Abstract
Causes of the progression of periodontitis such as an imbalance between the immune response by the host by the release of inflammatory mediators in the response of the oral pathogenic dysbiotic biofilm have been identified. New insights on specific cell signaling pathways that appear during periodontitis have attracted the attention of researchers in the study of new personalised approaches for the treatment of periodontitis. The gold standard of non-surgical therapy of periodontitis involves the removal of supra and subgingival biofilm through professional scaling and root planing (SRP) and oral hygiene instructions. In order to improve periodontal clinical outcomes and overcome the limitations of traditional SRP, additional adjuvants have been developed in recent decades, including local or systemic antibiotics, antiseptics, probiotics, anti-inflammatory and anti-resorptive drugs and host modulation therapies. This review is aimed to update the current and recent evolution of therapies of management of periodontitis based on the adjunctive and target therapies. Moreover, we discuss the advances in host modulation of periodontitis and the impact of targeting epigenetic mechanisms approaches for a personalised therapeutic success in the management of periodontitis. In conclusion, the future goal in periodontology will be to combine and personalise the periodontal treatments to the colonising microbial profile and to the specific response of the individual patient.
Keywords:
periodontitis; adjuvants; drugs; antimicrobials; chemotherapeutic agents; dysbiosis; bacteria 1. Introduction
Periodontitis is a disease with an infectious aetiology characterised by inflammation of the supporting tissues of a tooth that can lead, if not properly treated, to the destruction of both periodontal tissues and alveolar bone, and, in the long term, cause tooth loss [1].
However, periodontitis’s onset and subsequent progression also occur as a host’s unbalanced immune reaction to a dysbiotic organised biofilm. Among dysbiotic biofilms, the periodontopathogenic bacteria produce metabolites and enzymes that determine the alteration and destruction of parts of the extracellular matrix, including the collagen; and the increase of the permeability of the cellular membranes of the host in order to determine a subsequent tissue invasion which increases periodontal damage over time [1,2]. All this induces the host immune response leading to site-specific local inflammation of the tissue and increased immune cell response [2]. Among the main known periodontal pathogens, there are bacteria such as Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis (P.gingivalis), which produce multiple factors underlying the tissue damage found during periodontitis such as peptidoglycans, various integrins and outer membrane proteins, lipopolysaccharide and cellular superficial fimbriae degradation connective tissue [3,4,5,6,7]. Once these pathogenic bacteria trigger immune and inflammatory processes, the body induces leukocytes, fibroblasts or other inflammatory cells to release various substances in order to protect tissues from infection, including metalloproteinases, cytokines, transglutaminases, prostaglandins and proteolytic enzymes [8]. Proteases cause the degradation of collagen in periodontal tissues and, therefore, can create incursions for further infiltration of leukocytes [9]. Despite the production and release of several inflammatory mediators, tissue destruction occurs mainly due to an imbalance between the matrix metalloproteinase level and their endogenous inhibitors [10]. Subsequently, throughout the stimulation of some pro-inflammatory cytokines, such as including interleukin (IL)-1b, IL-6, tumor necrosis factor, and (TNF)-a, the inflammatory infiltrate from the periodontal tissues starts the tissue and alveolar bone destruction [7,10,11,12,13,14]. The inflammatory-related mediators can control the release of ligand-receptor factor Kappa B (NF-KB), RANKL and osteoprotegerin, which stimulate osteoclastic activity by inducing alveolar bone destruction [15].
Host modulation therapy is among the most widely used approaches to halting the progression of periodontitis and alveolar bone loss. This uses pharmacological therapies and conventional periodontal treatment to ameliorate the destructive aspects of the host inflammatory response by inhibiting microbial growth or modulating the host response [16,17,18], through reduction of the production of metalloproteinases or related inflammatory cytokines [19]. Several classes of anti-inflammatory drugs have been tested, including metalloproteinase inhibitors (doxycycline) [20] and NSAIDs (flurbiprofen) [21], but these have numerous limitations in clinical use [22].
In recent years, a better understanding of the pathways of inflammatory cells that result in the release of tissue-destroying proteins has led to an analysis of the possibility of developing new therapies for the long-term success of periodontitis [23], but there is still a long way to go before they are routinely used in clinical practice. Numerous studies have attempted to describe the main bacterial species that determine the tissue damage of periodontitis, which species are influenced by specific therapies, and what are the innovative therapeutic effects of reducing the bacterial and inflammatory mediators released during periodontitis, but there are still several aspects to be explored further.
The objectives of this review were to analyse and update the present knowledge of the conventional and adjunctive therapies for periodontitis. Furthermore, we discussed the impact of innovative and target therapies of periodontitis for long-term therapeutic success based on the latest scientific evidence. The data included in this review have been collected through a free literature search by three independent authors.
2. Conventional Therapies of Periodontitis: Endpoint, Strategies and Limitations
2.1. Endpoints
The success of periodontitis therapy, both in the short- and long term, depends on the effect of physical disintegration of the supra and subgingival periodontal pathogens presented in the biofilm [24]. The initial causal therapy mainly consisted of oral hygiene instruction and professional scaling and root planing (SRP). Both the supra- and subgingival pathogens and calculus are mechanically disrupted, with combined and synergic effects induced by periodontal debridement and domiciliary oral hygiene instructions [25]. In this regard, the gold standard of non-surgical therapy of periodontitis is represented by the SRP performed by both hand and ultrasonic instruments alone, with a demonstrated microbial and clinical effectiveness in the short-term period [26,27] (Figure 1).
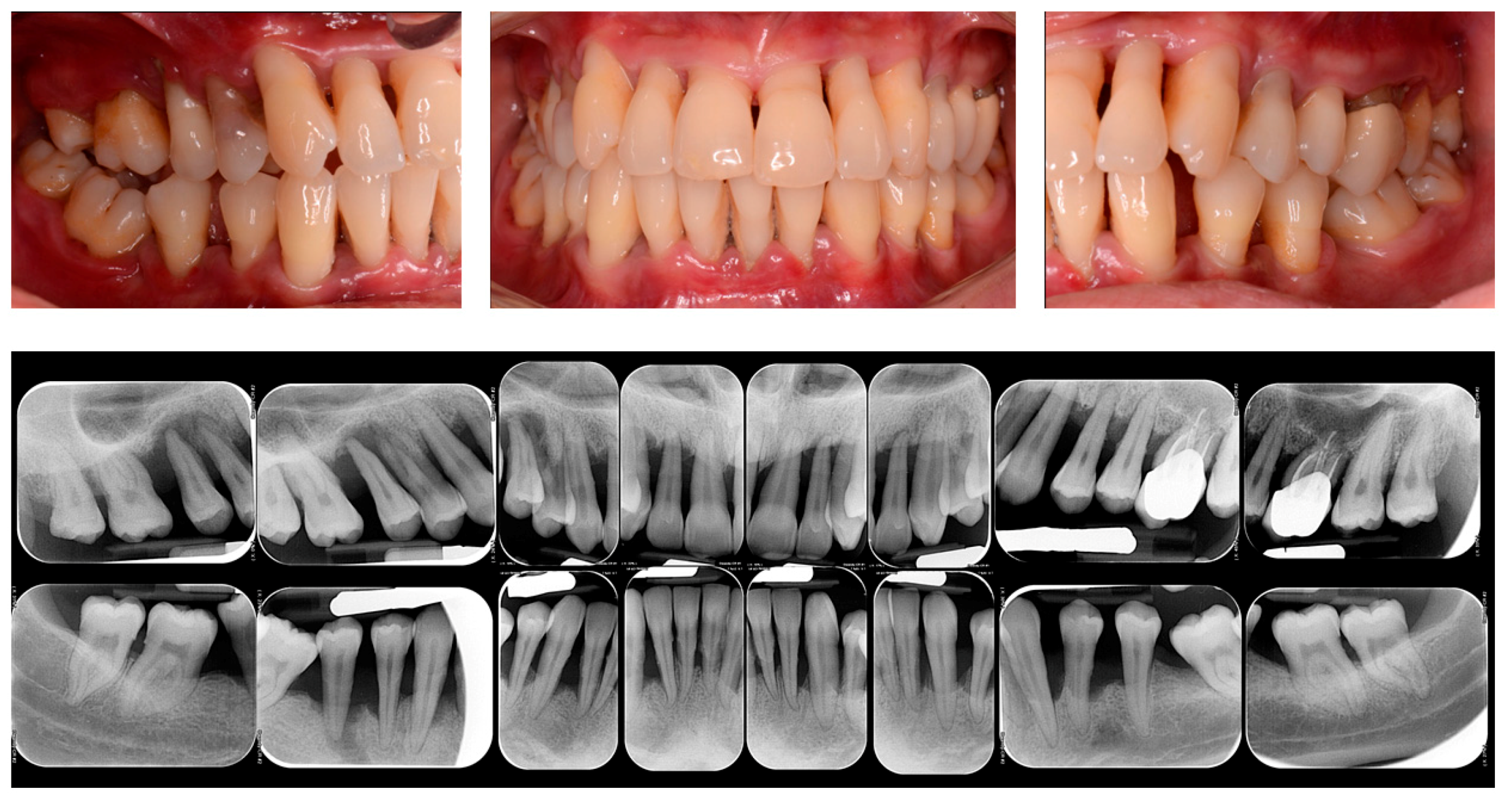
Figure 1.
Clinical and Rx images of a patient with periodontitis.
It has been previously shown that the mechanical disruption of supra- and subgingival biofilm by the non-surgical periodontal therapy performed by SRP alone can reduce the plaque index (PI) and the bleeding on probing (BOP) in around 45% of periodontal sites [28]. On this regard, following non-surgical periodontal therapy, a reduction in the probing depth (PD) has been demonstrated in a range of 1.29 mm for periodontal pockets with an initial PD of 3–4 mm and of 2.2 mm for the pockets ranged 5–6 mm and an improvement in the clinical attachment level (CAL) in a range of 0.5–2 mm [29,30].
The classic approach to periodontal disease may well change in the future. In fact, over the last few years, the concept of personalised dentistry has become increasingly popular, thanks to a broader knowledge of the characteristics of the oral microbiome and proteome. This has been made possible by the increased awareness that saliva and the crevicular fluid (GCF) represent an inexhaustible source of information, so the application of various “OMIC” approaches has made it possible to obtain information on the composition of the oral microbiome, the salivary proteome and the functional profile of the innate immune response. Despite the large amount of information obtained and the development of relevant microbial and biochemical information databases, this has not translated into new strategies for diagnosis, treatment, and follow-up, as there is a need for further longitudinal studies. This can only be achieved through meaningful and routine screening of changes in the microbiome, proteome or metabolome in health and disease, through the point-of-care (POC) technique. It is a minimally invasive and rapid technique that includes small-scale laboratory molecular assays integrated on a cartridge that could be performed during routine visits, yielding a wealth of information useful for the development of targeted therapies [31].
2.2. Strategies
Conventional strategies in the treatment of periodontitis can be classified by a non-surgical or surgical approach. The non-surgical procedure requires from one week to four or six appointments for 3–6 weeks [32,33]. The path through different therapeutic periodontal debridement sessions allows to obtain a meticulous treatment of a limited number of teeth together with a combined patient’s domiciliary plaque control. Conversely, the possible re-infection of recently treated periodontal sites caused by the entrainment of bacteria located in non-dental sites (tongue, mucosa, saliva) has been suggested [32,34]. During the last decades, a different approach was demonstrated to the non-surgical periodontal therapy performed with SRP alone. “Full mouth disinfection” (FMD), is a protocol aimed to treat the full mouth in order to reduce the periodontal pathogens in all periodontal pockets and also to prevent the transmission of bacteria from a periodontal site to another [35]. FMD includes, in addition to SRP, is performed in one or two appointments for a maximum of 24 h and can consist of the use of chlorhexidine. The one-stage full-mouth debridement approach was also proposed, performed in a single 1 h session, and involving the exclusive use of an ultrasound device with excellent points [36]. However, there is no proven indication that full mouth disinfection protocol offers supplementary benefits compared to the multi-staged non-surgical periodontal approach. Both therapeutic approaches are valid, and there were demonstrated only small differences between the two therapies; therefore, the choice of the strategy should be taken on the specific cases at the patient’s level [37].
2.3. Limitations
Only 10–15 bacterial species, among the over 700 present in the oral biofilm, have been related to the beginning and evolution of periodontitis dysbiosis [38]. Despite the high composition of the oral biofilm, the traditional periodontal therapy is non-specific, as it consists mainly of the mechanical disruption of the supra- and subgingival biofilms. However, SRP alone is sufficient to determine positive results in the long-term in some patients with a mild form of periodontitis [39]; nevertheless, a significant percentage of pocket sites may not have a good response in periodontal patients. This can be partly explained due to the inherent limitations of mechanical debridement, including the problematic access to deep and winding pockets, furcations, and vertical defects. Furthermore, therapy performed by SRP alone can represent limited effects on some periodontal pathogens, such as bacteria of the red complex of Socransky [40] and does not allow the elimination of periodontopathogens in non-native oral microflora (e.g., oral mucosa, tongue) [41]. SRP alone can also determine some minor side effects, such as gingival recession and dentinal hypersensitivity [42]. These limitations in therapy can be partially overcome through the use of a series of alternative technologies [43]. For these reasons, additional adjuvants, which includes local or systemic antibiotics, antiseptics, probiotics, and host modulation therapies, have been developed in the last few decades.
3. Microbial Modulation of Periodontitis
Almost all forms of periodontitis are determined by the presence of a pathogen biofilm located into the periodontal pocket, which can require therapy to modulate the microbial biofilm. In general, oral infections caused by periodontal pathogens are tricky to modulate with antibiotics, particularly if the biofilm is not mechanically removed [44]. Therefore, according to the current consensus, correct antimicrobial therapy should be led by an accurate periodontal debridement that destroys the supra and subgingival biofilm [45]. Adjuvant antimicrobial therapies are both administered at a local and systemic level. The advantages and limits of local and systemic antimicrobials are presented in Table 1.

Table 1.
Potential of antimicrobials in periodontology.
The potential advantage of the systemic dispensation of antimicrobials is the possibility of reducing all periodontopathogens present in the mouth and those located in other sites such as the tonsils and dorsum of the tongue. Nevertheless, this approach necessitates a strong collaboration by the patient and could determine undesirable side effects at a systemic level that could cause the onset of bacterial resistance [46]. On the contrary, local administration of antimicrobials does not request particular compliance of the patient and allows the application of the active principle directly at the local infection site at a high intensity that not be achieved systemically [46].
3.1. Chlorhexidine
Antiseptic solutions are often used for the control of biofilms, especially pathogenic ones, during the active phases of periodontal treatment. A recent review of the literature found a similar result in the comparison between chlorhexidine irrigation associated with SRP and SRP alone [47]. Chlorhexidine (CHX) gluconate is an antiseptic, antifungal and bactericidal chemical agent that acts successfully on both Gram-positive and -negative periodontopathogens. It also has a bacteriostatic effect that determines the inhibition of bacterial proliferation [48]. This bisbiguanide antiseptic is also used as a slow local release system available in the form of gel, paint or chip. In order to increase the effectiveness of non-surgical periodontal therapy, the concept of chemomechanical treatment based on sequential SRP and the additional subgingival administration of 35% of CHX paint [49] and subgingival positioning of a biodegradable CHX chip was introduced [50].
In the last few decades, several clinical studies have demonstrated that a CHX chip used after SRP is an effective protocol that allows the active reduction of periodontitis for about 6–9 months [51]. The use of CHX in the form of mouthwash has produced better clinical and microbial results compared with SRP alone or SRP associated with professional plaque control [52] or SRP associated with placebo [53]. However, the adverse effects of CHX, such as brown tooth and mucosal staining, altered taste perception, and augmented calculus deposition should be taken into account [54].
3.2. Antibiotics
Some clinical studies suggest the hypothesis that in specific clinical situations, including particular forms of active and rapidly evolving periodontitis, in the presence of deep pockets or with particular pathological profiles, the additional use of systemic antimicrobials associated with SRP may determine clinically relevant advantages in the medium and long term.
The combined use of amoxicillin and metronidazole for periodontitis was initially introduced to counteract Aggregatibacter actinomycetemcomitans (A. actinomicetemcomitans) on periodontal tissues [55], although subsequent studies have also shown a role for several other pathogens during the active phases of periodontitis [56]. Furthermore, not only a low prevalence of A. actinomicetemcomitans during periodontitis has been demonstrated [57], but moreover, it has been shown that the use of amoxicillin and metronidazole in addition to SRP is associated with adverse effects, including systemic resistance to antibiotics, and also could not be valid against other periodontopathogenic bacteria, especially P. gingivalis and other periodontopathogens, after 3–6 months of therapy [35].
Regarding the use of topical antibiotics in periodontics, some studies have shown limited usefulness to sites refractory to traditional therapy with SRP alone or in patients with localised lesions [58]. Antibiotics used topically (e.g., chips, gels, topical solutions), compared with antibiotics systemically, lead to fewer adverse events, including bacterial resistance, and are better tolerated by the patient [59]. The use of local antibiotics has been suggested, in some studies, as an alternative for surgical therapy [60], but at the same time, a real long-term efficacy of local applications has not been demonstrated, and some commercial products have been withdrawn from the market [61].
The most effective antimicrobial agents, such as tetracycline fibers, slow-release doxycycline, and minocycline, resulted in an average PD reduction of between 0.5 and 0.7 mm; while less effective agents such as CHX and metronidazole determined an average PD gain of 0.1–0.4 mm [62]. Some randomised controlled trials with a follow-up ≤ 6 months demonstrated a moderate increase in the CAL level of 0.40 mm using CHX chips, a mean percentage of 0.64 mm for doxycycline gel, and 0.24 mm microspheres of minocycline [63]. However, a precise evaluation of the usefulness of topical antimicrobials for routine clinical use is prevented by significant limitations and high acquisition costs [47,59]. More specifically, a 3-rear RCT study showed that the adjunctive use of locally delivered doxycycline gel determined positive short-term effects on BOP, PD and CAL, but repeated annual applications were not useful compared to SRP alone in maintenance patients [64]. The results of a recent study suggested that nanostructured doxycycline gel (nDOX) compared to conventional doxycycline gel and placebo used as an adjunct to SRP determined a significant PD and CAL reduction; however, these observations are limited to the short term [65].
Research is currently focusing on different therapeutic strategies to limit the use of antibiotics, especially systemic antibiotics, to extremely severe cases of periodontitis. Among the strategies being explored are antiviral strategies against leucotoxin (LtxA), which is produced by A. actinomycetemcomitans strains [66]. This toxin specifically kills human white blood cells, reducing the host’s ability to respond to infection [67]. Underlying this antivirulence strategy is the idea that by inhibiting the activity of this particular toxin, the virulence of the bacteria would be reduced, resulting in a decrease in the severity of the infection. Obvious advantages include slower development of resistance due to reduced selective pressure (compared to antibiotics) and targeted treatment that would not impact on the healthy microbiota. However, further studies and research are needed before this therapy can be used in clinical practice [66]. Several studies have pointed the role of Aggregatibacter actinomycetemcomitans in the development of periodontitis with rapid progression of attachment loss (AL) in adolescents, as they observed that subjects with a highly virulent clone of A. actinomycetemcomitans, called genotype JP2, have a pronounced risk for disease progression [67,68,69,70]. Therefore, therapies targeting specific virulence factors, such as the one against LtxA could have an important preventive effect in these individuals [68].
3.3. Antimicrobial Proteins
Peptides and antimicrobial proteins are classes of host defense particles that act in order to reduce the proportions and augmentation of oral bacteria and similar pathogens. These compounds have aroused huge attention in periodontal innate immunity medicine and as an alternative resource of typical antimicrobials (e.g., antibiotics) during the last decade [71].
Several antimicrobial peptides such as tissue-specific human beta-defensins (hBD)-3 and cathelicidin antimicrobial peptides (LL)-37 have been analysed against different types and different strains of oral periodontal pathogens [72]. Regarding main peptides involved against periodontitis, Streptococcus gordonii subtype M5 and 10,558 presents a full range susceptibility against P. gingivalis. On the other hand, LL-37 has been shown to be poorly sensitive against P. gingivalis, while hBD3 has been proven to possess a high susceptibility in some in vitro periodontitis models in which gingival epithelial cells were exposed to periodontal bacteria [72].
More than twenty systemic genetic diseases have been identified as associated with periodontitis related to alterations in the expression of the antimicrobial peptide similarly to periodontitis, which may have the same characteristics of augmented susceptibility to periodontal pathogen infections [73].
Several reports have analysed other antimicrobial peptides associated such as lactoferrin and mucin-7, both associated with periodontitis. Among these, mucin-7 (MG2) and lactoferrin have been shown to decrease significantly three times in subjects with periodontitis compared to healthy controls, thus suggesting antimicrobial properties of these peptides [74].
3.4. Probiotics
The term “probiotic” was defined in 2005 as a set of live microorganisms that, given in adequate quantities, confer benefits to the host [75]. There are likely to be slight changes in the definition of this term in the literature, which may be due to the new criteria of evaluation and new discoveries. For example, some reports have shown that certain inactivated microorganisms or the derivatives of their components have potentially beneficial effects on health, expanding the concept of probiotics as a therapeutic use [76].
In periodontology, probiotic strains have been evaluated in addition to SRP and have been demonstrated to interfere with bacterial recolonisation [77]. Most of the studies on probiotics have analysed Lactobacillus-derived products such as ones that contain Lactobacillus reuteri. A recent meta-analysis has demonstrated that the use of probiotics in periodontal therapy determined a CAL gain of 0.42 mm and PD reduction of 0.18 mm in moderate pockets and 0.67 mm in deep pockets after periodontal therapy [78]. Moreover, continuous intake of probiotics has been provided with the best favourable results when used, in periodontal treatment, as an adjuvant to SRP, even if the heterogeneity in the design of the analysed studies does not allow one to draw any important conclusions, especially as concerns clinical utility in the long term [79].
3.5. Lasers
In the last century, lasers have been introduced for the treatment of several diseases in the medical field. A laser device can produce electromagnetic radiations with a specific wavelength and a low radiation beam that determines important tissues effects (Figure 2).
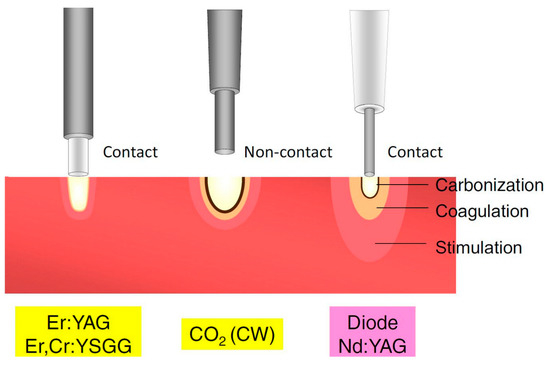
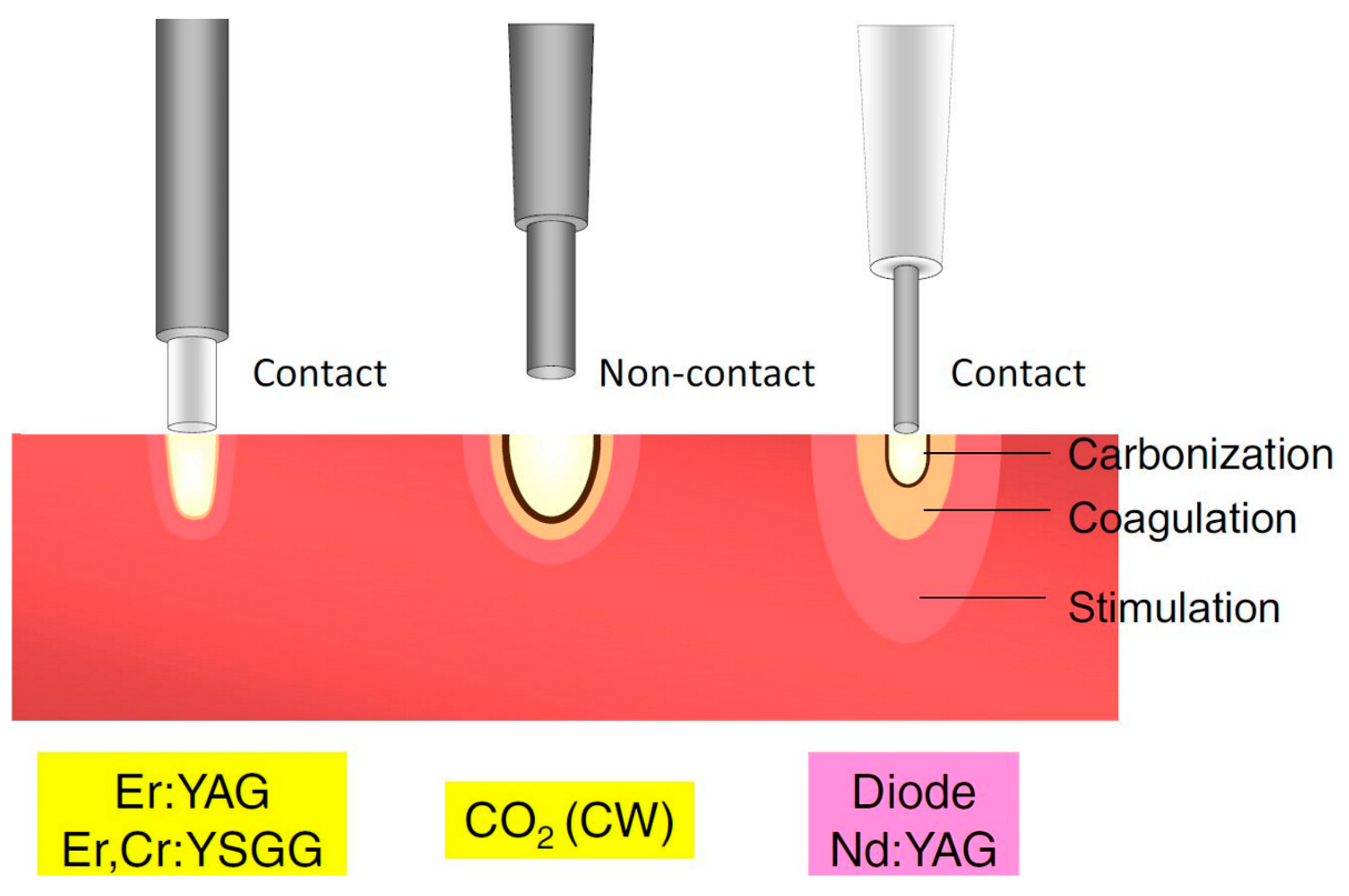
Figure 2.
Classification of lasers according to penetration depth in tissue. CO2, carbon dioxide; CW, continuous wave; Er,Cr:YSGG, erbium, chromium-doped yttrium-scandium-gallium-garnet; Er:YAG, erbium-doped yttrium-aluminium-garnet; Nd:YAG, neodymium-doped yttrium-aluminium-garnet. Adapted from Aoki et al. [80], Copyright 2015 Akira Aoki et al.
Medical lasers cause broad effects on soft and hard tissues, such as vaporisation, microbial ablation destruction, blood hemostasis, and biological influences, such as tissue biostimulation and biological responses, with beneficial therapeutic outcomes. Therefore, the use of lasers should be considered helpful for the treatment of various infectious and inflammatory disorders, including periodontal and peri-implant diseases [80,81].
More specifically, laser therapy has been used against periodontal and peri-implant bacteria through many effects, mainly bactericidal and detoxification effects on the gingival biofilm [82].
Various lasers have been used in the therapy of periodontitis, such as erbium-doped yttrium-aluminium-garnet (Er:YAG) laser (2.940 nm) and erbium, chromium-doped yttrium-scandium-gallium-garnet laser (Er,Cr:YSGG, 2.780 nm), that can be used on periodontal hard and soft tissues, as well as on peri-implant surfaces during periodontal and peri-implant diseases [81] (Table 2).

Table 2.
Lasers used for the treatment of oral and periodontal diseases.
Most surgical lasers when operated with certain power settings can produce a photothermal impact on the tissues, causing soft tissue thermal effects such as tissue evaporation. Specifically, Er:YAG and CO2 lasers cause photothermal effects like direct evaporation of soft tissues [83].
However, the primary function of the main function of most surgical lasers is the bactericidal effect obtained by their photothermal effects. The phenomena resulting in bacterial inactivation or destruction happen during the evaporation or denaturation phases of the laser irradiation [84]. Consequently, the advantageous bactericidal outcomes of laser therapy allow obtaining a state of disinfection useful during surgery [85]. For example, Nd:YAG lasers have been shown to possess selective absorption in pigments, effective in killing some of biofilms such as those of P. gingivalis in periodontitis models [84]. Furthermore, lasers can reduce the release of toxic substances, such as lipopolysaccharide endotoxins [86]. Thanks to these additional effects of decontamination and detoxification, lasers can promote better wound healing when compared to conventional therapies used in periodontal pockets.
Moreover, it can be argued that irradiation of the root surface by a laser may determine a microbe-inhibiting effect on the adhesion and colonisation of oral bacteria, an important stage in the stability of healing of the periodontal pocket [82].
It is also hypothesised that, following laser therapy, the effects of photobiomodulation are simultaneously determined, including the stimulation of proliferation and differentiation of cell tissues and the anti-inflammatory influences, and that this should promote tissue healing. However, the photobiomodulation effect induced by laser therapy on wound healing is not still well understood and has been demonstrated to vary between different types of lasers.
Yamasaki et al. [87] stated that the low-level laser therapy induced by CO2 laser led to the heat shock protein expression with different intensities and patterns to those expressed after surgery performed with the scalpel. In fact, on the day following laser application therapy, growth in the percentage of connective tissue cells marked with bromodeoxyuridine in the laser wound was demonstrated compared to the wound healing of traditional surgery, with a more quickly wound repair process induced by the laser therapy than through conventional surgery.
It seems that another significant effect induced by lasers through a low pulsed emission (especially with the CO2 laser) is the coagulation process that produces an acceleration in the repair process and also that promotes the progression remodelling of gingival tissues with a good influence on wound healing [88].
However, at present, clinical trials aimed at analysing the effectiveness of lasers on periodontal and tissue regeneration are still limited. Given these promising pivotal results, further research is needed to clarify better the possible useful effects of lasers on soft tissue wound healing.
3.6. Desiccant Agents
Given the key role of biofilms in the development of periodontitis, some additional treatments were developed to improve the effectiveness of SRP in subjects with periodontitis by selectively reducing periodontal pathogens.
Microorganisms in biofilms live in an extracellular polymeric substance (EPS) matrix composed of proteins, polysaccharides, and DNA that can adhere on both root surfaces or material surfaces (e.g., implants) [89]. The presence of EPS allows oral bacteria to survive and protects them, at the same time, from the degrading action of any antimicrobial agents that are unable to reach the bacteria present at the subgingival level [90]. For these reasons, some strategies have been developed in the last few decades, such as the local delivery agents.
In dentistry, a type of local delivery agent, a desiccant agent, has been employed for the treatment of oral aphthous stomatitis [91]. Successively, a further generation of desiccant had been developed in order to be applied in periodontal pockets. A desiccant agent is a liquid or gel blend containing a mixture of sulphonic and sulfuric acids [92]. The desiccant agents include aqueous mixture solutions of concentrated hydroxybenzenesulfonic and hydroxymethoxybenzenesulphonic and sulfuric acid that present a hygroscopic surface and a denaturing action. The sulfate group present in the desiccant mixture has a particular internal polar structure and oxygen atoms on the external surface, presenting a solid negative surface superficial charge. This feature allows this solution, thanks to their high effect, to constrain the water of the matrix of the oral bacterial biofilm, to adhere and detach, quickly destroy the biofilm, and eradicate it from the gingival surface and the root surface [93]. Encouraging results have been reported in a pivotal study by Bracke et al. [92], which demonstrated that the additional use of desiccant in the SRP was effective for reducing the mean levels of some periodontal clinical mediators after periodontal therapy. Moreover, in a further RCT study with a split-mouth protocol, the desiccant agent associated with SRP performed with ultrasonic and hand instruments was demonstrated to be efficacious in disrupting the biofilm and in reducing the microbial and inflammatory mediators when used in combination to SRP [94].
On this regard, a molecular report aimed at detecting the activity of the main periodontal bacteria such as P. gingivalis, T. forsythia, and T. denticola after 2 weeks of active periodontal therapy found a significant decrease in about 99% for bacteria of the red complex and 96% in the percentage of the total bacterial count after treatment with the adjunctive use of a desiccant agent plus SRP [95]. In accordance, another research reported favourable results of the desiccant solutions useful in the elimination of pathogenic biofilm on the peri-implant surfaces [96].
3.7. Anti-Inflammatory Drugs and Bisphosphonates
Different cell types in the periodontium (neutrophils, macrophages, fibroblasts and epithelial cells) in response to bacterial lipopolysaccharide release prostaglandins, potent pro-inflammatory mediators that are relevant for the progression of periodontal disease; for example, prostaglandin E2 is known to induce osteoclastic bone resorption [97]. Various studies showed that both topical and systemic non-steroidal anti-inflammatory drugs (NSAIDs) short-term application induced a reduction of gingival bleeding, whereas long-term intake promoted an improvement in terms of bone loss [98,99,100,101]. However a systematic review has pointed out some important limitations in their use for periodontitis: lacking of long-term observational studies, and systemic side effects (mainly gastrointestinal and cardiotoxic) [102].
Bisphosphonates are drugs capable of inhibiting bone resorption and are mainly used in patients with osteoporosis or bone metastases. The main side effect is osteonecrosis of the jaws, which is more common in patients taking intravenous bisphosphonates [103,104]. The anti-resorptive properties of bisphosphonates have been of interest among researchers on periodontal disease as an adjunctive therapy for the possible benefits that have been observed in animal and human studies [105,106,107]. However, although there may be the possibility that bisphosphonates can alter the severity of periodontal disease, their use is still debated both for the risk of osteonecrosis and for the potential equivocal data related to the fact that osteoporosis could influence the progression of periodontitis [106,108].
3.8. Other Adjunctive Therapies for Periodontitis
Statins are drugs used to lower cholesterol levels, but they also have anti-inflammatory properties that may be of interest in the management of periodontal disease. Large cohort studies adjusted for confounding factors have however shown little or no evidence on the potential beneficial effects of statins [109,110]. In contrast, improvements in periodontal parameters were reported in a smaller study, not adjusted for all possible confounders [111]. However, more recent studies have reported beneficial effects in the additional topical use of statins + SRP [112,113,114,115]. However, further studies are needed to confirm or not the possible use of statins as adjuvant therapy in periodontitis.
Metformin is a hypoglycaemic drug that inhibits hepatic gluconeogenesis and decreases peripheral insulin resistance. It has been shown that it is able to stimulate osteoblastic differentiation and bone formation [116,117]. Doses of 50 mg/kg induced a reduction of inflammation, oxidative stress and bone loss in induced periodontitis rats [117]. In humans local application of 1% metformin gel into the periodontal pockets induced an improvement in PD, CAL and intrabody defects reduction compared to placebo in adjunct to SRP [118]. Two recent meta-analysis concluded that the use of adjunctive metformin to SRP may induce additional benefits in the treatment of periodontal disease [119,120].
Omega-3 polyunsaturated fatty acids (PUFAs) have anti-inflammatory properties that have been studied in several diseases, including periodontal disease [121]. An RCT study demonstrated that SRP associated with dietary supplementation of omega-3 PUFAs (300 mg/die for twelve weeks) significantly reduced gingival inflammation, PD and CAL compared to SRP and placebo in patients with moderate-severe periodontitis [122]. In contrast, another group showed that omega-3 PUFAs dietary supplementation (+ SRP) induced a reduction of salivary TNF-α but without significant impact on periodontal clinical parameters in patients with periodontitis [123]. Further studies are needed to clarify their possible role in the treatment of periodontitis.
4. Conclusions
The treatment of periodontal disease in its various forms has evolved over the past century, initially on an empirical basis and then on an increasing number of studies based on scientific evidence. In periodontology, it has been recognised that the removal of supra and subgingival plaque deposits and tartar had a favourable influence on periodontal tissues. The biofilm removal, both through periodontal therapy using SRP and thorough home oral hygiene, has become the milestone of modern periodontal treatment.
However, it has been shown that changing the proportions of periodontopathogenic bacteria (especially the red and orange Socransky complex bacteria) and the speed of recolonisation of the biofilm is regulated and improved by the use of additional agents to traditional mechanical procedures. The addition of chemotherapeutic agents administered systemically or locally, measured by clinical, microbial, and inflammatory outcomes has been demonstrated to possess good results; however, in the short and mid-term.
In light of the scientific evidence analysed in this review, it is also clear that the subjects affected by periodontal diseases differ from each other in the composition and proportion of sub-gingival microorganisms (especially those that are most periodontal pathogens). This is because periodontal pathogens present in biofilms are colonised by different pathogenic species, which are influenced by various environmental factors. It can be assumed that the variances in the clinical, microbiological, and inflammatory response are important both for the host’s ability to react to the infection of periodontal bacteria and for the “cluster” typology of the supra and subgingival biofilm, species that are deposited on the periodontal tissues with particular specific pathways. Furthermore, such evidence suggests that each subject responds differently to equal periodontal treatment, and these studies are still unclear on the reason. Certainly, the goal of future research in periodontology will be to combine and prepare a periodontal treatment, personalising it to the colonising microbial profile and to the specific response of the individual patient.
The future of health care will surely be based on better prevention of disease and personalisation of the single therapy. Only through regular preventive care and periodic periodontal visits with the relative risk factors reduction (e.g., smoking, diabetes, cardiovascular disease prevention, etc.) at a patient-level can prevent the development of periodontitis and, at the same time, will reduce both from a perspective of economic and biological saving for the patient.
A better understanding of the epigenetic modification of periodontal tissues that interact with the dysbiotic biofilm, perhaps through the analysis of transgenerational genomic modifications, will be one of the fundamental steps better to understand the aetiology, development and progression of periodontitis.
The next challenge will be to carry out integrated studies that will combine basic and clinical research knowledge to explore in a combined way therapeutic paths that will surely have a synergistic therapeutic impact in the long-term therapy of periodontitis.
Author Contributions
Conceptualisation, G.I.; methodology, A.P., S.S.; validation, D.D., F.I.; data curation, M.M.; writing—review and editing, G.I., A.P., S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was performed through funds from the Department of General Surgery and Surgical-MedicalF Specialties, School of Dentistry, University of Catania, Catania, Italy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. S1), S159–S172. [Google Scholar] [CrossRef] [Green Version]
- Bainbridge, B.W.; Darveau, R.P. Porphyromonas gingivalis lipopolysaccharide: An unusual pattern recognition receptor ligand for the innate host defense system. Acta Odontol. Scand. 2001, 59, 131–138. [Google Scholar] [CrossRef]
- Sugita, N.; Kimura, A.; Matsuki, Y.; Yamamoto, T.; Yoshie, H.; Hara, K. Activation of transcription factors and IL-8 expression in neutrophils stimulated with lipopolysaccharide from Porphyromonas gingivalis. Inflammation 1998, 22, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Chen, T.; Tribble, G.D. Genetic exchange and reassignment in Porphyromonas gingivalis. J. Oral. Microbiol. 2018, 10, 1457373. [Google Scholar] [CrossRef] [Green Version]
- Olsen, I.; Singhrao, S.K. Is there a link between genetic defects in the complement cascade and Porphyromonas gingivalis in Alzheimer’s disease? J. Oral. Microbiol. 2020, 12, 1676486. [Google Scholar] [CrossRef] [Green Version]
- Olsen, I.; Singhrao, S.K. Importance of heterogeneity in Porhyromonas gingivalis lipopolysaccharide lipid A in tissue specific inflammatory signalling. J. Oral. Microbiol. 2018, 10, 1440128. [Google Scholar] [CrossRef] [Green Version]
- Olsen, I.; Yilmaz, O. Possible role of Porphyromonas gingivalis in orodigestive cancers. J. Oral. Microbiol. 2019, 11, 1563410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graves, D.T.; Jiang, Y.; Valente, A.J. The expression of monocyte chemoattractant protein-1 and other chemokines by osteoblasts. Front Biosci 1999, 4, D571–D580. [Google Scholar] [CrossRef] [Green Version]
- Graves, D.T.; Oskoui, M.; Volejnikova, S.; Naguib, G.; Cai, S.; Desta, T.; Kakouras, A.; Jiang, Y. Tumor necrosis factor modulates fibroblast apoptosis, PMN recruitment, and osteoclast formation in response to P. gingivalis infection. J. Dent. Res. 2001, 80, 1875–1879. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Shima, M.; Shimoaka, T.; Fujieda, A.; Obara, K.; Suzuki, H.; Nagai, Y.; Ikeda, T.; Yamato, H.; Kawaguchi, H. Regulation of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) by bone resorptive factors in osteoblastic cells. J. Cell Physiol. 2000, 185, 207–214. [Google Scholar] [CrossRef]
- Olsen, I. Relationship between serine dipeptide lipids of commensal Bacteroidetes and atherosclerosis. J. Oral. Microbiol. 2018, 10, 1453224. [Google Scholar] [CrossRef] [Green Version]
- Olsen, I. Organization of supragingival plaque at the micron scale. J. Oral. Microbiol. 2018, 10, 1438722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, I.; Singhrao, S.K.; Potempa, J. Citrullination as a plausible link to periodontitis, rheumatoid arthritis, atherosclerosis and Alzheimer’s disease. J. Oral. Microbiol. 2018, 10, 1487742. [Google Scholar] [CrossRef] [Green Version]
- Olsen, I.; Yamazaki, K. Can oral bacteria affect the microbiome of the gut? J. Oral. Microbiol. 2019, 11, 1586422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubin, J.E.; Bonnelye, E. Osteoprotegerin and its ligand: A new paradigm for regulation of osteoclastogenesis and bone resorption. Medscape Womens Health 2000, 5, 5. [Google Scholar] [CrossRef]
- Olsen, I.; Hajishengallis, G. Major neutrophil functions subverted by Porphyromonas gingivalis. J. Oral. Microbiol. 2016, 8, 30936. [Google Scholar] [CrossRef] [Green Version]
- Olsen, I.; Lambris, J.D.; Hajishengallis, G. Porphyromonas gingivalis disturbs host-commensal homeostasis by changing complement function. J. Oral. Microbiol. 2017, 9, 1340085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, I.; Hicks, S.D. Oral microbiota and autism spectrum disorder (ASD). J. Oral. Microbiol. 2020, 12, 1702806. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Olsen, I. Porphyromonas gingivalis and its CRISPR-Cas system. J. Oral. Microbiol. 2019, 11, 1638196. [Google Scholar] [CrossRef] [Green Version]
- Preshaw, P.M.; Hefti, A.F.; Jepsen, S.; Etienne, D.; Walker, C.; Bradshaw, M.H. Subantimicrobial dose doxycycline as adjunctive treatment for periodontitis: A review. J. Clin. Periodontol. 2004, 31, 697–707. [Google Scholar] [CrossRef]
- Williams, R.C.; Jeffcoat, M.K.; Howard Howell, T.; Rolla, A.; Stubbs, D.; Teoh, K.W.; Reddy, M.S.; Goldhaber, P. Altering the progression of human alveolar bone loss with the non-steroidal anti-inflammatory drug flurbiprofen. J. Periodontol. 1989, 60, 485–490. [Google Scholar] [CrossRef]
- Preshaw, P.M. Host modulation therapy with anti-inflammatory agents. Periodontol 2000 2018, 76, 131–149. [Google Scholar] [CrossRef]
- Rakmanee, T.; Calciolari, E.; Olsen, I.; Darbar, U.; Griffiths, G.S.; Petrie, A.; Donos, N. Expression of growth mediators in the gingival crevicular fluid of patients with aggressive periodontitis undergoing periodontal surgery. Clin. Oral Investig. 2019, 23, 3307–3318. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Socransky, S.S.; Nyman, S.; Haffajee, A.; Westfelt, E. “Critical probing depths” in periodontal therapy. J. Clin. Periodontol. 1982, 9, 323–336. [Google Scholar] [CrossRef]
- Laleman, I.; Cortellini, S.; De Winter, S.; Rodriguez Herrero, E.; Dekeyser, C.; Quirynen, M.; Teughels, W. Subgingival debridement: End point, methods and how often? Periodontol 2000 2017, 75, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Hallmon, W.W.; Rees, T.D. Local anti-infective therapy: Mechanical and physical approaches. A systematic review. Ann Periodontol 2003, 8, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Smiley, C.J.; Tracy, S.L.; Abt, E.; Michalowicz, B.S.; John, M.T.; Gunsolley, J.; Cobb, C.M.; Rossmann, J.; Harrel, S.K.; Forrest, J.L.; et al. Evidence-based clinical practice guideline on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J. Am. Dent. Assoc. 2015, 146, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.M. Clinical significance of non-surgical periodontal therapy: An evidence-based perspective of scaling and root planing. J. Clin. Periodontol. 2002, 29 (Suppl. S2), 6–16. [Google Scholar] [CrossRef] [Green Version]
- Cobb, C.M. Non-surgical pocket therapy: Mechanical. Ann. Periodontol. 1996, 1, 443–490. [Google Scholar] [CrossRef]
- Van der Weijden, G.A.; Timmerman, M.F. A systematic review on the clinical efficacy of subgingival debridement in the treatment of chronic periodontitis. J. Clin. Periodontol. 2002, 29 (Suppl. S3), 55–71, discussion 90–51. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Bostanci, N.; Marsh, P.D.; Zaura, E. Applications of the oral microbiome in personalized dentistry. Arch. Oral Biol. 2019, 104, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Badersten, A.; Nilveus, R.; Egelberg, J. Effect of nonsurgical periodontal therapy. I. Moderately advanced periodontitis. J. Clin. Periodontol. 1981, 8, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Badersten, A.; Nilveus, R.; Egelberg, J. Effect of nonsurgical periodontal therapy. II. Severely advanced periodontitis. J. Clin. Periodontol. 1984, 11, 63–76. [Google Scholar] [CrossRef] [PubMed]
- van Winkelhoff, A.J.; van der Velden, U.; de Graaff, J. Microbial succession in recolonizing deep periodontal pockets after a single course of supra- and subgingival debridement. J. Clin. Periodontol. 1988, 15, 116–122. [Google Scholar] [CrossRef]
- Keestra, J.A.; Grosjean, I.; Coucke, W.; Quirynen, M.; Teughels, W. Non-surgical periodontal therapy with systemic antibiotics in patients with untreated aggressive periodontitis: A systematic review and meta-analysis. J. Periodontal. Res. 2015, 50, 689–706. [Google Scholar] [CrossRef]
- Tomasi, C.; Bertelle, A.; Dellasega, E.; Wennstrom, J.L. Full-mouth ultrasonic debridement and risk of disease recurrence: A 1-year follow-up. J. Clin. Periodontol. 2006, 33, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P. Commentary: Bacteria play a critical role in the etiology of periodontal disease. J. Periodontol. 2014, 85, 211–213. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. The bacterial etiology of destructive periodontal disease: Current concepts. J. Periodontol. 1992, 63, 322–331. [Google Scholar] [CrossRef]
- Drisko, C.H. Nonsurgical periodontal therapy. Periodontol 2000 2001, 25, 77–88. [Google Scholar] [CrossRef]
- Renvert, S.; Wikstrom, M.; Dahlen, G.; Slots, J.; Egelberg, J. Effect of root debridement on the elimination of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis from periodontal pockets. J. Clin. Periodontol. 1990, 17, 345–350. [Google Scholar] [CrossRef]
- Danser, M.M.; Timmerman, M.F.; van Winkelhoff, A.J.; van der Velden, U. The effect of periodontal treatment on periodontal bacteria on the oral mucous membranes. J. Periodontol. 1996, 67, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Haffajee, A.D.; Cugini, M.A.; Dibart, S.; Smith, C.; Kent, R.L., Jr.; Socransky, S.S. The effect of SRP on the clinical and microbiological parameters of periodontal diseases. J. Clin. Periodontol. 1997, 24, 324–334. [Google Scholar] [CrossRef]
- Sanz, I.; Alonso, B.; Carasol, M.; Herrera, D.; Sanz, M. Nonsurgical treatment of periodontitis. J. Evid. Based Dent. Pract. 2012, 12, 76–86. [Google Scholar] [CrossRef]
- Marsh, P.D. Controlling the oral biofilm with antimicrobials. J. Dent. 2010, 38 (Suppl. S1), S11–S15. [Google Scholar] [CrossRef]
- Garcia Canas, P.; Khouly, I.; Sanz, J.; Loomer, P.M. Effectiveness of systemic antimicrobial therapy in combination with scaling and root planing in the treatment of periodontitis: A systematic review. J. Am. Dent. Assoc. 2015, 146, 150–163. [Google Scholar] [CrossRef]
- Quirynen, M.; Teughels, W.; van Steenberghe, D. Microbial shifts after subgingival debridement and formation of bacterial resistance when combined with local or systemic antimicrobials. Oral Dis. 2003, 9 (Suppl. S1), 30–37. [Google Scholar] [CrossRef]
- Hanes, P.J.; Purvis, J.P. Local anti-infective therapy: Pharmacological agents. A systematic review. Ann. Periodontol. 2003, 8, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Heasman, P.A.; Heasman, L.; Stacey, F.; McCracken, G.I. Local delivery of chlorhexidine gluconate (PerioChip) in periodontal maintenance patients. J. Clin. Periodontol. 2001, 28, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Cosyn, J.; Wyn, I.; De Rouck, T.; Sabzevar, M.M. Subgingival chlorhexidine varnish administration as an adjunct to same-day full-mouth root planing. I. Clinical observations. J. Periodontol. 2007, 78, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Jeffcoat, M.K.; Palcanis, K.G.; Weatherford, T.W.; Reese, M.; Geurs, N.C.; Flashner, M. Use of a biodegradable chlorhexidine chip in the treatment of adult periodontitis: Clinical and radiographic findings. J. Periodontol. 2000, 71, 256–262. [Google Scholar] [CrossRef]
- Soskolne, W.A.; Chajek, T.; Flashner, M.; Landau, I.; Stabholtz, A.; Kolatch, B.; Lerner, E.I. An in vivo study of the chlorhexidine release profile of the PerioChip in the gingival crevicular fluid, plasma and urine. J. Clin. Periodontol. 1998, 25, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Feres, M.; Gursky, L.C.; Faveri, M.; Tsuzuki, C.O.; Figueiredo, L.C. Clinical and microbiological benefits of strict supragingival plaque control as part of the active phase of periodontal therapy. J. Clin. Periodontol. 2009, 36, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Faveri, M.; Gursky, L.C.; Feres, M.; Shibli, J.A.; Salvador, S.L.; de Figueiredo, L.C. Scaling and root planing and chlorhexidine mouthrinses in the treatment of chronic periodontitis: A randomized, placebo-controlled clinical trial. J. Clin. Periodontol. 2006, 33, 819–828. [Google Scholar] [CrossRef]
- Loe, H.; Schiott, C.R.; Karring, G.; Karring, T. Two years oral use of chlorhexidine in man. I. General design and clinical effects. J. Periodontal. Res. 1976, 11, 135–144. [Google Scholar] [CrossRef]
- Pavicic, M.J.; van Winkelhoff, A.J.; Douque, N.H.; Steures, R.W.; de Graaff, J. Microbiological and clinical effects of metronidazole and amoxicillin in Actinobacillus actinomycetemcomitans-associated periodontitis. A 2-year evaluation. J. Clin. Periodontol. 1994, 21, 107–112. [Google Scholar] [CrossRef]
- Guerrero, A.; Nibali, L.; Lambertenghi, R.; Ready, D.; Suvan, J.; Griffiths, G.S.; Wilson, M.; Tonetti, M.S. Impact of baseline microbiological status on clinical outcomes in generalized aggressive periodontitis patients treated with or without adjunctive amoxicillin and metronidazole: An exploratory analysis from a randomized controlled clinical trial. J. Clin. Periodontol. 2014, 41, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.W.; Bissada, N.F. Clinical evaluation of systemic doxycycline and ibuprofen administration as an adjunctive treatment for adult periodontitis. J. Periodontol. 1998, 69, 772–776. [Google Scholar] [CrossRef]
- Bonito, A.J.; Lux, L.; Lohr, K.N. Impact of local adjuncts to scaling and root planing in periodontal disease therapy: A systematic review. J. Periodontol. 2005, 76, 1227–1236. [Google Scholar] [CrossRef]
- Etienne, D. Locally delivered antimicrobials for the treatment of chronic periodontitis. Oral Dis. 2003, 9 (Suppl. S1), 45–50. [Google Scholar] [CrossRef]
- Killoy, W.J. The clinical significance of local chemotherapies. J. Clin. Periodontol. 2002, 29 (Suppl. S2), 22–29. [Google Scholar] [CrossRef]
- Mombelli, A.; Cionca, N.; Almaghlouth, A. Does adjunctive antimicrobial therapy reduce the perceived need for periodontal surgery? Periodontol 2000 2011, 55, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Matesanz-Perez, P.; Garcia-Gargallo, M.; Figuero, E.; Bascones-Martinez, A.; Sanz, M.; Herrera, D. A systematic review on the effects of local antimicrobials as adjuncts to subgingival debridement, compared with subgingival debridement alone, in the treatment of chronic periodontitis. J. Clin. Periodontol. 2013, 40, 227–241. [Google Scholar] [CrossRef]
- Smiley, C.J.; Tracy, S.L.; Abt, E.; Michalowicz, B.S.; John, M.T.; Gunsolley, J.; Cobb, C.M.; Rossmann, J.; Harrel, S.K.; Forrest, J.L.; et al. Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J. Am. Dent. Assoc. 2015, 146, 508–524.e5. [Google Scholar] [CrossRef] [PubMed]
- Bogren, A.; Teles, R.P.; Torresyap, G.; Haffajee, A.D.; Socransky, S.S.; Wennström, J.L. Locally delivered doxycycline during supportive periodontal therapy: A 3-year study. J. Periodontol. 2008, 79, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Madi, M.; Pavlic, V.; Samy, W.; Alagl, A. The anti-inflammatory effect of locally delivered nano-doxycycline gel in therapy of chronic periodontitis. Acta Odontol. Scand. 2018, 76, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Krueger, E.; Brown, A.C. Aggregatibacter actinomycetemcomitans leukotoxin: From mechanism to targeted anti-toxin therapeutics. Mol. Oral Microbiol. 2020, 35, 85–105. [Google Scholar] [CrossRef]
- Johansson, A. Aggregatibacter actinomycetemcomitans leukotoxin: A powerful tool with capacity to cause imbalance in the host inflammatory response. Toxins 2011, 3, 242–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ennibi, O.K.; Claesson, R.; Akkaoui, S.; Reddahi, S.; Kwamin, F.; Haubek, D.; Johansson, A. High salivary levels of JP2 genotype of Aggregatibacter actinomycetemcomitans is associated with clinical attachment loss in Moroccan adolescents. Clin. Exp. Dent. Res. 2019, 5, 44–51. [Google Scholar] [CrossRef]
- Claesson, R.; Höglund-Åberg, C.; Haubek, D.; Johansson, A. Age-related prevalence and characteristics of Aggregatibacter actinomycetemcomitans in periodontitis patients living in Sweden. J. Oral. Microbiol. 2017, 9, 1334504. [Google Scholar] [CrossRef] [Green Version]
- Ennibi, O.K.; Benrachadi, L.; Bouziane, A.; Haubek, D.; Poulsen, K. The highly leukotoxic JP2 clone of Aggregatibacter actinomycetemcomitans in localized and generalized forms of aggressive periodontitis. Acta Odontol. Scand. 2012, 70, 318–322. [Google Scholar] [CrossRef]
- Gordon, Y.J.; Romanowski, E.G.; McDermott, A.M. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr. Eye Res. 2005, 30, 505–515. [Google Scholar] [CrossRef]
- Ji, S.; Hyun, J.; Park, E.; Lee, B.L.; Kim, K.K.; Choi, Y. Susceptibility of various oral bacteria to antimicrobial peptides and to phagocytosis by neutrophils. J. Periodontal. Res. 2007, 42, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Hart, T.C.; Atkinson, J.C. Mendelian forms of periodontitis. Periodontol 2000 2007, 45, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Groenink, J.; Ligtenberg, A.J.; Veerman, E.C.; Bolscher, J.G.; Nieuw Amerongen, A.V. Interaction of the salivary low-molecular-weight mucin (MG2) with Actinobacillus actinomycetemcomitans. Antonie Van Leeuwenhoek 1996, 70, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Perdigon, G.; Corthier, G.; Salminen, S.; Koletzko, B.; Morelli, L. Should yoghurt cultures be considered probiotic? Br. J. Nutr. 2005, 93, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Iniesta, M.; Herrera, D.; Montero, E.; Zurbriggen, M.; Matos, A.R.; Marin, M.J.; Sanchez-Beltran, M.C.; Llama-Palacio, A.; Sanz, M. Probiotic effects of orally administered Lactobacillus reuteri-containing tablets on the subgingival and salivary microbiota in patients with gingivitis. A randomized clinical trial. J. Clin. Periodontol. 2012, 39, 736–744. [Google Scholar] [CrossRef]
- Martin-Cabezas, R.; Davideau, J.L.; Tenenbaum, H.; Huck, O. Clinical efficacy of probiotics as an adjunctive therapy to non-surgical periodontal treatment of chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2016, 43, 520–530. [Google Scholar] [CrossRef]
- Matsubara, V.H.; Bandara, H.M.; Ishikawa, K.H.; Mayer, M.P.; Samaranayake, L.P. The role of probiotic bacteria in managing periodontal disease: A systematic review. Expert Rev. Anti. Infect. 2016, 14, 643–655. [Google Scholar] [CrossRef]
- Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Yukna, R.A.; Takasaki, A.A.; Romanos, G.E.; Taniguchi, Y.; Sasaki, K.M.; Zeredo, J.L.; et al. Periodontal and peri-implant wound healing following laser therapy. Periodontol 2000 2015, 68, 217–269. [Google Scholar] [CrossRef]
- Schwarz, F.; Aoki, A.; Becker, J.; Sculean, A. Laser application in non-surgical periodontal therapy: A systematic review. J. Clin. Periodontol. 2008, 35, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, I.; Okamoto, T.; Morita, S.; Shiramizu, F.; Fuma, Y.; Ichinose, S.; Okano, T.; Ando, T. Blue-violet light emitting diode (LED) irradiation immediately controls socket bleeding following tooth extraction: Clinical and electron microscopic observations. Photomed Laser Surg 2011, 29, 333–338. [Google Scholar] [CrossRef]
- Walsh, J.T., Jr.; Cummings, J.P. Effect of the dynamic optical properties of water on midinfrared laser ablation. Lasers Surg. Med. 1994, 15, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, F.; Aoki, A.; Miura-Uchiyama, M.; Sasaki, K.M.; Ichinose, S.; Umeda, M.; Ishikawa, I.; Izumi, Y. In vitro studies of the ablation mechanism of periodontopathic bacteria and decontamination effect on periodontally diseased root surfaces by erbium:yttrium-aluminum-garnet laser. Lasers Med. Sci. 2011, 26, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Shimada, K.; Iwasaki, H.; Ito, K. Inhibitory effects of a super pulsed carbon dioxide laser at low energy density on periodontopathic bacteria and lipopolysaccharide in vitro. J. Periodontal. Res. 2005, 40, 469–473. [Google Scholar] [CrossRef]
- Folwaczny, M.; Aggstaller, H.; Mehl, A.; Hickel, R. Removal of bacterial endotoxin from root surface with Er:YAG laser. Am. J. Dent. 2003, 16, 3–5. [Google Scholar]
- Yamasaki, A.; Ito, H.; Yusa, J.; Sakurai, Y.; Okuyama, N.; Ozawa, R. Expression of heat shock proteins, Hsp70 and Hsp25, in the rat gingiva after irradiation with a CO2 laser in coagulation mode. J. Periodontal. Res. 2010, 45, 323–330. [Google Scholar] [CrossRef]
- Yamasaki, A.; Tamamura, K.; Sakurai, Y.; Okuyama, N.; Yusa, J.; Ito, H. Remodeling of the rat gingiva induced by CO2 laser coagulation mode. Lasers Surg. Med. 2008, 40, 695–703. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental plaque: Biological significance of a biofilm and community life-style. J. Clin. Periodontol. 2005, 32 (Suppl. S6), 7–15. [Google Scholar] [CrossRef] [PubMed]
- Rhodus, N.L.; Bereuter, J. An evaluation of a chemical cautery agent and an anti-inflammatory ointment for the treatment of recurrent aphthous stomatitis: A pilot study. Quintessence Int. 1998, 29, 769–773. [Google Scholar]
- Bracke, J.; Basara, M.; Savord, E.; Dunaway, A.; Watkins, M. Pilot Evaluation of a Simple Adjunctive Method for Improved Removal of Oral Biofilm during Conventional Scaling and Root Planing Therapy. J. Biol. Regul. Homeost Agents 2015, 29, 6–9. [Google Scholar]
- Porter, S.R.; Al-Johani, K.; Fedele, S.; Moles, D.R. Randomised controlled trial of the efficacy of HybenX in the symptomatic treatment of recurrent aphthous stomatitis. Oral Dis. 2009, 15, 155–161. [Google Scholar] [CrossRef]
- Isola, G.; Matarese, G.; Williams, R.C.; Siciliano, V.I.; Alibrandi, A.; Cordasco, G.; Ramaglia, L. The effects of a desiccant agent in the treatment of chronic periodontitis: A randomized, controlled clinical trial. Clin. Oral Investig. 2018, 22, 791–800. [Google Scholar] [CrossRef]
- Antonelli, A.; Giovannini, L.; Baccani, I.; Giuliani, V.; Pace, R.; Rossolini, G.M. In Vitro Antimicrobial Activity of the Decontaminant HybenX((R)) Compared to Chlorhexidine and Sodium Hypochlorite against Common Bacterial and Yeast Pathogens. Antibiotic 2019, 8, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pini-Prato, G.; Magnani, C.; Rotundo, R. Treatment of Acute Periodontal Abscesses Using the Biofilm Decontamination Approach: A Case Report Study. Int. J. Periodontics Restor. Dent. 2016, 36, 55–63. [Google Scholar] [CrossRef]
- Howell, T.H.; Williams, R.C. Nonsteroidal antiinflammatory drugs as inhibitors of periodontal disease progression. Crit. Rev. Oral Biol. Med. 1993, 4, 177–196. [Google Scholar] [CrossRef]
- Heasman, P.; Seymour, R. An association between long-term non-steroidal anti-inflammatory drug therapy and the severity of periodontal disease. J. Clin. Periodontol. 1990, 17, 654–658. [Google Scholar] [CrossRef]
- Ali, T.T.; Waite, I. The effect of systemic ibuprofen on gingival inflammation in humans. J. Clin. Periodontol. 1993, 20, 723–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waite, I.; Saxton, C.; Young, A.; Wagg, B.; Corbett, M. The periodontal status of subjects receiving non-steroidal anti-inflammatory drugs. J. Periodontal Res. 1981, 16, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Heasman, P.; Seymour, R.; Boston, R. The effect of a topical non-steroidal anti-inflammatory drug on the development of experimental gingivitis in man. J. Clin. Periodontol. 1989, 16, 353–358. [Google Scholar] [CrossRef]
- Salvi, G.; Lang, N. The effects of non-steroidal anti-inflammatory drugs (selective and non-selective) on the treatment of periodontal diseases. Curr. Pharm. Des. 2005, 11, 1757–1769. [Google Scholar] [CrossRef]
- Mücke, T.; Krestan, C.R.; Mitchell, D.A.; Kirschke, J.S.; Wutzl, A. Bisphosphonate and medication-related osteonecrosis of the jaw: A review. In Seminars in Musculoskeletal Radiology; Thieme Medical Publishers: Lipsia, Germany, 2016; pp. 305–314. [Google Scholar]
- Sigua-Rodriguez, E.A.; da Costa Ribeiro, R.; de Brito, A.C.R.; Alvarez-Pinzon, N.; de Albergaria-Barbosa, J.R. Bisphosphonate-related osteonecrosis of the jaw: A review of the literature. Int. J. Dent. 2014, 192320. [Google Scholar] [CrossRef] [Green Version]
- Bhavsar, N.; Trivedi, S.; Dulani, K.; Brahmbhatt, N.; Shah, S.; Chaudhri, D. Clinical and radiographic evaluation of effect of risedronate 5 mg as an adjunct to treatment of chronic periodontitis in postmenopausal women (12-month study). Osteoporos. Int. 2016, 27, 2611–2619. [Google Scholar] [CrossRef]
- Akram, Z.; Abduljabbar, T.; Kellesarian, S.V.; Abu Hassan, M.I.; Javed, F.; Vohra, F. Efficacy of bisphosphonate as an adjunct to nonsurgical periodontal therapy in the management of periodontal disease: A systematic review. Br. J. Clin. Pharmacol. 2017, 83, 444–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badran, Z.; Kraehenmann, M.A.; Guicheux, J.; Soueidan, A. Bisphosphonates in periodontal treatment: A review. Oral Health Prev Dent 2009, 7, 3–12. [Google Scholar] [PubMed]
- Chambrone, L. Current status of the influence of osteoporosis on periodontology and implant dentistry. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Saver, B.G.; Hujoel, P.P.; Cunha-Cruz, J.; Maupomé, G. Are statins associated with decreased tooth loss in chronic periodontitis? J. Clin. Periodontol. 2007, 34, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Cruz, J.; Saver, B.; Maupome, G.; Hujoel, P.P. Statin use and tooth loss in chronic periodontitis patients. J. Periodontol. 2006, 77, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Meisel, P.; Kroemer, H.K.; Nauck, M.; Holtfreter, B.; Kocher, T. Tooth loss, periodontitis, and statins in a population-based follow-up study. J. Periodontol. 2014, 85, e160–e168. [Google Scholar] [CrossRef] [PubMed]
- Sinjab, K.; Zimmo, N.; Lin, G.H.; Chung, M.P.; Shaikh, L.; Wang, H.L. The effect of locally delivered statins on treating periodontal intrabony defects: A systematic review and meta-analysis. J. Periodontol. 2017, 88, 357–367. [Google Scholar] [CrossRef]
- Pradeep, A.; Garg, V.; Kanoriya, D.; Singhal, S. 1.2% rosuvastatin versus 1.2% atorvastatin gel local drug delivery and redelivery in treatment of intrabony defects in chronic periodontitis: A randomized placebo-controlled clinical trial. J. Periodontol. 2016, 87, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, D.R.; Andrade, C.X.; Chaparro, A.P.; Inostroza, C.M.; Ramirez, V.; Violant, D.; Nart, J. Short-Term Effects of 2% Atorvastatin Dentifrice as an Adjunct to Periodontal Therapy: A Randomized Double-Masked Clinical Trial. J. Periodontol. 2015, 86, 623–630. [Google Scholar] [CrossRef]
- Estanislau, I.M.G.; Terceiro, I.R.C.; Lisboa, M.R.P.; Teles, P.d.B.; Carvalho, R.d.S.; Martins, R.S.; Moreira, M.M.S.M. Pleiotropic effects of statins on the treatment of chronic periodontitis–a systematic review. Br. J. Clin. Pharmacol. 2015, 79, 877–885. [Google Scholar] [CrossRef]
- Jang, W.G.; Kim, E.J.; Bae, I.-H.; Lee, K.-N.; Kim, Y.D.; Kim, D.-K.; Kim, S.-H.; Lee, C.-H.; Franceschi, R.T.; Choi, H.-S. Metformin induces osteoblast differentiation via orphan nuclear receptor SHP-mediated transactivation of Runx2. Bone 2011, 48, 885–893. [Google Scholar] [CrossRef]
- Araújo, A.A.; Pereira, A.; Medeiros, C.; Brito, G.A.C.; Leitão, R.F.C.; Araújo, L.S.; Guedes, P.M.M.; Hiyari, S.; Pirih, F.Q.; Araújo Júnior, R.F. Effects of metformin on inflammation, oxidative stress, and bone loss in a rat model of periodontitis. PLoS ONE 2017, 12, e0183506. [Google Scholar] [CrossRef] [Green Version]
- Pradeep, A.; Rao, N.S.; Naik, S.B.; Kumari, M. Efficacy of varying concentrations of subgingivally delivered metformin in the treatment of chronic periodontitis: A randomized controlled clinical trial. J. Periodontol. 2013, 84, 212–220. [Google Scholar] [CrossRef]
- Akram, Z.; Vohra, F.; Javed, F. Locally delivered metformin as adjunct to scaling and root planing in the treatment of periodontal defects: A systematic review and meta-analysis. J. Periodontal Res. 2018, 53, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, A.C.; Grisa, T.A.; Muniz, F.W.M.G.; Rösing, C.K.; Cavagni, J. Effect of adjuvant use of metformin on periodontal treatment: A systematic review and meta-analysis. Clin. Oral Investig. 2019, 23, 2659–2666. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [Green Version]
- Deore, G.D.; Gurav, A.N.; Patil, R.; Shete, A.R.; NaikTari, R.S.; Inamdar, S.P. Omega 3 fatty acids as a host modulator in chronic periodontitis patients: A randomised, double-blind, palcebo-controlled, clinical trial. J. Periodontal Implant. Sci. 2014, 44, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keskiner, I.; Saygun, I.; Bal, V.; Serdar, M.; Kantarci, A. Dietary supplementation with low-dose omega-3 fatty acids reduces salivary tumor necrosis factor-α levels in patients with chronic periodontitis: A randomized controlled clinical study. J. Periodontal Res. 2017, 52, 695–703. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).