Compatibility of Different Formulations in Pentravan® and Pentravan® Plus for Transdermal Drug Delivery
Abstract
1. Introduction
- undergoes extensive gut metabolism;
- undergoes extensive first-pass (hepatic) metabolism;
- associated with increased adverse effects when given orally;
- may exert benefits from localized elevated concentrations.
2. Materials and Methods
2.1. Preparation of Active Pharmaceutical Ingredient Cream Samples
- The creams were prepared using the following general protocol with the formulations from Table 1. All the ingredients were provided by Fagron (St. Paul, MN, USA)
- The required quantity of each ingredient for the total amount to be prepared was calculated.
- Each ingredient was accurately weighed in a containment hood.
- The APIs and vehicle were placed into FagronLabTM mixing jars (FagronLabTM, Glinde, Germany) and stirred manually to initially combine the ingredients.
- The jar was placed in a FagronLabTM Mixing Pro (FagronLabTM, Glinde, Germany) and spun on the normal setting.
- The product was then transferred to an FagronLab™ TRM Ointment Mill (FagronLabTM, St. Paul, MN, USA) twice: first, on setting 3, and then, on setting 1.
- The product was then manually mixed an additional time.
- Finally, the creams were transferred to appropriately sized clean containers and labeled.
- The creams were then immediately assayed at T = 0 and stored at room temperature (20–25 °C) for the duration of the study.
2.2. Stability Study
2.3. Chromatographic Conditions
3. Results
4. Conclusions
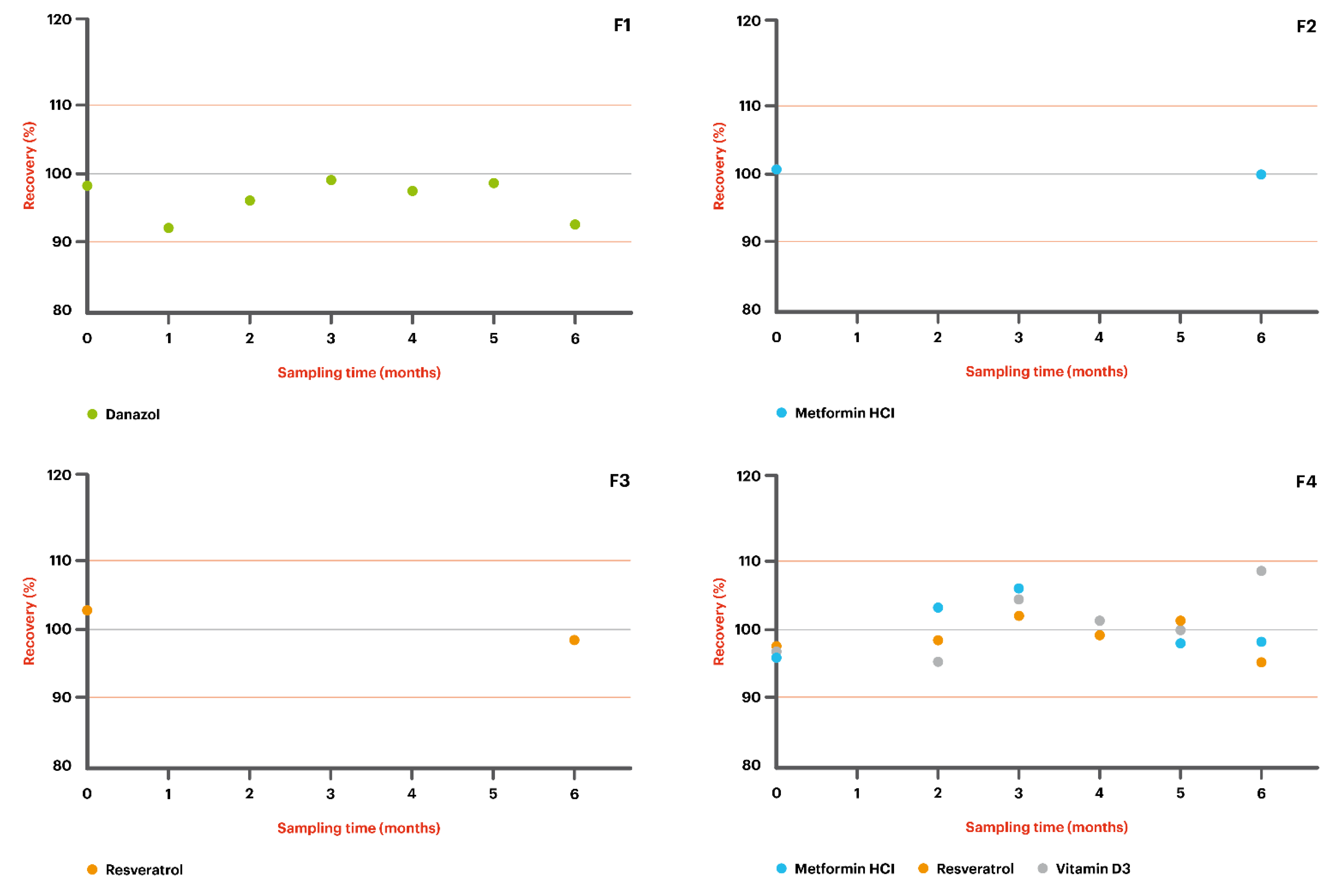
- F1: Danazol 50 mg/g + MiodesinTM 85 mg/g in Pentravan®;
- F2: Metformin HCl 200 mg/g in Pentravan®;
- F3: Resveratrol 200 mg/g in Pentravan®;
- F4: Metformin HCl 100 mg/g + Resveratrol 100 mg/g + Vitamin D3 5000 IU in Pentravan®;
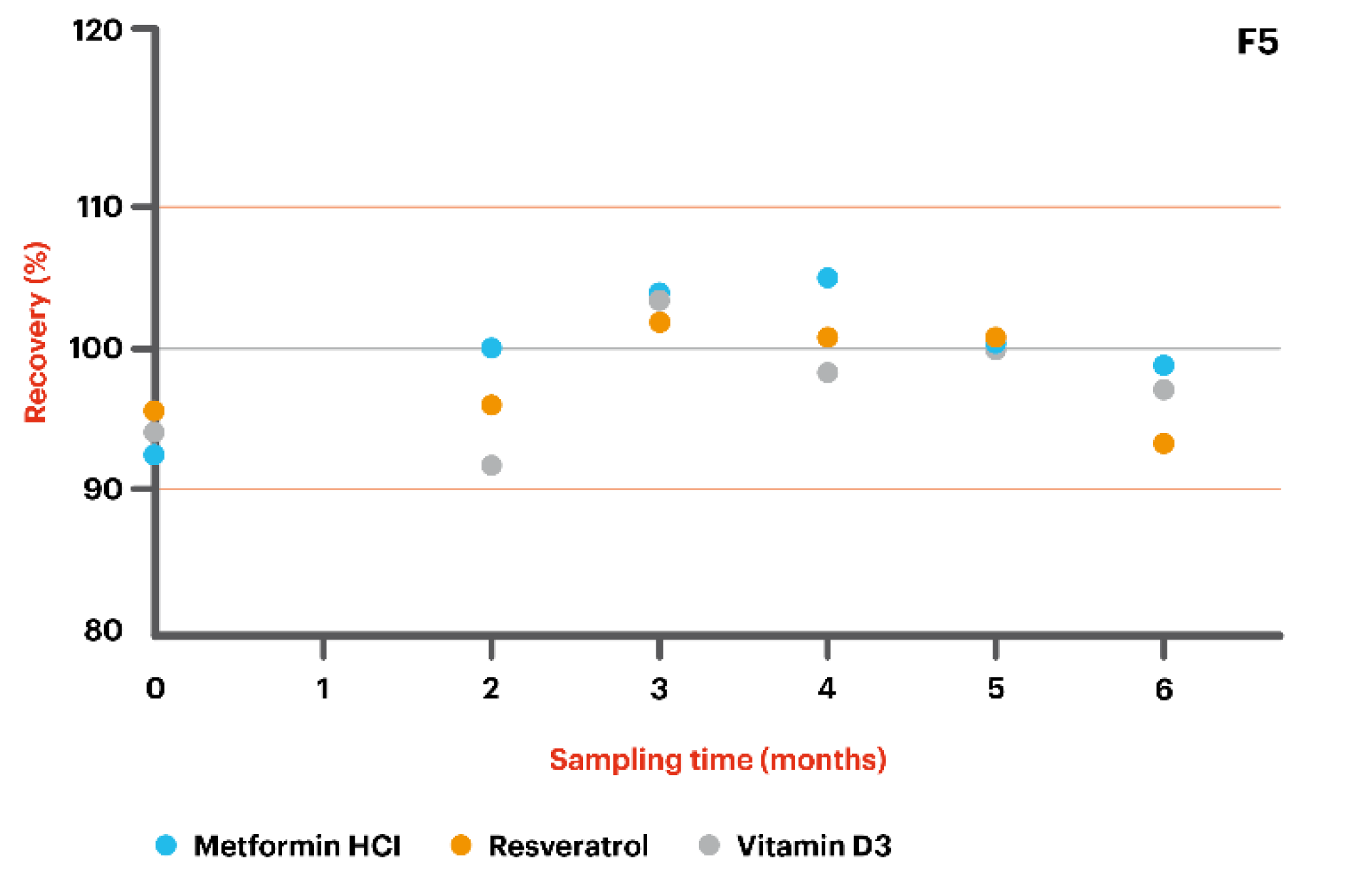
- F5: Metformin HCl 200 mg/g + Resveratrol 200 mg/g + Vitamin D3 5000 IU in Pentravan® Plus.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Alkilani, A.; McCrudden, M.T.; Donnelly, R. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.S.; Milewski, M.; Swadley, C.L.; Brogden, N.K.; Ghosh, P.; Stinchcomb, A.L. Challenges and opportunities in dermal/transdermal delivery. Ther. Deliv. 2010, 1, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2021, 1–34. [Google Scholar] [CrossRef]
- Klepser, T.B.; Kelly, M.W. Metformin hydrochloride: An antihyperglycemic agent. Am. J. Heal. Pharm. 1997, 54, 893–903. [Google Scholar] [CrossRef]
- Araoye, E.F.; Thomas, J.A.L.; Aguh, C.U.; Dallas, M. CASE REPORT Hair regrowth in 2 patients with recalcitrant central centrifugal cicatricial alopecia after use of topical metformin. JAAD Case Rep. 2020, 6, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Polonini, H.; Cândido, P.J.L.; Andrade, J.L.; Loures, S.; Raposo, N.R.; Brandão, M.A.F.; de Oliveira Ferreira, A. Transdermal Delivery of Metformin Hydrochloride from a Semisolid Vehicle. Int. J. Pharm. Compd. 2019, 23, 65–69. [Google Scholar] [PubMed]
- Carlyle, D.; Khader, T.; Lam, D.; Vadivelu, N.; Shiwlochan, D.; Yonghee, C. Endometriosis Pain Management: A Review. Curr. Pain Headache Rep. 2020, 24, 49. [Google Scholar] [CrossRef] [PubMed]
- Devalapally, H.; Silchenko, S.; Zhou, F.; McDade, J.; Goloverda, G.; Owen, A.; Hidalgo, I.J. Evaluation of a Nanoemulsion Formulation Strategy for Oral Bioavailability Enhancement of Danazol in Rats and Dogs. J. Pharm. Sci. 2013, 102, 3808–3815. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berretta, M.; Bignucolo, A.; Di Francia, R.; Comello, F.; Facchini, G.; Ceccarelli, M.; Iaffaioli, R.V.; Quagliariello, V.; Maurea, N. Resveratrol in Cancer Patients: From Bench to Bedside. Int. J. Mol. Sci. 2020, 21, 2945. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Zhang, J.; Yang, B.; Elias, P.M.; Man, M.-Q. Role of Resveratrol in Regulating Cutaneous Functions. Evid. Based Complement. Altern. Med. 2020, 2020, 2416837. [Google Scholar] [CrossRef] [PubMed]
- Alsaqr, A.; Rasoully, M.; Musteata, F.M. Investigating transdermal delivery of vitamin D3. AAPS PharmSciTech 2015, 16, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.; Sastry, K.S.; Al Ali, F.; Al-Khulaifi, M.; Wang, E.; Chouchane, A.I. Vitamin D and the Pathophysiology of Inflammatory Skin Diseases. Skin Pharmacol. Physiol. 2018, 31, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Lehman, P.A.; Raney, S.G. In vitro percutaneous absorption of ketoprofen and testosterone: Comparison of pluronic lecithin organogel vs. pentravan cream. Int. J. Pharm. Compd. 2012, 16, 248–252. [Google Scholar] [PubMed]
- Polonini, H.C.; Brandão, M.A.; Ferreira, A.O.; Ramos, C.; Raposo, N.R. Evaluation of percutaneous absorption performance for human female sexual steroids into pentravan cream. Int. J. Pharm. Compd. 2014, 18, 332–340. [Google Scholar] [PubMed]
- United States Pharmacopeial Convention. USP 40-NF 35 The United States Pharmacopeia and National Formulary; United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2017. [Google Scholar]
- Council of Europe. Uniformity of dosage units. In European Pharmacopoeia 10.0; Council of Europe: Strasbourg, France, 2019; pp. 398–400. [Google Scholar]
- United States Pharmacopeia. <795> Pharmaceutical Compounding–Nonsterile Preparations. In United States Pharmacopeia; United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2020. [Google Scholar]
| API | F1 | F2 | F3 | F4 | F5 | Pharmaceutical Action |
|---|---|---|---|---|---|---|
| Danazol | 50 mg | - | - | - | - | Androgen; treatment of endometriosis, fibrocystic breast disease, hereditary angioedema, and other conditions. |
| MiodesinTM | 85 mg | - | - | - | - | Natural anti-inflammatory; treatment of endometriosis; uterine leiomyomas; adenomyosis; inflammaging (chronic inflammation, in ageing, cardiovascular disease, and frailty. joint health); and other conditions. |
| Metformin HCl | - | 200 mg | - | 100 mg | 200 mg | Biguanide; treatment of diabetes mellitus. |
| Resveratrol | - | - | 200 mg | 100 mg | 200 mg | Polyphenolic phytoalexin; antioxidant. |
| Vitamin D3 | - | - | - | 5000 IU | 5000 IU | Vitamin; treatment and prevention of bone disorders and low levels of calcium or phosphate. |
| Pentravan® | q.s. 1 g | q.s. 1 g | q.s. 1 g | q.s. 1 g | - | Ready-to-use transdermal vehicle. |
| Pentravan® Plus | - | - | - | - | q.s. 1 g | Ready-to-use transdermal vehicle. |
| Active Pharmaceutical Ingredient | Mobile Phase Composition | Work Concentration (μg/mL)/Injection Volume (µL) | Column | Flux (mL/min) | Ultraviolet Detection Wavelength (nm) |
|---|---|---|---|---|---|
| F1 | 60 mL acetonitrile + 40 mL water | 400/5.0 | C18, 150 × 4.6 mm | 1.5 | 290 |
| F2 | 450 mL water + 0.25 g HeptaneSO3Na + 0.25 g NaCl + 0.6 g NaH2PO4 + 14 µL H3PO4 + 50 mL acetonitrile | 1000/3.0 | C18, 150 × 4.6 mm, at 30 °C | 2.0 | 245 |
| F3 | 400 mL 50 mM KH2PO4 + 100 mL acetonitrile | 2000/4.0 | C18, 300 × 4.6 mm | 2.0 | 350 |
| F4/F5 * | 90 mL methanol + 10 mL isopropyl alcohol | 200/10.0 | C18, 250 × 4.6 mm | 2.0 | 269 |
| Elapsed Time (Days) | % Assay (Room Temperature, 20–25 °C) |
|---|---|
| Danazol 50 mg/g—compounded in combination with Miodesin 85mg/g in Pentravan® | |
| T = 0 | 98.5 |
| T = 32 | 92.7 |
| T = 62 | 96.6 |
| T = 90 | 99.4 |
| T = 120 | 97.7 |
| T = 153 | 98.8 |
| T = 183 | 93.0 |
| Metformin HCl 200 mg/g—in Pentravan® | |
| T = 0 | 100.5 |
| T = 176 | 100.1 |
| Resveratrol 200 mg/g—in Pentravan® | |
| T = 0 | 102.8 |
| T = 178 | 98.7 |
| Metformin HCl 100 mg/g—compounded in combination with Resveratrol 100mg/g and Vitamin D3 5000 IU in Pentravan® | |
| T = 0 | 95.8 |
| T = 63 | 103.1 |
| T = 89 | 105.7 |
| T = 118 | 99.4 |
| T = 154 | 97.9 |
| T = 183 | 98.1 |
| Resveratrol 100mg/g—compounded in combination with Metformin HCl 100 mg/g and Vitamin D3 5000 IU in Pentravan® | |
| T = 0 | 97.4 |
| T = 63 | 98.4 |
| T = 89 | 102.0 |
| T = 118 | 99.4 |
| T = 154 | 101.0 |
| T = 183 | 95.5 |
| Vitamin D3 5000 IU—compounded in combination with Metformin HCl 100 mg/g and Resveratrol 100mg/g in Pentravan® | |
| T = 0 | 96.7 |
| T = 63 | 95.3 |
| T = 89 | 104.5 |
| T = 118 | 101.1 |
| T = 154 | 99.9 |
| T = 183 | 108.4 |
| Metformin HCl 200 mg/g—compounded in combination with Resveratrol 200 mg/g and Vitamin D3 5000 IU in Pentravan® Plus | |
| T = 0 | 93.7 |
| T = 63 | 99.9 |
| T = 89 | 103.0 |
| T = 125 | 103.6 |
| T = 154 | 100.1 |
| T = 187 | 98.9 |
| Resveratrol 200 mg/g—compounded in combination with Metformin HCl 200 mg/g and Vitamin D3 5000 IU in Pentravan® Plus | |
| T = 0 | 95.3 |
| T = 63 | 95.8 |
| T = 89 | 101.8 |
| T = 125 | 100.5 |
| T = 154 | 100.6 |
| T = 187 | 93.1 |
| Vitamin D3 5000 IU—compounded in combination with Metformin HCl 200 mg/g and Resveratrol 200 mg/g in Pentravan® Plus | |
| T = 0 | 94.6 |
| T = 63 | 92.7 |
| T = 89 | 102.8 |
| T = 125 | 98.2 |
| T = 154 | 99.8 |
| T = 187 | 97.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polonini, H.; Taylor, S.; Zander, C. Compatibility of Different Formulations in Pentravan® and Pentravan® Plus for Transdermal Drug Delivery. Sci. Pharm. 2021, 89, 51. https://doi.org/10.3390/scipharm89040051
Polonini H, Taylor S, Zander C. Compatibility of Different Formulations in Pentravan® and Pentravan® Plus for Transdermal Drug Delivery. Scientia Pharmaceutica. 2021; 89(4):51. https://doi.org/10.3390/scipharm89040051
Chicago/Turabian StylePolonini, Hudson, Sarah Taylor, and Clark Zander. 2021. "Compatibility of Different Formulations in Pentravan® and Pentravan® Plus for Transdermal Drug Delivery" Scientia Pharmaceutica 89, no. 4: 51. https://doi.org/10.3390/scipharm89040051
APA StylePolonini, H., Taylor, S., & Zander, C. (2021). Compatibility of Different Formulations in Pentravan® and Pentravan® Plus for Transdermal Drug Delivery. Scientia Pharmaceutica, 89(4), 51. https://doi.org/10.3390/scipharm89040051






