Contribution of Topical Antioxidants to Maintain Healthy Skin—A Review
Abstract
1. Introduction
2. Aggressive Skin Agents
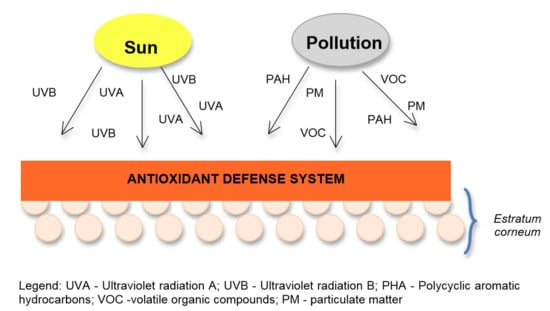
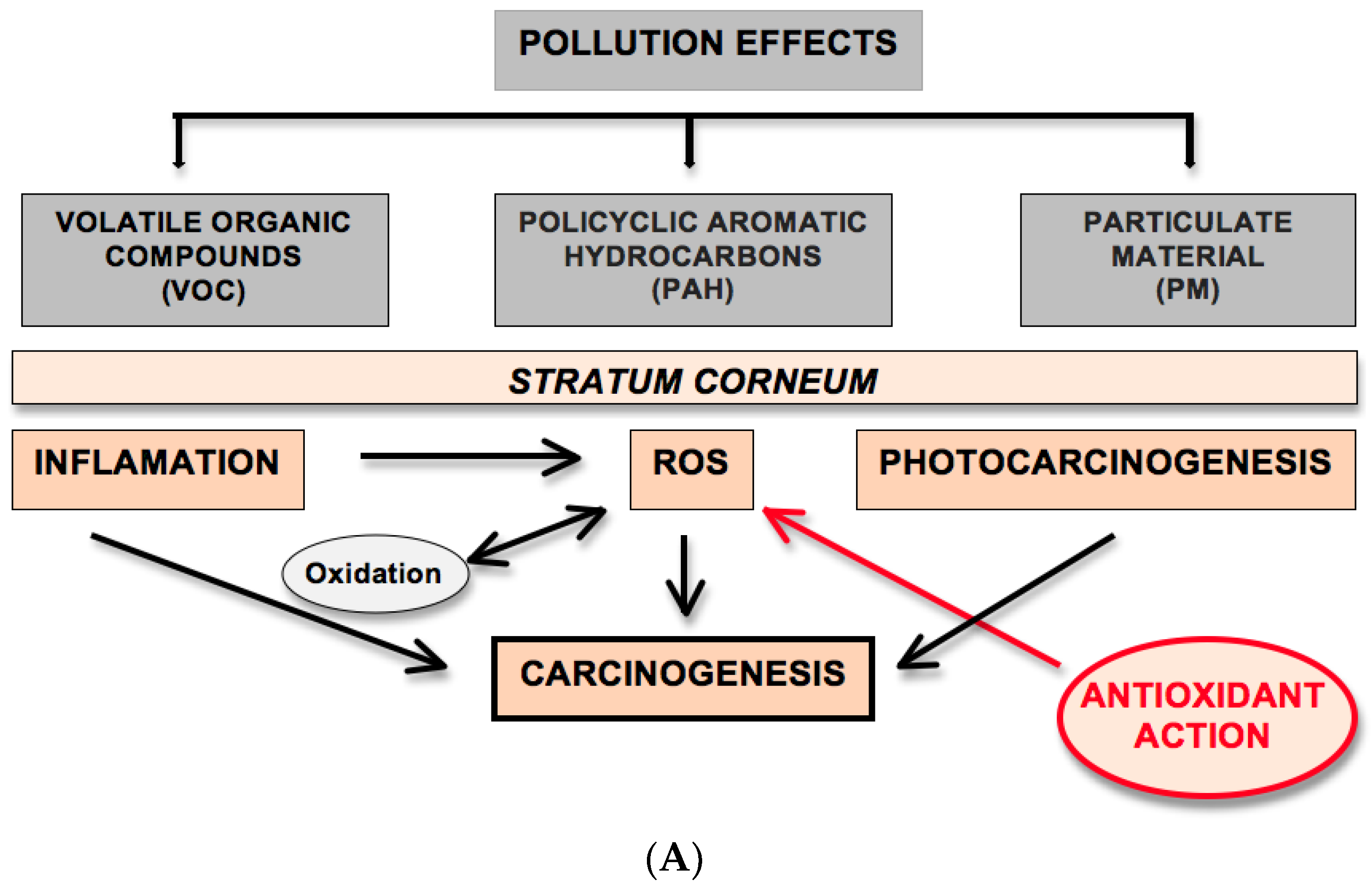
2.1. Pollution Effects
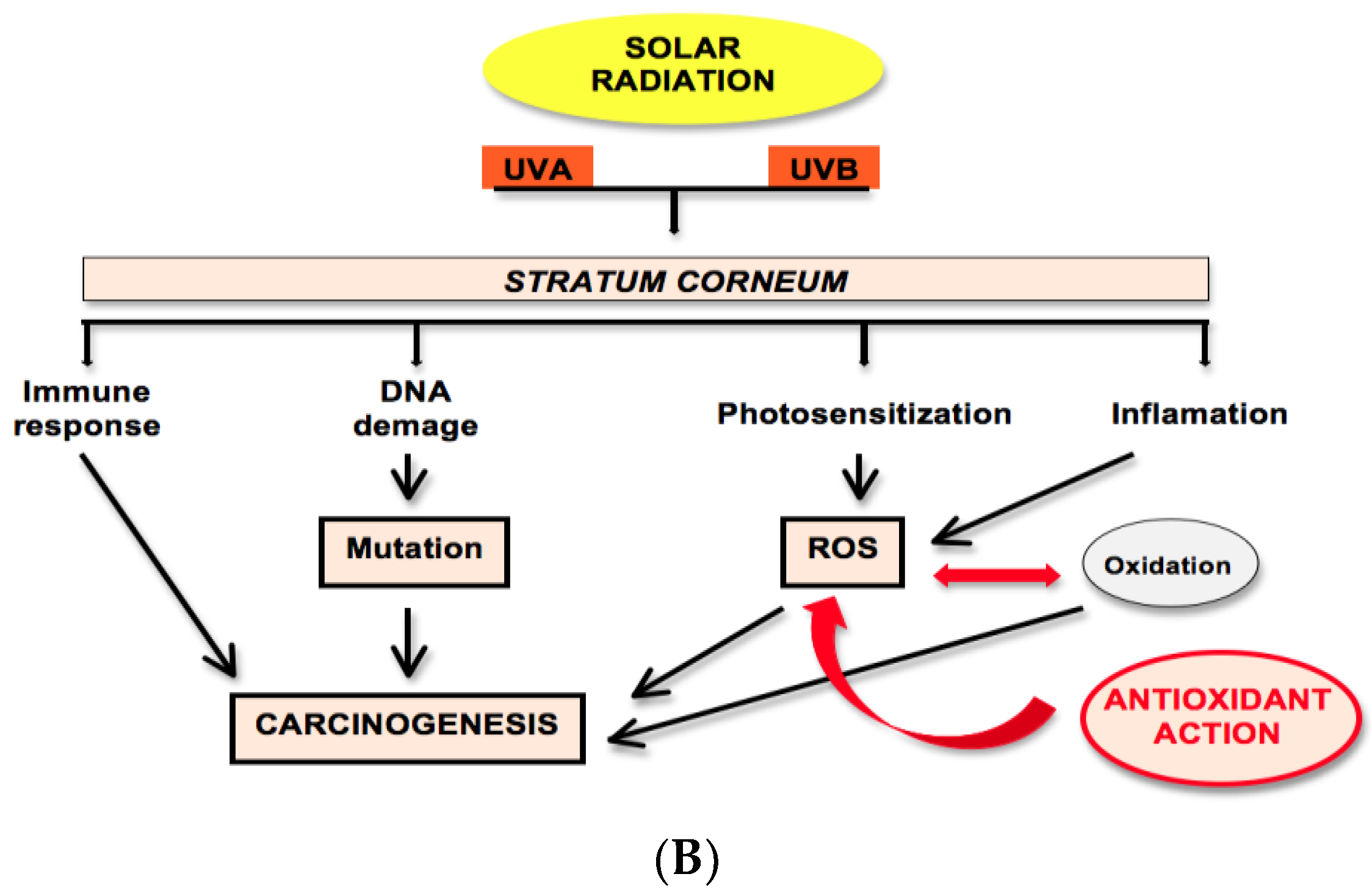
2.2. Solar Radiation
3. Skin Peroxidation Process


4. Transcutaneous Penetration and Antioxidants Products
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Song, S.; Lee, K.; Lee, Y.-M.; Lee, J.-H.; Lee, S.I.; Yu, S.-D.; Paek, M. Acute health effects of urban fine and ultrafine particles on children with atopic dermatitis. Environ. Res. 2011, 111, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K. The role of air pollutants in atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Kular, J.K.; Basu, S.; Sharma, R.I. The extracellular matrix: Structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J. Tissue Eng. 2014, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kamble, P.; Sadarani, B.; Majumdar, A.; Bhullar, S. Nanofiber based drug delivery systems for skin: A promising therapeutic approach. J. Drug Deliv. Sci. Technol. 2017, 41, 124–133. [Google Scholar] [CrossRef]
- Velasco, M.V.R.; Sauce, R.; Oliveira, C.; Pinto, C.A.D.O.; Martinez, R.M.; Baah, S.; Almeida, T.S.; Rosado, C.; Baby, A.R. Active ingredients, mechanisms of action and efficacy tests of antipollution cosmetic and personal care products. Braz. J. Pharm. Sci. 2018, 54, 54. [Google Scholar] [CrossRef]
- Marrot, L. Pollution and Sun Exposure: A Deleterious Synergy. Mechanisms and Opportunities for Skin Protection. Curr. Med. Chem. 2019, 25, 5469–5486. [Google Scholar] [CrossRef] [PubMed]
- Balogh, T.S.; Velasco, M.V.R.; Pedriali, C.; Kaneko, T.M.; Baby, A.R. Proteção à radiação ultravioleta: Recursos disponíveis na atualidade em fotoproteção. Bras. De Derm. 2011, 86, 732–742. [Google Scholar] [CrossRef]
- American Cancer Society (ACS). Learn About Cancer. What Causes Cancer. Sun and UV Exposure. Skin Cancer Prevention and Early Detection. What Is Ultraviolet (UV) Radiation? 2013. Available online: https://www.cdc.gov/cancer/skin/basic_info/what-is-skin-cancer.htm (accessed on 2 January 2020).
- Soeur, J.; Belaïdi, J.-P.; Chollet, C.; Denat, L.; Dimitrov, A.; Jones, C.; Perez, P.; Zanini, M.; Zobiri, O.; Mezzache, S.; et al. Photo-pollution stress in skin: Traces of pollutants (PAH and particulate matter) impair redox homeostasis in keratinocytes exposed to UVA1. J. Derm. Sci. 2017, 86, 162–169. [Google Scholar] [CrossRef]
- Maverakis, E.; Miyamura, Y.; Bowen, M.P.; Correa, G.; Ono, Y.; Goodarzi, H. Light, including ultraviolet. J. Autoimmun. 2009, 34, J247–J257. [Google Scholar] [CrossRef]
- Valacchi, G.; Sticozzi, C.; Pecorelli, A.; Cervellati, F.; Cervellati, C.; Maioli, E. Cutaneous responses to environmental stressors. Ann. N. Y. Acad. Sci. 2012, 1271, 75–81. [Google Scholar] [CrossRef]
- Portugal-Cohen, M.; Oron, M.; Cohen, D.; Ma’or, Z. Antipollution skin protection—A new paradigm and its demonstration on two active compounds. Clin. Cosmet. Investig. Dermatol. 2017, 10, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.C.; Latorre, M.D.R.D.D.O.; Cardoso, M.R.A.; Gonçalves, F.L.T.; Saldiva, P.H.; Braga, A.L.F. Poluição atmosférica e atendimentos por pneumonia e gripe em São Paulo, Brasil. Rev. Saúde Púb. 2002, 36, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, L.F.C.; Gavinier, S.S. Air pollutants and hospital admissions due to stroke. Ambient. Agua Interdiscip. J. Appl. Sci. 2014, 9, 390–401. [Google Scholar] [CrossRef][Green Version]
- Wisthaler, A.; Weschler, C.J. Reactions of ozone with human skin lipids: Sources of carbonyls, dicarbonyls, and hydroxycarbonyls in indoor air. Proc. Natl. Acad. Sci. USA 2010, 107, 6568–6575. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Cho, D.; Park, H. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef]
- Falcon-Rodriguez, C.I.; Osornio-Vargas, A.R.; Sada-Ovalle, I.; Segura-Medina, P. Aeroparticles, Composition, and Lung Diseases. Front. Immunol. 2016, 7, 89. [Google Scholar] [CrossRef]
- Prow, T.W.; Grice, J.E.; Lin, L.L.; Faye, R.; Butler, M.; Becker, W.; Wurm, E.M.; Yoong, C.; Robertson, T.A.; Soyer, H.P.; et al. Nanoparticles and microparticles for skin drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 470–491. [Google Scholar] [CrossRef]
- Filon, F.L.; Mauro, M.; Adami, G.; Bovenzi, M.; Crosera, M. Nanoparticles skin absorption: New aspects for a safety profile evaluation. Regul. Toxicol. Pharm. 2015, 72, 310–322. [Google Scholar] [CrossRef]
- Baby, A.R. Avaliação In Vitro da Permeabilidade Cutânea da Rutina em Emulsões Cosméticas. Ph.D. Thesis, Universidade de Sao Paulo, Agencia USP de Gestao da Informacao Academica (AGUIA), São Paulo, Brazil, 10 September 2007. [Google Scholar]
- Krutmann, J.; Liu, W.; Li, L.; Pan, X.; Crawford, M.; Sore, G.; Seite, S. Pollution and skin: From epidemiological and mechanistic studies to clinical implications. J. Derm. Sci. 2014, 76, 163–168. [Google Scholar] [CrossRef]
- Tanaka, K.; Asamitsu, K.; Uranishi, H.; Iddamalgoda, A.; Ito, K.; Kojima, H.; Okamoto, T. Protecting Skin Photoaging by NF-κB Inhibitor. Curr. Drug Metab. 2010, 11, 431–435. [Google Scholar] [CrossRef]
- Jux, B.; Kadow, S.; Luecke, S.; Rannug, A.; Krutmann, J.; Esser, C. The Aryl Hydrocarbon Receptor Mediates UVB Radiation–Induced Skin Tanning. J. Investig. Derm. 2011, 131, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.-M.; Boussouira, B.; Moyal, D.; Nguyen, Q. Oxidization of squalene, a human skin lipid: A new and reliable marker of environmental pollution studies. Int. J. Cosmet. Sci. 2015, 37, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Sousa, E.T.; Lopes, W.A.; De Andrade, J.B. Sources, formation, reactivity and determination of quinones in the atmosphere. Quím. N. 2016, 39, 486–495. [Google Scholar] [CrossRef]
- Kostyuk, V.A.; Potapovich, A.; Stancato, A.; DeLuca, C.; Lulli, D.; Pastore, S.; Korkina, L. Photo-Oxidation Products of Skin Surface Squalene Mediate Metabolic and Inflammatory Responses to Solar UV in Human Keratinocytes. PLoS ONE 2012, 7, e44472. [Google Scholar] [CrossRef] [PubMed]
- Boussouira, B.; Pham, D.M. Squalene and Skin Barrier Function: From Molecular Target to Biomarker of Environmental Exposure. Ski. Stress Response Pathw. 2016, 29–48. [Google Scholar] [CrossRef]
- Nakamura, M.; Ueda, Y.; Hayashi, M.; Kato, H.; Furuhashi, T.; Morita, A. Tobacco smoke-induced skin pigmentation is mediated by the aryl hydrocarbon receptor. Exp. Derm. 2013, 22, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Hocquaux, M.; Loing, E.; Bedos, P. Compounds, Use Thereof in Cosmetic and Cosmeceutic Applications, and Compositions Comprising Same. U.S. Patent 9,115,176, 25 August 2015. [Google Scholar]
- Rodrigues, N.D.N.; Staniforth, M.; Stavros, V.G. Photophysics of sunscreen molecules in the gas phase: A stepwise approach towards understanding and developing next-generation sunscreens. Proc. R. Soc. Lond. 2016, 472, 20160677. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, L.F.D.; Dos Santos, E.P.; De Aguiar, A.P. Organic Sunscreens. Research, Innovation and the Organic Synthesis Importance. Rev. Virtual Quím. 2014, 6, 190–223. [Google Scholar] [CrossRef]
- Gueymard, C.A. Parameterized transmittance model for direct beam and circumsolar spectral irradiance. Sol. Energy 2001, 71, 325–346. [Google Scholar] [CrossRef]
- Oliveira, C.; Peres, D.D.; Rugno, C.M.; Kojima, M.; Pinto, C.A.S.D.O.; Consiglieri, V.O.; Kaneko, T.M.; Rosado, C.; Mota, J.; Velasco, M.V.R.; et al. Functional photostability and cutaneous compatibility of bioactive UVA sun care products. J. Photochem. Photobiol. B Biol. 2015, 148, 154–159. [Google Scholar] [CrossRef]
- Velasco, M.V.R.; Balogh, T.S.; Pedriali, C.A.; Sarruf, F.D.; Pinto, C.A.S.O.; Baby, A.R. Novas metodologias analíticas para avaliação da eficácia fotoprotetora (in vitro)—Revisão. J. Basic Appl. Pharm. Sci. 2011, 32, 27–34. [Google Scholar]
- Bezerra, S.M.D.F.M.D.C.; Sotto, M.N.; Orii, N.M.; Alves, C.; Duarte, A.J.D.S. Efeitos da radiação solar crônica prolongada sobre o sistema imunológico de pescadores profissionais em Recife (PE), Brasil. Bras. Derm. 2011, 86, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Popim, R.C.; Corrente, J.E.; Marino, J.A.G.; De Souza, C.A. Câncer de pele: Uso de medidas preventivas e perfil demográfico de um grupo de risco na cidade de Botucatu. Ciência Saúde Coletiva 2008, 13, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Velasco, M.V.R.; Balogh, T.S.; Pedriali, C.A.; Sarruf, F.D.; Pinto, C.A.S.O.; Kaneko, T.M.; Baby, A.R. Associação da rutina com p-Metoxicinamato de Octila e Benzofenona-3: Avaliação In vitro da eficácia fotoprotetora por espectrofotometria de refletância. Lat. Am. J. Pharm. 2007, 27, 23–27. [Google Scholar]
- Svobodová, A.R.; Walterova, D.; Vostálová, J. Ultraviolet light induced alteration to the skin. Biomed. Pap. 2006, 150, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.A.S.O. Influência da Rutina na Fotoestabilização da Avobenzona (Filtro UVA) e Do r-Metoxicinamato de Octila (Filtro UVB). Ph.D. Thesis, Faculty of Pharmaceutical Sciences, University of São Paulo, São Paulo, Brazil, 2014. [Google Scholar]
- Repetto, M.; Semprine, J.; Boveris, A. Lipid Peroxidation: Chemical Mechanism, Biological Implications and Analytical Determination. In Lipid Peroxidation; IntechOpen: London, UK, 2012; pp. 1–28. [Google Scholar]
- Barreiros, A.L.B.S.; David, J.M.; David, J.P. Estresse oxidativo: Relação entre geração de espécies reativas e defesa do organismo. Química Nova 2006, 29, 113–123. [Google Scholar] [CrossRef]
- Kohen, R.; Nyska, A. Invited Review: Oxidation of Biological Systems: Oxidative Stress Phenomena, Antioxidants, Redox Reactions, and Methods for Their Quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef]
- Barbosa, K.B.F.; Costa, N.M.B.; Alfenas, R.D.C.G.; De Paula, S.O.; Minim, V.P.R.; Bressan, J. Estresse oxidativo: Conceito, implicações e fatores modulatórios. Rev. Nutr. 2010, 23, 629–643. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 1–31. [Google Scholar] [CrossRef]
- Pospíšil, P.; Prasad, A.; Rác, M. Mechanism of the Formation of Electronically Excited Species by Oxidative Metabolic Processes: Role of Reactive Oxygen Species. Biomolecules 2019, 9, 258. [Google Scholar] [CrossRef]
- Silva, S.A.M.E.; Michniak-Kohn, B.; Leonardi, G.R. An overview about oxidation in clinical practice of skin aging. Anais Bras. Derm. 2017, 92, 367–374. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Taube, H. Mechanisms of Oxidation with Oxygen. J. Gen. Physiol. 1965, 49, 29–50. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.K.; Kumar, S.; Choi, E.-H.; Chaudhary, S.; Kim, M.-H. Molecular dynamic simulations of oxidized skin lipid bilayer and permeability of reactive oxygen species. Sci. Rep. 2019, 9, 4496. [Google Scholar] [CrossRef]
- Schäfer, M.; Werner, S.; Sch, M. The cornified envelope: A first line of defense against reactive oxygen species. J. Investig. Derm. 2011, 131, 1409–1411. [Google Scholar] [CrossRef]
- Van Smeden, J.; Bouwstra, J. Stratum Corneum Lipids: Their Role for the Skin Barrier Function in Healthy Subjects and Atopic Dermatitis Patients. Telemed. Teledermatol. 2016, 49, 8–26. [Google Scholar] [CrossRef]
- Haque, T.; Talukder, M.U. Chemical Enhancer: A Simplistic Way to Modulate Barrier Function of the Stratum Corneum. Adv. Pharm. Bull. 2018, 8, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Osborne, D.W.; Musakhanian, J. Skin Penetration and Permeation Properties of Transcutol®—Neat or Diluted Mixtures. AAPS Pharmscitech. 2018, 19, 3512–3533. [Google Scholar] [CrossRef] [PubMed]
- Saija, A. In vitro and in vivo evaluation of caffeic and ferulic acids as topical photoprotective agents. Int. J. Pharm. 2000, 199, 39–47. [Google Scholar] [CrossRef]
- Wróblewska, K.B.; Baby, A.R.; Guaratini, M.T.G.; Moreno, P.R.H. In vitro antioxidant and photoprotective activity of five native Brazilian bamboo species. Ind. Crop. Prod. 2019, 130, 208–215. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Selim, M.; Shea, C.R.; Grichnik, J.M.; Omar, M.M.; Monteiro-Riviere, N.A.; Pinnell, S.R. UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J. Am. Acad. Derm. 2003, 48, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Keen, M.A.; Hassan, I. Vitamin E in dermatology. Indian Derm. Online J. 2016, 7, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Pinnell, S.R.; Yang, H.; Omar, M.; Monteiro-Riviere, N.A.; DeBuys, H.V.; Walker, L.C.; Wang, Y.; Levine, M. Topical L-ascorbic acid: Percutaneous absorption studies. Derm. Surg. 2001, 27, 137–142. [Google Scholar] [CrossRef]
- Juturu, V.; Bowman, J.P.; Deshpande, J. Overall skin tone and skin-lightening-improving effects with oral supplementation of lutein and zeaxanthin isomers: A double-blind, placebo-controlled clinical trial. Clin. Cosmet. Investig. Derm. 2016, 9, 325–332. [Google Scholar] [CrossRef]
- Freire, S.T.; Gomes, H.C.; Ferreira, L.M.; Percario, S. Uric acid as a monitor of oxidative stress in a random skin flap in rats. Acta Cir. Bras. 2003, 18, 18. [Google Scholar] [CrossRef][Green Version]
- Tišma, V.S.; Basta-Juzbasic, A.; Jaganjac, M.; Brcic, L.; Dobrić, I.; Lipozencic, J.; Tatzber, F.; Zarkovic, N.; Poljak-Blaži, M. Oxidative stress and ferritin expression in the skin of patients with rosacea. J. Am. Acad. Derm. 2009, 60, 270–276. [Google Scholar] [CrossRef]
- Torti, F.M.; Torti, S.V. Regulation of ferritin genes and protein. Blood 2002, 99, 3505–3516. [Google Scholar] [CrossRef]
- Tuong, W.; Kuo, S.; Sivamani, R. Botanicals and Cosmeceuticals for Sun Protection. Cosmeceutic. Act. Cosmet. 2015, 269–280. [Google Scholar] [CrossRef]
- Lopes, L.B.; VanDeWall, H.; Li, H.T.; Venugopal, V.; Li, H.K.; Naydin, S.; Hosmer, J.; Levendusky, M.; Zheng, H.; Bentley, M.V.L.; et al. Topical Delivery of Lycopene using Microemulsions: Enhanced Skin Penetration and Tissue Antioxidant Activity. J. Pharm. Sci. 2010, 99, 1346–1357. [Google Scholar] [CrossRef]
- Tournas, J.A.; Lin, F.-H.; Burch, J.A.; Selim, M.A.; Monteiro-Riviere, N.A.; Zielinski, J.E.; Pinnell, S.R. Ubiquinone, Idebenone, and Kinetin Provide Ineffective Photoprotection to Skin when Compared to a Topical Antioxidant Combination of Vitamins C and E with Ferulic Acid. J. Investig. Derm. 2006, 126, 1185–1187. [Google Scholar] [CrossRef]
- Darvin, M.E.; Gersonde, I.; Albrecht, H.; Gonchukov, S.A.; Sterry, W.; Lademann, J. Determination of beta carotene and lycopene concentrations in human skin using resonance Raman spectroscopy. Laser Phys. 2005, 2, 295–299. [Google Scholar]
- Mein, J.R.; Lian, F.; Wang, X.-D. Biological activity of lycopene metabolites: Implications for cancer prevention. Nutr. Rev. 2008, 66, 667–683. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.W.; Khoo, H.E.; Prasad, K.N.; Ismail, A.; Tan, C.; Rajab, N.F. Revealing the Power of the Natural Red Pigment Lycopene. Molecules 2010, 15, 959–987. [Google Scholar] [CrossRef] [PubMed]
- Castro, I.A.; Barros, S.M.; Marquez, U.L.; Motizuki, M.; Sawada, T.H. Optimization of the antioxidant capacity of a mixture of carotenoids and α-tocopherol in the development of a nutritional supplement. Food Res. Int. 2005, 38, 861–866. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Carotenoids and flavonoids contribute to nutritional protection against skin damage from sunlight. Mol. Biotechnol. 2007, 37, 26–30. [Google Scholar] [CrossRef]
- Iovine, B.; Iannella, M.L.; Gasparri, F.; Monfrecola, G.; Bevilacqua, M.A. Synergic Effect of Genistein and Daidzein on UVB-Induced DNA Damage: An Effective Photoprotective Combination. J. Biomed. Biotechnol. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Lawal, O.; Ogunwande, I.; Kasali, A.A.; Opoku, A.R.; Oyedeji, A.O. Chemical Composition, Antibacterial and Cytotoxic Activities of Essential Oil from the Leaves of Helichrysum odoratissimum grown in South Africa. J. Essent. Oil Bear. Plants 2015, 18, 236–241. [Google Scholar] [CrossRef]
- Twilley, D.; Lall, N. Extracts and Composition of Helichrysumodoratissimum for Preventing and Treating Skin Cancer. U.S. Patent WO 2015049666 A1, 9 April 2015. [Google Scholar]
- Hubner, A.; Sobreira, F.; Neto, A.V.; Pinto, C.A.S.O.; Dario, M.F.; Díaz, I.E.C.; Lourenço, F.R.; Rosado, C.; Baby, A.R.; Bacchi, E.M. The synergistic behavior of antioxidant phenolic compounds obtained from winemaking waste´s valorization, increased the efficacy of a sunscreen system. Antioxidants 2019, 8, 530. [Google Scholar] [CrossRef]
- Balboa, E.; Soto, M.L.; Nogueira-Librelotto, D.R.; González-López, N.; Conde, E.; Moure, A.; Vinardell, M.P.; Mitjans, M.; Domínguez, H. Potential of antioxidant extracts produced by aqueous processing of renewable resources for the formulation of cosmetics. Ind. Crop. Prod. 2014, 58, 104–110. [Google Scholar] [CrossRef]
- Rosado, C.; Tokunaga, V.K.; Sauce, R.; De Oliveira, C.A.; Sarruf, F.D.; Parise-Filho, R.; Maurício, E.; De Almeida, T.S.; Velasco, M.V.R.; Baby, A.R. Another Reason for Using Caffeine in Dermocosmetics: Sunscreen Adjuvant. Front. Physiol. 2019, 10, 519. [Google Scholar] [CrossRef]
- Trommer, H.; Neubert, R.H. Screening for new antioxidative compounds for topical administration using skin lipid model systems. J. Pharm. Pharm. Sci. 2005, 8, 494–506. [Google Scholar] [PubMed]
- Baby, A.R.; Haroutiounian-Filho, C.A.; Sarruf, F.D.; Tavante-Júnior, C.R.; Pinto, C.A.S.D.O.; Zague, V.; Arêas, E.P.G.; Kaneko, T.M.; Velasco, M.V.R. Estabilidade e estudo de penetração cutânea in vitro da rutina veiculada em uma emulsão cosmética através de um modelo de biomembrana alternativo. Rev. Bras. De Ciências Farm. 2008, 44, 233–248. [Google Scholar] [CrossRef]
- Caparica, R.; Júlio, A.; Araújo, M.E.M.; Baby, A.R.; Fonte, P.; Costa, J.G.; De Almeida, T.S. Anticancer Activity of Rutin and Its Combination with Ionic Liquids on Renal Cells. Biomolecules 2020, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Health Observatory; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Peres, D.A.; Sarruf, F.D.; Oliveira, C.A.; Velasco, M.V.R.; Baby, A.R. Ferulic acid photoprotectivepropeties in association with UV filters: Multifuncional sunscreen with improved SPF and UVA-PF. J. Photochem. Photobiol. B Biol. 2018, 185, 46–49. [Google Scholar] [CrossRef]
- Candido, T.M.; De Oliveira, C.A.; Ariede, M.B.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. Safety and Antioxidant Efficacy Profiles of Rutin-Loaded Ethosomes for Topical Application. AAPS Pharmscitech. 2018, 19, 1773–1780. [Google Scholar] [CrossRef]
- Gonçalves, P.V. Avaliação Ex Vivo da Inibição da Peroxidação Lipídica do Estrato Córneo Promovida por Filtro UVB. Master’s Thesis, Faculty of Pharmaceutical Sciences, University of São Paulo, São Paulo, Brazil, 20 March 2019. [Google Scholar]
| Name | Structural Formulas | Types of Compound(s) | Main Effect(s) | Method | Refs. |
|---|---|---|---|---|---|
| Ascorbic Acid | 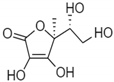 | Water soluble phenolic | Protection skin against erythema produced by UVB and UVA irradiation | Ex vivosolar simulator | [56] |
| Vitamin E (α-tocopherol) | 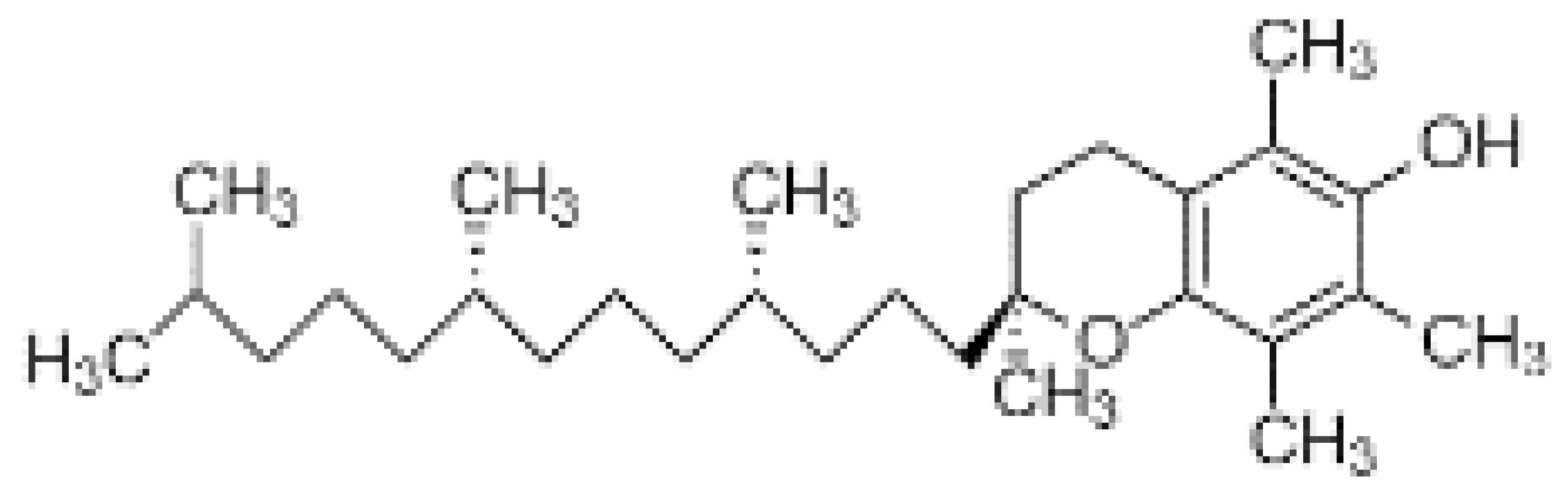 | Fat soluble phenolic | Prevents UVB radiation | Ex vivosolar simulator | [56] |
| Helichrysumodoratissimum (L.) Sweet | N/A | Flavonoid, chalcones | Increases SPF and reduces UV-induced erythema | In vivo-SANS 1557:2013 and ISO 24444:2010. | [72,73] |
| Genistein |  | Isoflavone | Agents against UV-induced photodamage | In vitroHuman skin grown in 3D. | [63] |
| Lycopene |  | Carotenoid |
microemulsion increased lycopene penetration and antioxidant activity | Ex vivoporcineearskin | [64] |
| Vitisvinifera L. |  | Phenolic | Increased SPF value and broad protection spectrum | In vitro-diffuse reflectance spectrophotometry, DPPH | [74] |
| Extracts of Brazilian species of bamboo | N/A | Phenolic | Increased efficiency and photo-stability of UV sunscreens | In vitro-diffuse reflectance spectrophotometry, DPPH | [55] |
| Buckwheat extract | N/A | Flavonoids, flavones, phytosterols | Lipid Protection | In vivoTBARS | [77] |
| Rutin |  | Polyphenol, flavonoid | Prevent UV irradiation induced oxidative stress | In vivo | [79] |
| Caffeine | 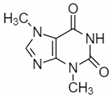 | Phenolic | ±25% increase in anti-UVB protection in vivo | In vivo-International SPF Test Method (Cosmetics Europe, 2006). In vitro-diffuse reflectance spectrophotometers | [76] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo Martins, T.E.; Sales de Oliveira Pinto, C.A.; Costa de Oliveira, A.; Robles Velasco, M.V.; Gorriti Guitiérrez, A.R.; Cosquillo Rafael, M.F.; Tarazona, J.P.H.; Retuerto-Figueroa, M.G. Contribution of Topical Antioxidants to Maintain Healthy Skin—A Review. Sci. Pharm. 2020, 88, 27. https://doi.org/10.3390/scipharm88020027
Azevedo Martins TE, Sales de Oliveira Pinto CA, Costa de Oliveira A, Robles Velasco MV, Gorriti Guitiérrez AR, Cosquillo Rafael MF, Tarazona JPH, Retuerto-Figueroa MG. Contribution of Topical Antioxidants to Maintain Healthy Skin—A Review. Scientia Pharmaceutica. 2020; 88(2):27. https://doi.org/10.3390/scipharm88020027
Chicago/Turabian StyleAzevedo Martins, Tércio Elyan, Claudinéia Aparecida Sales de Oliveira Pinto, Andressa Costa de Oliveira, Maria Valéria Robles Velasco, Arilmí Rosa Gorriti Guitiérrez, Martha Francisca Cosquillo Rafael, Jossimar Paúl Huamaní Tarazona, and Mónica Guadalupe Retuerto-Figueroa. 2020. "Contribution of Topical Antioxidants to Maintain Healthy Skin—A Review" Scientia Pharmaceutica 88, no. 2: 27. https://doi.org/10.3390/scipharm88020027
APA StyleAzevedo Martins, T. E., Sales de Oliveira Pinto, C. A., Costa de Oliveira, A., Robles Velasco, M. V., Gorriti Guitiérrez, A. R., Cosquillo Rafael, M. F., Tarazona, J. P. H., & Retuerto-Figueroa, M. G. (2020). Contribution of Topical Antioxidants to Maintain Healthy Skin—A Review. Scientia Pharmaceutica, 88(2), 27. https://doi.org/10.3390/scipharm88020027