B (121) |
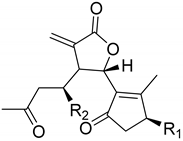 | 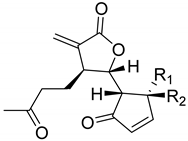 | ||||
|---|---|---|---|---|---|
| R1 | R2 | R1 | R2 | ||
| Arteludovicinolide A (122) | H | OH | Seco-tanapartholide A (126) | OH | CH3 |
| Iso-seco-tanapartholide (123) | OH | H | Seco-tanapartholide B (127) | CH3 | OH |
| 3-Methoxy-tanapartholide (124) | OCH3 | H | |||
| 3-Acetyl-iso-seco-tanapartholide (125) | OAc | H | |||
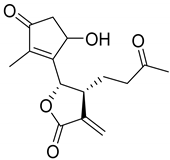 | 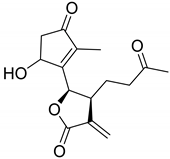 | 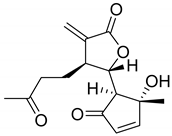 | |||
| Tanaphillin (128) | Τanaphillin (2α epimer) (129) Τanaphillin (2β epimer) (130) | 5-Epi-seco-tanapartholide A (131) | |||
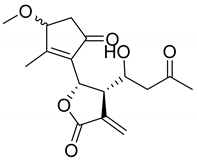 | 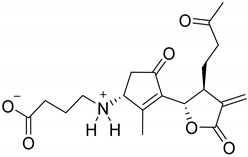 | ||||
| 8-Hydroxy-3-methoxy-iso-seco-tanaparatholide (132) | Wilhelmsin (133) | ||||
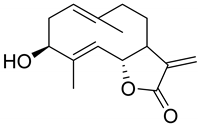 | 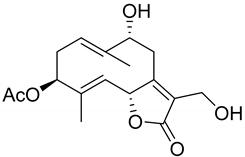 | ||||
| Hanphyllin (134) | Wilhelmsolide (135). | ||||
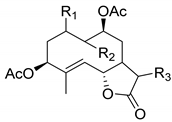 | 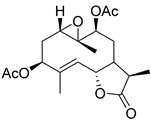 | ||||
|---|---|---|---|---|---|
| R1 | R2 | R3 | |||
| Sintenin (136) | H | Δ9(10) CH3 | αH βCH3 | Micranthin (139) | |
| 3β,9β-Diacetoxy-1β-hydroperoxy-6β,7αH-germacra-4,10(14),11(13)-trien-12,6α-olide (137) | αH βOH | Δ10(14) CH2 | CH2 | ||
| 3,9-Diacetoxy-1-hydroxy-6β,7α,11H-germacra-4,10(14)-dien-12,6α-olide (138) | αH βOH | Δ10(14) CH2 | CH3 | ||
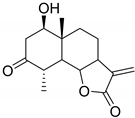 | 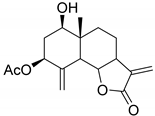 | |
|---|---|---|
| Artecalin (140) | 3-Acetylridentin (141) | |
3.1.5. Other Compounds
4. Ethnopharmacological Relevance: Traditional Uses of the Genus Achillea and Established Biological Effects
| Species | Traditional Uses | Ref. |
|---|---|---|
| A. aleppica DC. | Diuretic, carminative, emmenagogue, antiasthmatic, cardiotonic, stomachic, tonic effects, for colds, nephralgia, gynaecologic ailments and wound healing | [70,71] |
| A. alpina L. | Stomach disorders | [18] |
| Detox, clearing dampness, bloodstream promotion, and analgesic | [57] | |
| A. asiatica Serg. | Intestinal and stomach disorders, persistent fever, ulcers, wounds, inflammations and rheumatism | [20] |
| A. atrata L. | Respiratory disorders | [72] |
| A. bibersteinii Afan. | Diuretic, wound healing, gastrointestinal disorders, including abdominal pains and haemorrhoids | [67] |
| Colds, nephralgia, gynaecologic conditions such as women’ sterility, emmenagogue or jaundice, and astringent for skin conditions such as oedema and erythema | [73] [74] | |
| A. cappadocica Hausskn. & Bornm. | Astringent, emmenagogue, stomachic effects and to treat oedema | [71] |
| A. coarctata Poir. | Gastrointestinal ailments and hypertension, due to its diuretic effects | [7] |
| Menstrual effects | [74] | |
| A. collina Becker ex. Rchb. | Bedwetting by children and contradictory to increased diuresis and excretion of urinary stone blood purification, skin condition such as injuries, rashes and psoriasis, purulent ulcers, liver ailments, regulation of menstruation, bronchitis, asthma and throat ache | [75,76] |
| A. cretica L. | Gynaecological disorders and pathological symptoms analogous to endometriosis, wound healing, urogenital and respiratory disorders | [28] |
| A. clavennae L. | Abdominal pain, common cold, influenza, and respiratory disorders | [60,77] |
| A. falcata L. | Internal haemorrhage, uterine haemorrhoid, stomach ailment, and bladder stones | [56,78] |
| A. filipendulina Lam. | Hypoglycaemia | [56] |
| Arthritis, cardiovascular diseases, congestions, gastrointestinal disorders, gout, malaria, and as a diuretic, anthelmintic, and purgative agent | [79] | |
| A. fragrantissima (Forssk.) Sch. Bip. | Hypoglycaemia | [56] |
| Gastrointestinal disturbances, eye infections, and smallpox, fever caused by viral infection, chronic diseases, such as arthritis and diabetes | [30,80] | |
| Diabetes | [31] | |
| Respiratory diseases and gastrointestinal disturbances | [81] | |
| A. ligustica All. | Gastrointestinal disorders | [82] |
| Anthelmintic, against stomach-ache, chronic diseases such as rheumatism and skin disorders or inflammation, antimicrobial and haemostatic agent | [35,83] | |
| A. lanulosa Nutt. | Wound healing, as dermatological aid for sprains and swollen tissues, common cold, head and earache, indigestion problems, haemorrhoids and laxative agent | [56] |
| A. millefolium L. | Bleeding, stomach complaints, menstrual spasm | [8] |
| Gastrointestinal ailments, urinary, respiratory, and dermatological disorders | [84] | |
| Haemostatic and anti-inflammatory agent | [85] | |
| Anti-inflammatory and emmenagogue properties, for regulation of menstrual cycle and gynaecological disorders including endometriosis, urogenital and respiratory disorders, and as wound-healing agent | [28] | |
| Haemorrhoids, dyspepsia, dysmenorrhoea and gastric problems and fever | [86,87] | |
| Anti-inflammatory, emmenagogue, antipyretic, diuretic, and analgesic properties | [88] | |
| Wounds, cold, influenza and for stomach or urinary problems, as antiseptic, antitussive, for abdominal pain | [56,71] | |
| A. magnifica Hub.-Mor. | Stomach ailments | [36,89] |
| A. moschata Wulfen | Dyspepsia, abdominal bloating, flatulence, gastric pains and, in general, to affect the digestive system, cold, cough, dysmenorrhea, earache, fever, gout, headache, hypertension, insomnia, menopausal disorders, neuralgia, oliguria, skin inflammations, urinary tract inflammations and vaginitis, veterinary use | [44] |
| A. nobilis L. | Animal parasites, skin wounds and infections | [90] |
| A. santolina L. | Hypoglycaemia | [56] |
| Carminative, antispasmodic, depurative properties, as well as for stomach-aches and diabetes | [56] | |
| A. setacea Schwein. | Emmenagogue and stomachic | [71] |
| A. tenuifolia Lam. | Hypercholesterolemia, diabetes, asthma, bronchitis and cough | [71] |
| A. vermicularis Trin. | Stomachic problems | [71] |
| Anti-tumour effect | [49] | |
| A. wilhelmsii K. Koch. | Gastrointestinal and pulmonary complaints | [91] |
| Diuretic, for abdominal pain, as stomachic and emmenagogues and for women’ sterility, antihaemorrhoidal | [71] | |
| Body- and stomach-ache, blood coagulation, diabetes, hypertension, kidney stone and constipation | [50] |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Turkmenoglu, F.P.; Agar, O.T.; Akaydin, G.; Hayran, M.; Demirci, B. Characterization of volatile compounds of eleven Achillea species from turkey and biological activities of essential oil and methanol extract of A. hamzaoglui Arabacı & Budak. Molecules 2015, 20, 11432–11458. [Google Scholar] [CrossRef] [PubMed]
- Ehrendorfer, F.; Guo, Y.P. Multidisciplinary studies on Achillea sensu lato (Compositae-Anthemideae): New data on systematics and phylogeography. Willdenowia 2006, 36, 69–87. [Google Scholar] [CrossRef][Green Version]
- Guo, Y.P.; Ehrendorfer, F.; Samuel, R. Phylogeny and systematics of Achillea (Asteraceae—Anthemideae) inferred from the nrITS and plastid trnL-F DNA sequences. Taxon 2004, 53, 657–672. [Google Scholar] [CrossRef]
- Botanical Database. Available online: http://plantea.myspecies.info/ (accessed on 1 September 2021).
- Totelin, L.M.V. Hippocratic Recipes: Oral and Written Transmission of Pharmacological Knowledge in Fifth- and Fourth-Century Greece; Brill: Leiden, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Papakosta, K.; Grafakou, M.E.; Barda, C.; Kostopoulos, I.V.; Tsitsilonis, O.; Skaltsa, H. Cytotoxicity and anti-cancer activity of the genus Achillea L. Curr. Med. Chem. 2020, 27, 6910–6925. [Google Scholar] [CrossRef] [PubMed]
- Applequist, W.L.; Moerman, D.E. Yarrow (Achillea millefolium L.): A neglected panacea? A review of ethnobotany, bioactivity, and biomedical research. Econ. Bot. 2011, 65, 209. [Google Scholar] [CrossRef]
- World Health Organization. Available online: www.who.int (accessed on 1 September 2021).
- European Medicines Agency. Available online: www.ema.europa.eu (accessed on 1 September 2021).
- Si, X.T.; Zhang, M.L.; Shi, Q.W.; Kiyota, H. Chemical constituents of the plants in the genus Achillea. Chem. Biodivers. 2006, 3, 1163–1180. [Google Scholar] [CrossRef]
- Salehi, B.; Selamoglu, Z.; Sevindik, M.; Fahmy, N.M.; Al-Sayed, E.; El-Shazly, M.; Csupor-Löffler, B.; Csupor, D.; Yazdi, S.E.; Sharifi-Rad, J.; et al. Achillea spp.: A comprehensive review on its ethnobotany, phytochemistry, phytopharmacology and industrial applications. Cell Mol. Biol. 2020, 66, 78–103. [Google Scholar] [CrossRef]
- Mohammadhosseini, M.; Sarker, S.D.; Akbarzadeh, A. Chemical composition of the essential oils and extracts of Achillea species and their biological activities: A review. J. Ethnopharmacol. 2017, 6, 257–315. [Google Scholar] [CrossRef]
- Saeidnia, S.; Gohari, A.; Mokhber-Dezfuli, N.; Kiuchi, F. A review on phytochemistry and medicinal properties of the genus Achillea. DARU J. Pharm. Sci. 2011, 19, 173–186. [Google Scholar]
- Blumenthal, M.; Goldberg, A.; Brinckmann, J. Herbal Medicine, Expanded Commission E monographs; The American Botanical Council: Austin TX, USA, 2000; pp. 419–423. [Google Scholar]
- Kaczorová, D.; Karalija, E.; Dahija, S.; Bešta-Gajević, R.; Parić, A.; Ćavar Zeljković, S. Influence of extraction solvent on the phenolic profile and bioactivity of two Achillea species. Molecules 2021, 26, 1601. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Z.; Ren, T.; Ge, Y.; Zheng, Y.; Yao, D.; He, X.; Gu, Y.; Shi, Q.; Huo, C. Chemical composition of Achillea alpina. Chem. Nat. Compd. 2014, 50, 534–536. [Google Scholar] [CrossRef]
- Lee, H.; Sim, M.; Jeong Da-Eun, K.; Jung, H.; An, B.; Cho, H. Antioxidant and anti-melanogenic activities of compounds isolated from the aerial parts of Achillea alpina L. Chem. Biodivers. 2019, 16, e1900033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhou, Q.; Huo, C.; Wang, Y.; Wu, Y.; Zhang, M.; Li, L.; Jin, S.; Shi, Q.; Gu, Y. Phenolic components of the aerial parts of Achillea alpina. Chem. Nat. Compd. 2019, 55, 337–339. [Google Scholar] [CrossRef]
- Dorjsembe, B.; Lee, H.J.; Kim, M.; Dulamjav, B.; Jigjid, T.; Nho, C.W. Achillea asiatica extract and its active compounds induce cutaneous wound healing. J. Ethnopharmacol. 2017, 206, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Apel, L.; Lorenz, P.; Urban, S.; Sauer, S.; Spring, O.; Stintzing, F.C.; Kammerer, D.R. Phytochemical characterization of different yarrow species (Achillea sp.) and investigations into their antimicrobial activity. ZNC 2020, 76, 55–65. [Google Scholar] [CrossRef]
- Salomon, L.; Lorenz, P.; Bunse, M.; Spring, O.; Stintzing, F.C.; Kammerer, D.R. Comparison of the phenolic compound profile and antioxidant potential of Achillea atrata L. and Achillea millefolium L. Molecules 2021, 26, 1530. [Google Scholar] [CrossRef] [PubMed]
- Afshari, M.; Rahimmalek, M.; Miroliaei, M. Variation in polyphenolic profiles, antioxidant and antimicrobial activity of different Achillea species as natural sources of antiglycative compounds. Chem. Biodivers. 2018, 15, 1–15. [Google Scholar] [CrossRef]
- Abd-Alla, H.; Shalaby, N.; Hamed, M.; El-Rigal, N.S.; Al-Ghamdi, S.; Bouajila, J. Phytochemical composition, protective and therapeutic effect on gastric ulcer and a-amylase inhibitory activity of Achillea biebersteinii Afan. Arch. Pharm. Res. 2015, 39, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Şabanoğlu, S.; Gökbulut, A.; Altun, M.L. Characterization of phenolic compounds, total phenolic content and antioxidant activity of three Achillea species. J. Res. Pharm. 2019, 23, 567–576. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Strzępek-Gomółka, M.; Czop, M.; Sakipova, Z.; Głowniak, K.; Kukula-Koch, W. Achillea millefolium L. and Achillea biebersteinii Afan. hydroglycolic extracts-bioactive ingredients for cosmetic use. Molecules 2020, 24, 3368. [Google Scholar] [CrossRef]
- Agar, O.T.; Dikmen, M.; Ozturk, N.; Yilmaz, M.A.; Temel, H.; Turkmenoglu, F.P. Comparative studies on phenolic composition, antioxidant, wound healing and cytotoxic activities of selected Achillea L. species growing in turkey. Molecules 2015, 20, 17976–18000. [Google Scholar] [CrossRef] [PubMed]
- Bina, F.; Daglia, M.; Santarcangelo, C. Phytochemical profiling and ameliorative effects of Achillea cretica L. on rat model of endometriosis. J. Ethnopharmacol. 2020, 254, 112747. [Google Scholar] [CrossRef]
- Benedec, D.; Vlase, L.; Oniga, I.; Mot, A.C.; Damian, G.; Hanganu, D.; Duma, M.; Silaghi-Dumitrescu, R. Polyphenolic composition, antioxidant and antibacterial activities for two Romanian subspecies of Achillea distans Waldst. et Kit. ex Willd. Molecules 2013, 18, 8725–8739. [Google Scholar] [CrossRef]
- Awad, B.M.; Habib, E.S.; Ibrahim, A.K.; Wanas, A.S.; Radwan, M.M.; Helal, M.A.; ElSohly, M.A.; Ahmed, S.A. Cytotoxic activity evaluation and molecular docking study of phenolic derivatives from Achillea fragrantissima (Forssk.) growing in Egypt. Med. Chem. Res. 2017, 26, 2065–2073. [Google Scholar] [CrossRef]
- Ezzat, S.M.; Salama, M.M. A new α-glucosidase inhibitor from Achillea fragrantissima (Forssk). Sch. Bip. growing in Egypt. Nat. Prod. Res. 2014, 28, 812–881. [Google Scholar] [CrossRef]
- Skafa, J.; Hamarshehb, O.; Berningera, M.; Balasubramanianc, S.; Oelschlaegerc, T.; Holzgrabea, U. Improving anti-trypanosomal activity of alkamides isolated from Achillea fragrantissima. Fitoterapia 2018, 125, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Fattah, A.; Ali, S.; Aly, H.; Abd-Alla, H.; Shalaby, N.; Saleh, M. Therapeutic potential of Achillea fragrantissima extracts in amelioration of high-fat diet and low dose streptozotocin diabetic rats. J. Altern. Complement. Med. 2018, 7, 115–130. [Google Scholar] [CrossRef]
- Taşkın, D.; Alkaya, D.B.; Dölen, E. Analysis of natural dyestuffs in Achillea grandifolia Friv. using HPLC-DAD and Q-TOF LC/MS. NISCAIR-CSIR 2017, 16, 83–88. [Google Scholar]
- Venditti, A.; Guarcini, L.; Bianco, A.; Rosselli, S.; Bruno, M.; Senatore, F. Phytochemical analysis of Achillea ligustica All. from Lipari Island (Aeolian Islands). Nat. Prod. Res. 2015, 30, 912–919. [Google Scholar] [CrossRef]
- Taşkın, T.; Taşkın, D.; Rayaman, E.; Dikpınar, T.; Süzgeç-Selçuk, S.; Arabacı, T. Characterization of the biological activity and phenolics in Achillea lycaonica. Anal. Lett. 2018, 51, 33–48. [Google Scholar] [CrossRef]
- Taskin, T.; Dogan, M.; Arabaci, T. Bioassay-guided isolation and antiproliferative efficacy of extract loaded in chitosan nanoparticles and LC-QTOF-MS/MS analysis of Achillea magnifica. S. Afr. J. Bot. 2020, 133, 236–244. [Google Scholar] [CrossRef]
- Sevindik, H.G.; Guvenalp, Z.; Yerdelen, K.O.; Yuca, H. The discovery of potential anticholinesterase compounds from Achillea millefolium L. Ind. Crops Prod. 2015, 76, 873–879. [Google Scholar] [CrossRef]
- Huo, C.-H.; Li, Y.; Zhang, M.-L.; Wang, Y.-F.; Zhang, Q.; Qin, F.; Shi, Q.-W.; Kiyota, H. Cytotoxic flavonoids from the flowers of Achillea millefolium L. Chem. Nat. Compd. 2013, 48, 958–962. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Dueñas, M.; Pereira, E.; Carvalho, A.M.; Alves, R.C.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical composition of wild and commercial Achillea millefolium L. and bioactivity of the methanolic extract, infusion and decoction. Food Chem. 2013, 141, 4152–4160. [Google Scholar] [CrossRef]
- Bhat, M.H.; Bhat, K.A.; Prabha, S.; Hamid, A. Antioxidant and cytotoxic activities of Achillea millefolium from Kashmir. JAIR 2014, 2, 487–491. [Google Scholar]
- Taşkın, T.; Güler, E.M.; Şentürk, S.; Çelik, D.D.; Arabacı, T.; Gürer, Ü.S. Cytotoxic activity-guided isolation from Achillea monocephala, and biological activities of its different extracts. Compd. J. 2020, 8, 7–14. [Google Scholar] [CrossRef]
- Vitalini, S.; Madeo, M.; Tava, A.; Iriti, M.; Vallone, L.; Avato, P.; Argentieri, M.P. Chemical profile, antioxidant and antibacterial activities of Achillea moschata Wulfen, an endemic species from the alps. Molecules 2016, 21, 1–15. [Google Scholar] [CrossRef]
- Argentieri, M.P.; Madeo, M.; Avato, P.; Iriti, M.; Vitalini, S. Polyphenol content and bioactivity of Achillea moschata from the Italian and Swiss Alps. Z. Für Nat. C 2020, 75, 57–64. [Google Scholar] [CrossRef]
- Taşkın, D.; Taşkın, T.; Rayamanc, E. Phenolic composition and biological properties of Achillea nobilis L. subsp. neilreichii (Kerner) Formanek. Ind. Crops Prod. 2018, 111, 555–562. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.; Saeidia, G.; Talebib, M.; Matkowskic, A. The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech. Phytochemistry 2019, 162, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Benedec, D.; Hanganu, D.; Oniga, I.; Filip, L.; Bischin, C.; Silaghi-Dumitrescu, R.; Tiperciuc, B.; Vlase, L. Achillea schurii Flowers: Chemical, antioxidant, and antimicrobial investigations. Molecules 2016, 21, 1050. [Google Scholar] [CrossRef] [PubMed]
- Moradkhani, S.; Kobarfard, F.; Ayatollahi, S.A.M. Phytochemical investigations on chemical constituents of Achillea tenuifolia Lam. IJPR 2014, 13, 1049–1054. [Google Scholar] [PubMed]
- Taskin, T.; Balkan, I.; Tankin, D.; Dogan, A. Characterization of phenolic constituents and pharmacological activity of Achillea vermiculari. IJPS 2019, 81, 293–301. [Google Scholar] [CrossRef]
- Khazneh, E.; Hribová, P.; Hošek, J.; Suchý, P.; Kollár, P.; Pražanová, G.; Muselík, J.; Hanaková, Z.; Václavík, J.; Miłek, M.; et al. The chemical composition of Achillea wilhelmsii C. Koch and its desirable effects on hyperglycemia, inflammatory mediators and hypercholesterolemia as risk factors for cardiometabolic disease. Molecules 2016, 21, 404. [Google Scholar] [CrossRef]
- Serino, E.; Chahardoli, A.; Badolati, N.; Sirignano, C.; Jalilian, F.; Mojarrab, M.; Farhangi, Z.; Rigano, D.; Stornaiuolo, M.; Shokoohinia, Y.; et al. Salvigenin, a trimethoxylated flavone from Achillea Wilhelmsii C. Koch, exerts combined lipid-lowering and mitochondrial stimulatory effects. Antioxidants 2021, 10, 1042. [Google Scholar] [CrossRef]
- Valant-Vetschera, K.M.; Wollenweber, E. Comparative analysis of leaf exudate flavonoids in Achillea subsect. Filipendulinae. Biochem. Syst. Ecol. 1996, 24, 435–446. [Google Scholar] [CrossRef]
- Wollenweber, E.; Valant-Vetschera, K.; Ivanceva, S.; Kuzmanov, B. Flavonoid aglycones from the leaf surfaces of some Achillea species. Phytochemistry 1987, 26, 181–182. [Google Scholar] [CrossRef]
- Ivanceva, S.; Kuzmanov, B. Flavonoids in genus Achillea, A. nobilis group. Bull Liais. Groupe Polyphén. 1986, 13, 576–579. [Google Scholar]
- Ivanceva, S.; Kuzmanov, B. Epicuticular flavonoids in Achillea sect. Filipendulinae; A comparative study. Farmacija 1990, 40, 20–24. [Google Scholar]
- Nemeth, E.; Bernath, J. Biological activities of yarrow species (Achillea spp.). Curr. Pharm. Des. 2008, 14, 3151–3167. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-W.; Wang, R.-R.; Sun, X.-Y.; Fan, J.-H.; Qi, H.; Liu, Y.; Qin, G.-Q.; Wang, W. Identification of transient receptor potential vanilloid 3 antagonists from Achillea alpina L. and separation by liquid-liquid-refining extraction and high-speed counter-current chromatography. Molecules 2020, 25, 2025. [Google Scholar] [CrossRef] [PubMed]
- Taşkın, T.; Özakpınar, Ö.B.; Gürbüz, B.; Uras FGürer, U.S.; Bitiş, L. Identification of phenolic compounds and evaluation of antioxidant, antimicrobial and cytotoxic effects of the endemic Achillea multifida. IJTK 2016, 15, 594–603. [Google Scholar]
- Mohamed, A.E.-H.H.; Mohamed, N.S.; Hamed, A.R.; Hegazy, M.-E.F. Anti-inflammatory activity of highly oxygenated terpenoids from Achillea biebersteinii Afan. Z. Für Nat. C 2016, 71, 429–432. [Google Scholar] [CrossRef]
- Trifunovic, S.; Isaković, A.M.; Isaković, A.; Vučković, I.; Mandić, B.; Novaković, M.; Vajs, V.; Milosavljević, S.; Trajković, V. Isolation, characterization, and in vitro cytotoxicity of new sesquiterpenoids from Achillea clavennae. Planta Med. 2014, 80, 297–305. [Google Scholar] [CrossRef]
- Hichri, F.; Znatia, M.; Janneta, H.B.; Bouajilac, J. A new sesquiterpene lactone and secoguaianolides from Achillea cretica L. growing in Tunisia. Ind. Crops Prod. 2015, 77, 735–740. [Google Scholar] [CrossRef]
- Saikali, M.; Ghantous, A.; Halawi, R.; Talhouk, S.N.; Saliba, N.A.; Darwiche, N. Sesquiterpene lactones isolated from indigenous Middle Eastern plants inhibit tumor promoter-induced transformation of JB6 cells. BMC Complement. Altern. Med. 2012, 12, 89. [Google Scholar] [CrossRef]
- Tohme, R.; Al Aaraj, L.; Ghaddar, T.; Gali-Muhtasib, H.; Saliba, N.A.; Darwiche, N. Differential growth inhibitory Eeffects of highly oxygenated guaianolides isolated from the Middle Eastern indigenous plant Achillea falcata in HCT-116 colorectal cancer cells. Molecules 2013, 18, 8275–8288. [Google Scholar] [CrossRef]
- Li, Y.; Ni, Z.-Y.; Zhu, M.-C.; Zhang, K.; Wu, Y.-B.; Dong, M.; Shi, Q.-W.; Huo, C.-H.; Sauriol, F.; Kiyota, H.; et al. Millifolides A–C. New 1,10-Seco-guaianolides from the Flowers of Achillea millefolium. Z. Für Nat. B 2012, 67, 438–446. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, M.-L.; Cong, B.; Wang, S.-M.; Dong, M.; Sauriol, F.; Huo, C.-H.; Shi, Q.-W.; Gu, Y.-C.; Kiyota, H. Achillnin A, a Cytotoxic Guaianolide from Flower of yarrow, Achillea millefolium. Biosci. Biotechnol. Biochem. 2011, 75, 1554–1556. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, M.C.; Zhang, M.L.; Wang, Y.F.; Dong, M.; Shi, Q.W.; Huo CHSauriol, F.; Kiyota, H.; Gu, Y.-G.; Cong, B. Achillinin B and C, new sesquiterpene dimers isolated from Achillea millefolium. Tetr. Lett. 2012, 53, 2601–2603. [Google Scholar] [CrossRef]
- Arias-Durán, L.; Estrada-Soto, S.; Hernández-Morales, M.; Chávez-Silva, F.; Navarrete-Vázquez, G.; León-Rivera, I.; Perea-Arango, I.; Villalobos-Molina, R.; Ibarra-Barajas, M. Tracheal relaxation through calcium channel blockade of Achillea millefolium hexanic extract and its main bioactive compounds. J. Ethnopharmacol. 2020, 253, 112643. [Google Scholar] [CrossRef] [PubMed]
- Althaus, J.B.; Kaiser, M.; Brun, R.; Schmidt, T.J. Antiprotozoal activity of Achillea ptarmica (Asteraceae) and its main alkamide constituents. Molecules 2014, 19, 6428–6438. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, D.; He, L.; Mao, Q.; Hu, X. A new lignan and a new terpenoid from Achillea millefolium L. Phytochem. Lett. 2017, 22, 247–250. [Google Scholar] [CrossRef]
- Sezik, E.; Yesilada, E.; Honda, G. Traditional medicine in Turkey X. Folk medicine in Central Anatolia. J. Ethnopharmacol. 2001, 75, 95–115. [Google Scholar] [CrossRef]
- Altundag, E.; Ozturk, M. Ethnomedicinal studies on the plant resources of east Anatolia, Turkey. Procedia Soc. Behav. Sci. 2011, 19, 756–777. [Google Scholar] [CrossRef]
- Ristić, M.; Soković, M.; Grubišić, D.; Kovacević, N. Chemical analysis and antifungal activity of the essential oil of Achillea atrata L. J. Essent. Oil Res. 2004, 16, 75–78. [Google Scholar] [CrossRef]
- Ghasemi Pirbalouti, A.; Hossayni, I.; Shirmardi, H.A. Essential oil variation, antioxidant and antibacterial activity of mountain fennel (Zaravschanica membranacea (Boiss.) M. Pimen.). Ind. Crops Prod. 2013, 50, 443–448. [Google Scholar] [CrossRef]
- Naghibi, F.; Esmaeili, S.; Malekmohammadi, M.; Hassanpour, A.; Mosaddegh, M. Ethnobotanical survey of medicinal plants used traditionally in two villages of Hamedan, Iran. Res. J. Pharmacogn. 2014, 1, 7–14. [Google Scholar]
- Saric-Kundalić, B.; Fritz, E.; Dobeš, C.; Saukel, J. Traditional medicine in the pristine village of Prokoško lake on Vranica mountain, Bosnia and Herzegovina. Sci. Pharm. 2010, 78, 275–290. [Google Scholar] [CrossRef]
- Saric-Kundalic, B.; Dobes, C.; Klatte-Asselmeyer, V.; Saukel, J. Ethnobotanical study on medicinal use of wild and cultivated plants in middle, south and west Bosnia and Herzegovina. J. Ethnopharmacol. 2010, 131, 33–55. [Google Scholar] [CrossRef]
- Skocibusić, M.; Bezić, N.; Dunkić, V.; Radonić, A. Antibacterial activity of Achillea clavennae essential oil against respiratory tract pathogens. Fitoterapia. 2004, 75, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Ghantous, A.; Nasser, N.; Saab, I.; Darwiche, N.; Saliba, N.A. Structure–activity relationship of seco-tanapartholides isolated from Achillea falcata for inhibition of HaCaT cell growth. Eur. J. Med. Chem. 2009, 44, 3794–3797. [Google Scholar] [CrossRef] [PubMed]
- Aminkhani, A.; Sharifi, S.; Ekhtiyari, S. Achillea filipendulina Lam.: Chemical constituents and antimicrobial activities of essential oil of stem, leaf, and flower. Chem. Biodivers. 2020, 17, e2000133. [Google Scholar] [CrossRef] [PubMed]
- Choucry, M.A. Chemical composition and anticancer activity of Achillea fragrantissima (Forssk.) Sch. Bip. (Asteraceae) essential oil from Egypt. J. Pharmacogn. Phytother. 2017, 9, 1–5. [Google Scholar] [CrossRef]
- Elmann, A.; Mordechay, S.; Erlank, H.; Telerman, A.; Rindner, M.; Ofir, R. Anti-neuroinflammatory effects of the extract of Achillea fragrantissima. BMC Complement. Altern. Med. 2011, 1, 98. [Google Scholar] [CrossRef]
- Freires, I.A.; Denny, C.; Benso, B.; De Alencar, S.M.; Rosalen, P.L. Antibacterial activity of essential oils and their isolated constituents against cariogenic bacteria: A systematic review. Molecules 2015, 20, 7329–7358. [Google Scholar] [CrossRef]
- Maggi, F.; Bramucci, M.; Cecchini, C.; Coman, M.M.; Cresci, A.; Cristalli, G.; Lupidi, G.; Papa, F.; Quassinti, L.; Sagratini, G.; et al. Composition and biological activity of essential oil of Achillea ligustica All. (Asteraceae) naturalized in central Italy: Ideal candidate for anti-cariogenic formulations. Fitoterapia 2009, 80, 313–319. [Google Scholar] [CrossRef]
- Lin, L.-T.; Liu, L.-T.; Chiang, L.-C.; Lin, C.-C. In vitro anti-hepatoma activity of fifteen natural medicines from Canada. Phytother. Res. 2002, 16, 440–444. [Google Scholar] [CrossRef]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Wagner, H.; Verpoorte, R.; Heinrich, M. Medicinal plants of the Russian pharmacopoeia; their history and applications. J. Ethnopharmacol. 2014, 146, 481–536. [Google Scholar] [CrossRef]
- Miraldi, E.; Ferri, S.; Mostaghimi, V. Botanical drugs and preparations in the traditional medicine of West Azerbaijan (Iran). J Ethnopharmacol. 2001, 75, 77–87. [Google Scholar] [CrossRef]
- Sharma, P.K.; Chauhan, N.S.; Lal, B. Observations on the traditional phytotherapy among the inhabitants of Parvati valley in western Himalaya, India. J. Ethnopharmacol. 2004, 92, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A.; Bano, H. Brinjasif (Achillea millefolium Linn): An efficacious unani medicine. Int. J. Herb. Med. 2018, 6, 25–28. [Google Scholar]
- Demirci, B.; Baser, K.H.C.; Aytac, Z.; Khan, S.I.; Jacob, M.R.; Tabanca, N. Comparative study of three Achillea essential oils from eastern part of Turkey and their biological activities. Rec. Nat. Prod. 2018, 12, 195–200. [Google Scholar] [CrossRef]
- Ghani, A.; Azizi, M.; Hassanzadeh-Khayyat, M.; Pahlavanpour, A.A. Comparison of chemical composition of Achillea eriophora and A. wilhelmsii grown in wild and cultivated conditions in Iran. J. Essent. Oil Bear. Plants 2011, 14, 617–624. [Google Scholar] [CrossRef]
- Maffei, M.; Mucciarelli, M.; Scannerini, S. Essential oils from Achillea species of different geographic origin. Biochem. Syst. Ecol. 1994, 22, 679–687. [Google Scholar] [CrossRef]
- Kizil, M.; Kizil, G.; Yavuz, M.; ÇeKen, B. Protective activity of ethanol extract of three Achillea species against lipid peroxidation, protein oxidation and DNA damage in vitro. Acta Aliment. 2010, 39, 457–470. [Google Scholar] [CrossRef]
- Barış, D.; Kızıl, M.; Aytekin, C.; Kızıl, G.; Yavuz, M.; Çeken, B.; Selçuk Ertekin, A. In vitro antimicrobial and antioxidant activity of ethanol extract of three Hypericum and three Achillea species from Turkey. Int. J. Food Prop. 2011, 14, 339–355. [Google Scholar] [CrossRef]
- Qi, H.; Shi, Y.; Wu, H.; Niu, C.; Sun, X.; Wang, K. Inhibition of temperature-sensitive TRPV3 channel by two natural isochlorogenic acid isomers for alleviation of dermatitis and chronic pruritus. Acta Pharm. Sin. B 2021. [Google Scholar] [CrossRef]
- Aljančić, I.; Vajs, V.; Menković, N.; Karadžić, I.; Juranić, N.; Milosavljević, S.; Macura, S. Flavones and sesquiterpene lactones from Achillea atrata subsp. multifida: Antimicrobial activity. J. Nat. Prod. 1999, 62, 909–911. [Google Scholar] [CrossRef]
- Zengin, G.; Aktumsek, A.; Ceylan, R.; Uysal, S.; Mocan, A.; Guler, G.O. Shedding light on the biological and chemical fingerprints of three Achillea species (A. biebersteinii, A. millefolium and A. teretifolia). Food Funct. 2017, 8, 1152–1165. [Google Scholar] [CrossRef]
- Akkol, E.K.; Koca, U.; Pesin, I.; Yilmazer, D. Evaluation of the wound healing potential of Achillea biebersteinii Afan. (Asteraceae) by in vivo excision and incision models. Evid. Based Complement. Altern. Med. 2011, 2011, 474026. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.A.; Jaffal, S.M.; Al-Najjar, B.O. Analgesic and anxiolytic activities of Achillea biebersteinii: Evidence for the involvement of GABAergic systems. Orient. J. Chem. 2019, 35, 1433–1442. [Google Scholar] [CrossRef]
- Ertaş, A.; Boğa, M.; Haşimi, N.; Yeşil, Y.; Gören, A.C.; Topçu, G.; Kolak, U. Antioxidant, anticholinesterase, and antimicrobial activities and fatty acid constituents of Achillea cappadocica Hausskn. et Bornm. Turk. J. Chem. 2014, 38, 592–599. [Google Scholar] [CrossRef]
- Giorgi, A.; Bombelli, R.; Luini, A.; Speranza, G.; Cosentino, M.; Lecchini, S.; Cocucci, M. Antioxidant and cytoprotective properties of infusions from leaves and inflorescences of Achillea collina Becker ex Rchb. Phytother. Res. 2009, 23, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Gherase, F.; Pavelescu, M.D.; Stănescu, U.; Grigorescu, E. The experimental evaluation regarding analgesic activity of some extracts isolated from Achillea collina J. Becker ex Reichenb. Rev. Med. Chir. Soc. Med. Nat. Iasi 2002, 106, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, G.; Radulović, N.; Hashimoto, T.; Palić, R. In vitro antimicrobial activity of extracts of four Achillea species: The composition of Achillea clavennae L. (Asteraceae) extract. J. Ethnopharmacol. 2005, 101, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Karaalp, C.; Yurtman, A.N.; Yavasoglu, N.U.K. Evaluation of antimicrobial properties of Achillea L. flower head extracts. Pharm. Biol. 2009, 47, 86–91. [Google Scholar] [CrossRef]
- Hammad, M.H.; Carmen Litescu, S.; Matar, S.A.; Al-Jaber, I.H.; Afifi, F.U. Biological activities of the hydro-alcoholic and aqueous extracts of Achillea falcata L. (Asteraceae) grown in Jordan. EJMP 2013, 4, 259–270. [Google Scholar] [CrossRef]
- Gharibi, S.; Sayed Tabatabaei, B.E.; Saeidi, G. Comparison of essential oil composition, flavonoid content and antioxidant activity in eight Achillea Species. J. Essent. Oil Bear. Plants 2015, 18, 1382–1394. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.F.; Alqasoumi, S.I.; El-Desoky, A.H.; Soliman, G.A.; Paré, P.W.; Hegazy, M.-E. Evaluation of the anti-inflammatory, analgesic and anti-ulcerogenic potentials of Achillea fragrantissima (Forssk.). S. Afr. J. Bot. 2015, 98, 122–127. [Google Scholar] [CrossRef]
- Tuberoso, C.I.; Montoro, P.; Piacente, S.; Corona, G.; Deiana, M.; Dessì, M.A.; Pizza, C.; Cabras, P. Flavonoid characterization and antioxidant activity of hydroalcoholic extracts from Achillea ligustica All. J. Pharm. Biomed. Anal. 2009, 50, 440–448. [Google Scholar] [CrossRef]
- Bader, A.; Martini, F.; Schinella, G.R.; Rios, J.L.; Prieto, J.M. Modulation of COX-1, 5-, 12- and 15-LOX by popular herbal remedies used in southern Italy against psoriasis and other skin diseases. Phytother. Res. 2015, 29, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Loizzo, M.R.; Statti, G.A.; Menichini, F. Comparative radical scavenging and antidiabetic activities of methanolic extract and fractions from Achillea ligustica ALL. Biol. Pharm. Bull. 2005, 28, 1791–1794. [Google Scholar] [CrossRef] [PubMed]
- Tadić, V.; Arsić, I.; Zvezdanović, J.; Zugić, A.; Cvetković, D.; Pavkov, S. The estimation of the traditionally used yarrow (Achillea millefolium L. Asteraceae) oil extracts with anti-inflamatory potential in topical application. J. Ethnopharmacol. 2017, 199, 138–148. [Google Scholar] [CrossRef]
- Benedek, B.; Kopp, B. Achillea millefolium L. s.l. revisited: Recent findings confirm the traditional use. Wien. Med. Wochenschr. 2007, 157, 312–314. [Google Scholar] [CrossRef]
- Baretta, I.P.; Felizardo, R.A.; Bimbato, V.F.; dos Santos, M.G.J.; Kassuya, C.A.L.; Gasparotto Junior, A.; da Silva, C.R.; de Oliveira, A.M.; Ferreira, J.; Andreatini, R. Anxiolytic-like effects of acute and chronic treatment with Achillea millefolium L. extract. J. Ethnopharmacol. 2012, 140, 46–54. [Google Scholar] [CrossRef]
- Kasabri, V.; Afifi, F.U.; Hamdan, I. In vitro and in vivo acute antihyperglycemic effects of five selected indigenous plants from Jordan used in traditional medicine. J. Ethnopharmacol. 2011, 133, 888–896. [Google Scholar] [CrossRef]
- Yazdanparast, R.; Ardestani, A.; Jamshidi, S. Experimental diabetes treated with Achillea santolina: Effect on pancreatic oxidative parameters. J. Ethnopharmacol. 2007, 112, 13–18. [Google Scholar] [CrossRef]
- Zitterl-Eglseer, K.; Jurenitsch, J.; Korhammer, S.; Haslinger, E.; Sosa, S.; Della Loggia, R.; Kubelka, W.; Franz, C. Entzündungshemmende Sesquiterpenlactone von Achillea setacea. Sesquiterpenelactones of Achillea setacea with antiphlogistic activity. Planta Med. 1991, 57, 444–446. [Google Scholar] [CrossRef]
- Bagheri, Y.; Fathi, E.; Maghoul, A.; Moshtagh, S.; Mokhtari, K.; Abdollahpour, A.; Montazersaheb, S.; Bagheri, A. Effects of Achillea tenuifolia Lam. hydro-alcoholic extract on anxiety-like behavior and reproductive parameters in rat model of chronic restraint stress. Hum. Exp. Toxicol. 2021, 25, 9603271211026723. [Google Scholar] [CrossRef]
- Hamzeloo-Moghadam, M.; Khalaj, A.; Malekmohammadi, M.; Mosaddegh, M. Achillea vermicularis a medicinal plant from Iranian Traditional Medicine induces apoptosis in MCF-7 cells. RJP 2015, 2, 1–5. [Google Scholar]
- Bashi, D.S.; Bazzaz, B.S.F.; Sahebkar, A.; Karimkhani, M.M.; Ahmadi, A. Investigation of optimal extraction, antioxidant, and antimicrobial activities of Achillea biebersteinii and A. wilhelmsii. Pharm. Biol. 2012, 50, 1168–1176. [Google Scholar] [CrossRef]
- Amjad, L.; Mohammadi-Sichani, M.; Mohammadi-Kamalabadi, M. Potential activity of the Achillea wilhelmsii leaves on bacteria. Int. J. Biosci. Biochem. Bioinform. 2011, 1, 216–218. [Google Scholar] [CrossRef]
- Niazmand, S.; Khooshnood, E.; Derakhshan, M. Effects of Achillea wilhelmsii on rat’s gastric acid output at basal, vagotomized, and vagal-stimulated conditions. Pharmacogn. Mag. 2010, 6, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Niaz, A.; Syed Wadood, A.S.; Ghayour, A.; Ismail, S.; Mohammad, S.; Muhammad, J.; Waqar, A. Acute toxicity and antispasmodic activities of Achillea wilhelmsii C. Koch. Pak. J. Pharm. Sci. 2014, 27, 309–315. [Google Scholar]
- Mohammadmehdi, H.-T.; Hassanpour-Fard, M.; Ahani, A.; Hosseini, M. Anti-Nociceptive activity of Achillea wilhelmsii ethanolic extract in mice. J. Complement. Med. Altern. Healthc. 2018, 6, 555685. [Google Scholar] [CrossRef][Green Version]
| a | ||||
|---|---|---|---|---|
| Keyword/Phrase | Number of Hits | |||
| PubMed | ScienceDirect | Reaxys | Google Scholar | |
| Achillea | 384 | 2.238 | 1.819 | 19.700 |
| Achillea genus | 21 | 558 | 73 | 14.400 |
| Achillea species | 150 | 1.678 | 678 | 17.000 |
| Achillea medicinal uses | 25 | 857 | 616 | 13.000 |
| Achillea pharmacological | 169 | 517 | 98 | 8.600 |
| Achillea traditional uses | 21 | 956 | 62 | 15.500 |
| Achillea compounds | 122 | 1.091 | 750 | 14.300 |
| Achillea phytochemicals | 39 | 437 | 103 | 7.110 |
| Achillea biological | 249 | 1.075 | 193 | 16.900 |
| Achillea bioassays | 25 | 144 | 20 | 2.560 |
| b | ||||
| Most co-occurred authors | Formari Tiziana; Amsilinger Sabine; Venditti Alessandro; Akram Muhammad; Skaltsa Helen | |||
| Most cited documents | Saednia et al., 2011; Vitalini et al., 2011; Mohammadhosseini et al., 2017; Baretta, et al., 2012; Agar et al., 2015 | |||
| Most used keywords | Achillea/Chemistry, A. milefolium, plan extracts/pharmacology, phytotherapy, medicinal plants | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barda, C.; Grafakou, M.-E.; Tomou, E.-M.; Skaltsa, H. Phytochemistry and Evidence-Based Traditional Uses of the Genus Achillea L.: An Update (2011–2021). Sci. Pharm. 2021, 89, 50. https://doi.org/10.3390/scipharm89040050
Barda C, Grafakou M-E, Tomou E-M, Skaltsa H. Phytochemistry and Evidence-Based Traditional Uses of the Genus Achillea L.: An Update (2011–2021). Scientia Pharmaceutica. 2021; 89(4):50. https://doi.org/10.3390/scipharm89040050
Chicago/Turabian StyleBarda, Christina, Maria-Eleni Grafakou, Ekaterina-Michaela Tomou, and Helen Skaltsa. 2021. "Phytochemistry and Evidence-Based Traditional Uses of the Genus Achillea L.: An Update (2011–2021)" Scientia Pharmaceutica 89, no. 4: 50. https://doi.org/10.3390/scipharm89040050
APA StyleBarda, C., Grafakou, M.-E., Tomou, E.-M., & Skaltsa, H. (2021). Phytochemistry and Evidence-Based Traditional Uses of the Genus Achillea L.: An Update (2011–2021). Scientia Pharmaceutica, 89(4), 50. https://doi.org/10.3390/scipharm89040050









