Abstract
Medicinal plants hold a significant place as alternative treatments available for inflammatory diseases, with many phytoconstituents being frequently tested in vitro for their biological activities. In the current study, we investigated the in vivo anti-inflammatory properties of a novel active gel formulation, combining Achillea millefolium and Taxodium distichum essential oils with extracts of Aesculus hippocastanum seeds and Plantago lanceolata leaves. The toxicity of the obtained extracts and volatile oils was determined using the invertebrate model based on Daphnia magna. Anti-inflammatory potential was evaluated by the plethysmometric method on Wistar rats, expressed as the inhibition of the inflammatory oedema (%IIO), while the antinociceptive response was determined on NMRI mice, according to the tail-flick latency method. The tested gel’s efficacy was similar to the 5% diclofenac standard (maximal %IIO of 42.01% vs. 48.70%, respectively), with the anti-inflammatory effect being observed sooner than for diclofenac. Our active gel also produced a significant prolongation of tail-flick latencies at both 60 and 120 min, comparable to diclofenac. Consequently, we can imply that the active constituents present in vivo anti-inflammatory properties, and the prepared gel may be suited for use as an alternative treatment of topical inflammatory conditions.
1. Introduction
Inflammation comprises a plethora of intricate biological responses induced by the presence of various harmful agents that lead to cellular and tissue damage. Serving a protective function, its mechanisms aim at removing the cause of cell injury and initiating tissue repair [1]. The disruption of tissue homeostasis marks the onset of the inflammatory cascade, as the immune system produces a series of pro-inflammatory mediators, e.g., interleukins, tumor necrosis factor alpha (TNF-α), reactive oxygen species, nitric oxide and prostaglandins [2,3]. The local microcirculation is thus affected, with other blood proteins and blood cells that mediate inflammatory response being attracted to the damaged tissue site through vasodilation and increased vascular permeability [4].
From a historical perspective, we know that humans have been preoccupied for millennia with treating a number of injuries and diseases, including the ones of an inflammatory nature. Egyptian papyruses from as early as 3400 B.C. listed various plants of medicinal importance, a number of them addressing conditions with a direct inflammatory component (e.g., dried myrtle infusion, recommended for rheumatic pain) [5]. A similar case of medicinal plants being employed for ailments characterized by acute inflammation was supported by Hippocrates (Greece, 450–370 BC), who used extracts from willow bark to relieve pain and fever [6].
The modern status quo in inflammation treatment varies from drugs such as nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, metformin, and statins, to nutraceuticals such as ginger root, turmeric, hyssop, devil’s claw (Harpagophytum procumbens) [7], or capsaicin [8].
NSAIDs are widely prescribed for treating both acute and chronic pain and inflammation, most of them being available as over-the-counter drugs (e.g., acetylsalicylic acid, diclofenac, ibuprofen, ketoprofen). Their underlying mechanism of action is that of cyclooxygenase (COX) inhibition—both COX-1 and COX-2 for the classical ones, and mainly COX-2 for the selective ones [9]. Inhibition of COX-1 consequently lowers the levels of beneficial prostaglandins, giving rise to many adverse effects, most importantly of a gastro-intestinal (GI) nature, but also circulatory, respiratory, hepatic or renal, while the more selective “coxibs” are characterized by cardiovascular toxicity [7,10,11]. Corticosteroids, more precisely glucocorticoids (GCs), are potent anti-inflammatory drugs that act in a similar manner, inhibiting phospholipase A2 and, indirectly, lypooxigenase (LOX), and both COX-1 and COX-2. They are included as the standard treatment for many inflammatory systemic or skin diseases such as arthritis, lupoid syndrome, asthma, and psoriasis, but their use is associated with a wide array of mild to severe adverse effects [7,12].
Dermal inflammation is as a disruptor for many of the skin’s functions, most notably its protective function. Therefore, prevention and cure of cutaneous inflammation should aim at resolving the cause or symptoms without further damaging the skin. For example, prolonged use of topical GCs is marked by iatrogenic reactions such as skin atrophy, defective scarring and an increase in infection risk [13], or may be prone to glucocorticoid resistance [14].
Cutaneous use of NSAIDs may result in skin rashes [11], and one Cochrane review from 2016 found they have limited efficacy in reducing muscular/skeletal pain, with only 10% of the patients receiving topical ketoprofen or diclofenac, the most commonly used topical NSAIDs, reporting better pain relief vs. placebo [15]. Another meta-analysis concluded that even though the classical GI or renal adverse effects may be greatly reduced for topical NSAIDs, the effect on the cardiovascular system should be subject to a more in-depth clinical scrutiny [16].
An alternative path, through the use of medicinal plants, appears to present some advantages over conventional pharmaceuticals, such as a better safety profile and lower costs [17,18]. The natural remedies are characterized by an abundance in bioactive phytochemicals, like polyphenols, carotenoids, flavonoids, lignans, omega-3 fatty acids, stillbenoids or phytosterols [18,19,20], substances possessing anti-inflammatory and antioxidant effects [19,21,22].
One of the plants employed traditionally for a variety of inflammatory and non-inflammatory diseases, is Achillea millefolium L., commonly referred to as yarrow. It is an herbaceous perennial from the Asteraceae family with a rich history of medicinal use in various conditions across the globe (Brazil, Europe, China, India) [23,24,25]. Achillea millefolium contains phytochemicals from a large array of classes, and its volatile oil constituents are for the most part represented by terpenes or other closely related compounds (1,8-cineole, camphor, borneol, thymol, carvacrol, α-pinene, artemisia ketone or sesquiterpene lactones like matricin). Some of these constituents were shown to contribute to its anti-inflammatory effects.
The seeds of Aesculus hippocastanum L., known as horse-chestnut, are used for treating various circulatory or venous conditions, as well as inflammation of the joints or muscles; horse-chestnut seeds contain flavonoids [26], coumarins [27], tannins, sterols and a mixture of saponins, generally known as aescin or escin [28]. It has vasoprotective, antioxidative and free radical scavenging properties, that are well suited for treating acute inflammation [29]. The anti-inflammatory potential of various horse chestnut seed extracts has been already proven in biochemical and animal models [30,31].
Many species of Plantago genus from the Plantaginaceae family, collectively named plantains, proved a valuable source of traditional medicine [32]. They are used internally or externally in many skin afflictions, like wounds or inflammations, for their beneficial properties that are rooted in the phytochemicals they contain, e.g., terpenoids, flavonoids, steroids or polysaccharides [33]. The anti-inflammatory effect of some plantains has been assessed and associated with COX-1, COX-2 and LOX inhibition [34,35]. An aqueous extract of Plantago holosteum was shown to dose-dependently reduce nitric oxide levels [36], and one recent article described two water and ethanol extracts from Plantago major as significantly anti-inflammatory, after an in vitro NF-kB (Nuclear factor-kB) assay [37,38].
Taxodium distichum (L.) Rich. is a coniferous tree in the redwood family (Cupressaceae) known as the bald cypress [39] and used as a natural remedy for the treatment of skin lesions, varicose veins, spasms, inflammation and pain due to rheumatic disorders, to respiratory or GI infectious diseases [40,41]. The volatile oil is considerably high in monoterpenoids, chiefly α-pinene, followed by myrcene, β-pinene, and limonene, and has marked anti-inflammatory and antispasmodic properties [42]. Another, more recent study, employed a form of structure–activity relationship (SAR) online software and successfully predicted the anti-inflammatory characteristic of some aromatic steroids that were identified in Taxodium species [43].
The present study was planned to assess the analgesic and anti-inflammatory potential of a gel containing a mixture of Plantago lanceolata extract, Aesculus hippocastanum extract, Achillea millefolium oil and Taxodium distichum oil, employing kaolin-induced inflammation and thermal stimulus induced pain as animal models.
2. Materials and Methods
2.1. Reagents
All the solvents used were of commercial grade. Carbopol 940, sodium benzoate, and triethanolamine were purchased from Sigma-Aldrich (Sigma-Aldrich, Taufkirchen, Germany). The compounds administered to experimental animals were as follows: urethane, kaolin (Sigma-Aldrich, Taufkirchen, Germany) and diclofenac sodium 5% gel (Hexal AG, Holzkirchen, Germany).
2.2. Plant Material and Extracts Preparation
Achillea millefolium (dry aerial plant harvested during blooming) was purchased from a local store (batch no. 31910952/2019), Plantago lanceolata leaves and Aesculus hippocastanum seeds were provided by Hofigal SA (Romania) and Taxodium distichum fresh female cones were harvested in Bucharest. Achillea millefolium and Plantago lanceolata materials were ground and used in the extraction process. Prior to grinding, the seminal tegument of Aesculus hippocastanum seeds was removed and then the material used to extraction. The fresh cones of Taxodium distichum were maintained in laboratory conditions for 48 h to remove excess moisture, then crushed and used further to obtain the volatile oil. The plant materials’ identities were verified, and voucher specimens were stored at the Pharmaceutical Botany and Cell Biology Department, “Carol Davila” University of Medicine and Pharmacy (voucher specimens no. 19-0702-Achillea millefolium, 18-0601-Plantago lanceolata, 18-0901-Aesculus hippocastanum and 18-1004-Taxodium distichum).
Leaves of Plantago lanceolata (50 g) were ground and extracted with a 10% ethanol in water using Soxhlet method. The obtained extractive solution was further concentrated using a rotary evaporator (RVO 004; Ingos, Prague, Czech Republic) and the concentrate was added in small portions into a flask with acetone. The precipitate was separated by vacuum filtration and dissolved in a small quantity of water. The operation was repeated two times. The resulting aqueous solution was lyophilized at −55 °C (CoolSafe ScanVac 55; LaboGene, Lynge, Denmark). Aesculus hippocastanum extract was obtained by extraction from 50 g of plant material with ethanol in the ratio of 1:10 (m:v) under reflux for 30 min, followed by vacuum filtration, concentration using a rotary evaporator (RVO 004; Ingos, Prague, Czech Republic) and then lyophilized at −55 °C (CoolSafe ScanVac 55; LaboGene, Lynge, Denmark). The volatile oils from Achillea millefolium and Taxodium distichum were obtained by steam distillation of 800 g plant material using a Neo Clevenger apparatus.
2.3. Active Gel Preparation
A 1% carbopol base gel was obtained using carbopol 940, water, sodium benzoate (0.1%), triethanolamine (q.s.), and glycerol (24%). The aqueous phase of the active gel consisted of tween 20, Plantago lanceolata extract, Aesculus hippocastanum extract, and ethanol. All were taken in a beaker and added in the 1% carbopol gel and then the oil phase (Achillea millefolium oil and Taxodium distichum oil) was added to the mixture drop by drop along with continuous stirring using an overhead mechanical stirrer (RZR1, Heidolph, Schwabach, Germany). A blank gel was prepared as a control for the pharmacological experiments containing the carbopol base, tween 20 and ethanol. The quantitative formulas of both gels are presented in Table 1.

Table 1.
Composition of prepared gels.
Physical appearance such as color and overall appearance were checked under normal day light. The pH of all gel formulations was determined using a Hanna HI-98127 pH Tester. The spreadability was determined by the extensometric method.
2.4. Toxicity Assay on Daphnia magna
Daphnia magna Straus were maintained parthenogenetically at “Carol Davila” University of Medicine and Pharmacy. The bioassay was performed on 10 daphnids in polypropylene tissue culture with 12-well plates (Greiner Bio-One) according to the protocol described in our previous studies [44,45,46]. The final volume in each well was 4 mL and the negative control was 1% DMSO. Each component of the active gel was tested at 6 concentration levels ranging from 12.37 to 375 µg/mL for the extracts, and from 2.5 to 100 nL/mL for the volatile oils. A mixture of all plant components was tested at 9 concentrations ranging from 1.25 to 500.5 µg/mL. The composition of the mixture was the same used for active gel preparation. Diclofenac (4.12–125 µg/mL) and α-pinene (2.5–100 nL/mL) were used as positive controls, because α- and β-pinene are the main components of bald cypress and yarrow volatile oils [47,48]. All determinations were performed in duplicate.
The lethality was recorded at 24 and 48 h of exposure and the 50% lethal concentrations (LC50) were estimated by interpolation on lethality curves plotted between average lethality (%) and the logarithm of concentrations using the least square fit method. Using the same method, 95% confidence intervals (CI95%) of LC50 were also calculated. All calculations were performed using GraphPad Prism v 5.1 software.
2.5. Animals
All experimental procedures were performed in accordance with bioethics norms proposed by the WMA Declaration of Helsinki and the NIH Guide for the Care and Use of Laboratory Animals. The experimental protocol (number FFC01/2020) was approved by the Bioethics Commission of the Faculty of Pharmacy, University of Medicine and Pharmacy Carol Davila, Bucharest.
Laboratory animals (NMRI mice and Wistar rats) were purchased from the biobase of the University of Medicine and Pharmacy “Carol Davila”, Bucharest, and were accustomed for two weeks to the new habitat. They were housed 8 animals per cage, in a ventilated cage system, with a bedding of wood sawdust, under controlled light/dark cycle conditions (12 h light/12 h dark; lights on at 6:00 a.m.), with free access to water and food pellets (Cantacuzino Institute, Bucharest). The animals were housed on a light/dark cycle of 12 h, under constant humidity and temperature, monitored with a thermohigrometer. The recorded values were between 35% and 45% for humidity and 20 and 22 °C for temperature. The sample size was calculated using the “resource equation” method, as our purpose was to observe the difference between groups, with E slightly exceeding the higher limit [49]. We took into account the fact that urethane-induced anesthesia used in the inflammation assay has a high degree of variability, with a lethal effect in some cases [50].
2.6. Anti-Inflammatory Effect
The anti-inflammatory activity of the active gel was determined using the plethysmometric method, as described by [31]. Inflammation was induced in 24 male Wistar rats, distributed into 3 equal experimental groups (n = 8). The animals were anesthetized intraperitoneally with a 13% urethane solution, 1300 mg/kg b.w. After general anesthesia induction, the initial hind paw volume was determined with the plethysmometer (Ugo Basile, Gemonio, Italy). Thereafter, 0.2 g of each treatment gel was applied to the surface of the right paw and massaged gently 50 times with the index finger, while the left paw was only massaged (control). The topical gels that were used as treatments are: group I—blank gel (control), group II—diclofenac 5%, group III—plant extracts-based gel (active gel)
Oedema was induced by intraplantar administration of 0.2 mL kaolin 10% suspension in the hind paws of each animal. The initial volume of the paws and the inflamed paws’ volume at 1, 2, 4, 12 and 24 h after kaolin administration were determined.
For the active group the percentage of oedema inhibition was calculated according to the formula:
where Xt represents the mean inflammatory oedema for the active gel group (in mL) and Xc is the mean inflammatory oedema of the control group (in mL).
2.7. Tail-Flick Test
From the three tested hydrogels, the hydrogel with the most pronounced anti-inflammatory effect was selected for further testing of the analgesic effect, using the tail-flick test [51]. A total of 32 NMRI mice were distributed in four equal groups (n = 8). Three repetitive measurements evaluated the baseline latency for each mouse one day before testing the gel formulation. The average was used as the baseline latency for each mouse. A quantity of 0.5 g of the test substances (group 1: control, group 2: base, group 3: diclofenac gel 5% and group 4: plant extracts-based gel) was then applied and massaged 50 times into the distal portion of the tail. Any excess test substance remaining on the tail was removed using a clean cloth.
The antinociceptive response was determined by the tail-flick latency method [52]. One day before administration and on the testing day, following topical administration, the distal mouse tail was exposed to the radiant heat source (infrared radiation). An intensity of 50% was maintained throughout the experiment. The latency time was recorded. A maximum tail-flick latency of 10 s was used to minimize tissue damage to the tail. Each mouse was tested thrice at 60 min and 120 min post-topical administration.
The antinociceptive response is represented as the percentage of analgesia index (%AI) using the following ratio:
2.8. Statistical Analysis
The evaluation of the obtained experimental data was performed using the GraphPad Prism v.5.1 software (GraphPad Software Inc., San Diego, CA, USA). The distribution of the biological responses was tested using the D’Agostino Pearson normality test. Parametric statistical tests were applied using the predefined 95% confidence interval, and the statistical significance threshold was set at 0.05. The experimental results are expressed as arithmetic mean ± standard deviation (SD).
3. Results
3.1. Gel Preparation
The plant material was extracted following the described procedures. The yield of extraction was 9.26% for the Plantago lanceolata leaves and 7.41% for the Aesculus hippocastanum seeds. The yield of extraction was 0.96% for Achillea millefolium oil and 1.17% for Taxodium distichum oil. Achillea millefolium oil presents as a dark blue liquid with distinctive smell and Taxodium distichum oil has a pale-brown color and a distinctive smell. The emulgel was obtained following the described preparation method. It was opaque, dark blue in color, and had a pH of 7.3 ± 0.3 (Hanna HI-98127 pH Tester). The visual inspection revealed a homogeneous product with good spreadability properties.
3.2. Toxicity Assay on Daphnia Magna
The results of LC50 are presented in Table 2. With the exception of Plantago lanceolata extract, all components exhibited moderate to high toxicity. The LC50 of Aesculus hippocastanum extract is comparable with diclofenac at both moments of determination.

Table 2.
Assessment of toxicity on Daphnia magna model.
The volatile components showed high toxicity and, taking into account the values of CI95% at 48 h, the values of LC50 are comparable. Both volatile oils exhibited a high toxicity compared with α-pinene. The high toxicity exhibited on Daphnia magna by the mixture of the components could be explained by the amounts of volatile components which are dissolved in it.
The average lethality in percent values was plotted against the logarithm of tested concentrations (µg/mL) using the least square fit method. Goodness of fit was over 0.8 for all curves, showing a good correlation between concentration and lethality. The lethality curves are presented in Figure 1.
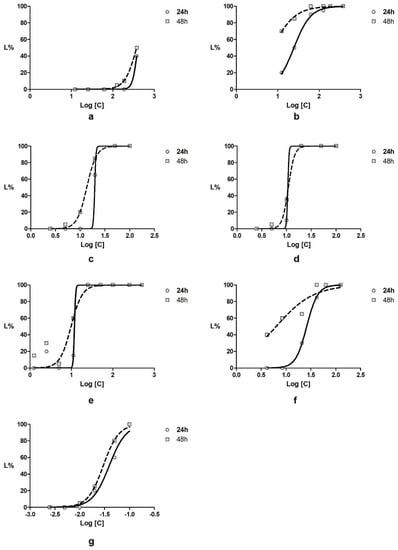
Figure 1.
Daphnia magna lethality curves for (a) Plantago lanceolata extract; (b) Aesculus hippocastanum extract; (c) Achillea millefolium volatile oil; (d), Taxodium distichum volatile oil; (e) mixture of the four active components in ratio of 1.5:0.5:1:1 (g:g:mL:mL); (f) diclofenac and (g) α-pinene.
3.3. Anti-Inflammatory Activity
The mean average volume of the control and treated paws prior to the induction of the oedema and at different intervals of time afterwards are summarized in Table 3.

Table 3.
Average paw volume in the intraplantar kaolin model.
A localized inflammatory reaction as a result of kaolin administration, as observed by the increase in paw volume, started within 1 h after administration and intensified constantly during the observation period of 24 h for the control and all treatment groups. As seen in Figure 2, the percentual inhibition of the inflammatory oedema was highest for diclofenac gel 5%, with a maximal efficacy observed after 12 h (%IIO = 48.70%). A similar efficacy was observed for the active gel (%IIO = 42.01%). Furthermore, for the active gel, the anti-inflammatory effect was observed sooner than for diclofenac (2 vs. 4 h, respectively). No anti-inflammatory activity was observed for the base used for the preparation of hydrogels.
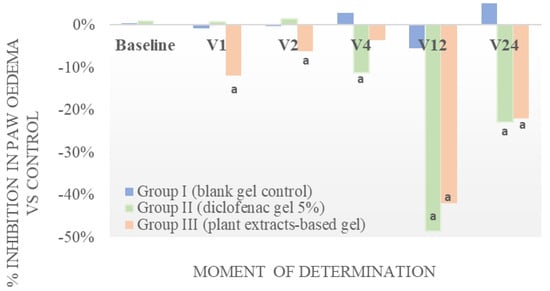
Figure 2.
Evaluation of anti-inflammatory effect of the tested gel on kaolin-induced paw oedema in rats vs. control. a Significantly different from control (all p < 0.01, paired tTest Student, CI = 95%).
3.4. Anti-Nociceptive Efficacy
No significant difference in the reaction time in the tail-flick assay was observed for the control group (no treatment) throughout the testing period. Diclofenac 5% as well as the tested plant-based gel (active gel) produced a significant prolongation of tail-flick latencies at both 60 and 120 min after application, as seen in Table 4. An increase in the tail-flick latency was also observed for the base, maybe due to a barrier effect; however, this effect disappeared 120 min after the application. The antinociception produced by the reference substance and the tested active gel persisted 120 min after the topical administration, even though it was slightly diminished in time.

Table 4.
Tail-flick latency test.
4. Discussion
The phytotherapeutic products were chosen in order to capitalize on native species that can be easily cultivated and to associate active principles with anti-inflammatory action leading to a synergism of action. The composition of the plant mixture was based on the results on Daphnia magna and the literature data on each plant’s anti-inflammatory potential. The ratio of 3 to 1 for the solid extracts was chosen based on the low toxicity of Plantago lanceolata leaves showed by the high values of LC50 compared to the Aesculus hippocastanum seeds extract. The low toxicity of the Plantago lanceolata extract can be explained by the presence of polysaccharides. These phytochemicals have high molecular mass and possess a low absorption rate [32]. Aesculus hippocastanum toxicity could be exhibited by escine and coumarin derivatives which are highly soluble in ethanol, the solvent in which the extract was prepared. Although these compounds exhibit some toxic effects, they have also showed good anti-inflammatory effects. Comparing with diclofenac, a frequently used NSAID drug, Aesculus hippocastanum extract has a similar toxicity, but its concentration in the active gel formulation is lower. Both volatile components are complex mixtures of highly active phytochemicals, and previous studies demonstrated that Daphnia magna is highly sensitive to similar essential oils [53,54]. Achillea millefolium oil main components are chamazulene, pinene, cineol and borneol, whereas in T. distichum oil the main terpenes are α-pinene, myrcene and thujone [55]. Although found in small quantities, they are potent and could induce toxicity on Daphnia magna. Moreover, they have showed several pharmacological activities, including anti-inflammatory effects [56]. Overall, the mixture of the active components has a LC50 dose (after 24 h) almost half of that of diclofenac.
Pain and inflammation are the main symptoms in a great variety of pathological states, both acute, e.g., trauma due to sprains, strains and bruises, as well as chronical, such as chronic venous insufficiency, osteoarthritis of superficial joints and localized forms of soft tissue rheumatism. The aim of this research was to investigate the anti-inflammatory and analgesic properties of a multi-component emulgel in order to identify an effective natural alternative for the treatment of the above-mentioned symptoms.
The kaolin oedema model was employed to assess the anti-inflammatory potential of the active gel. Our tested gel showed an anti-inflammatory profile similar to diclofenac. Furthermore, the short latency of the active gel may be a consequence of the essential oils contained in the formulation, which significantly improve the skin penetration of active substances. Kaolin is an aluminosilicate with a strong, long-lasting inflammatory effect mediated by prostaglandins, not by histamine or by serotonin [57], suggesting that the tested gel interferes with prostaglandin activity.
The tail-flick test is a thermal latency assay that has been widely used to assess the anti-nociceptive effect of various drugs in rodents [58]. The radiant tail-flick model involves nociceptive processing at spinal circuits with supraspinal modulation; however, several authors reported that NSAIDs applied topically increase the tail-flick latency, in a dose-dependent manner [59]. Our results also indicate that topical diclofenac and the new plant-based active gel led to a significant prolongation of tail-flick latencies at 60 and 120 min after administration. These results demonstrate that the gel formulation possesses significant antiedematogenic and analgesic activities after topical application. These results are in accordance with data from the literature. Several authors reported that hydroalcoholic extracts obtained from several Plantago species possess significant antiedematogenic, analgesic and wound healing activities, both after systemic administration [60,61] as well as after topical application [62,63]. The anti-inflammatory and antinociceptive effect was considered to be at least partially mediated by flavonoids such as baicalein and hispidulin and iridoid glycosides such as aucubin [32], due to an inhibition of LOX and COX-1 and by reducing the levels of proinflammatory cytokines [64]. An anti-inflammatory effect almost equal to that of naproxen was also reported for alcoholic and glycerolic Achillea millefolium extracts [23], while the volatile oil was shown to strongly inhibit the nitric oxide production in lipopolysaccharide-treated macrophages [65,66]. In a recent comprehensive study, using supercritical anti-solvent fractionation, Villava et al. observed that the fractions most abundant in the usual volatile components were the ones that best reduced the amount of secreted TNF-α [67].
Taking into account that the gel is to be applied on injured or inflamed skin, which favors absorption in the systemic circulation, the lack of tolerability data could be considered a limitation of this study. However, these compounds are highly used in traditional medicine and the literature abounds with safety data for each extract and volatile oil within the composition of the gel, demonstrating a high safety profile in the case of application on inflamed skin. Another aspect to be considered in the future is employing several concentrations of volatile oils in the hydrogel, in order to determine the product with the best anti-inflammatory profile.
5. Conclusions
The experimental results obtained in the kaolin-induced inflammation model and in thermal stimulus-induced pain indicate that the tested gel possesses an anti-inflammatory and analgesic effect, similar to that of diclofenac, one of the most used topical NSAIDs. The high efficacy of the tested gel may be partly explained by the various mixture of active constituents, already reported in the literature to have a significant antiedematogenic and analgesic effect, such as flavonoids, steroids or polysaccharides, monoterpenoides, as well as that of other components who need further study. Overall, our results suggest that the tested gel could be a valuable tool in treating the inflammatory reaction seen in both chronic and sub-acute pain syndromes.
Author Contributions
Conceptualization, G.M.N. and O.T.O.; Data curation, S.N.; Funding acquisition, G.M.N.; Investigation, A.Z., G.N., G.M.N., G.S., D.R., C.T., S.N. and O.T.O.; Methodology, A.Z., G.N., S.N. and O.T.O.; Project administration, O.T.O.; Writing—original draft, A.Z., G.N., G.M.N., G.S., S.N. and O.T.O.; Writing—review & editing, G.M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ji, R.; Chamessian, A.; Zhang, Y. Pain regulation by non-neuronal cells and inflammation. Pain Res. 2016, 354, 572–577. [Google Scholar] [CrossRef]
- Ambriz-Perez, D.L.; Leyva-Lopez, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar]
- Miyasaka, M.; Takatsu, K. Chronic Neuroinflammation Mechanism and Regulation; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Ashley, N.T.; Weil, Z.M.; Nelson, R.J. Inflammation: Mechanisms, Costs, and Natural Variation. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 385–406. [Google Scholar] [CrossRef]
- Duthie, G.G.; Wood, A.D. Natural salicylates: Foods, functions and disease prevention. Food Funct. 2011, 2, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Desborough, M.J.R.; Keeling, D.M. The aspirin story–from willow to wonder drug. Br. J. Haematol. 2017, 177, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, R.; Jialal, I. Chronic Inflammation-StatPearls-NCBI Bookshelf. In Stat Pearls. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493173/ (accessed on 24 May 2020).
- Persson, M.S.M.; Stocks, J.; Walsh, D.A.; Doherty, M.; Zhang, W. The relative efficacy of topical non-steroidal anti-inflammatory drugs and capsaicin in osteoarthritis: A network meta-analysis of randomised controlled trials. Osteoarthr. Cartil. 2018, 26, 1575–1582. [Google Scholar] [CrossRef]
- Brune, K.; Patrignani, P. New insights into the use of currently available non-steroidal anti-inflammatory drugs. J. Pain Res. 2015, 8, 105–118. [Google Scholar] [CrossRef]
- Green, G.A. Understanding NSAIDs: From aspirin to COX-2. Clin. Cornerstone 2001, 3, 50–58. [Google Scholar] [CrossRef]
- Harirforoosh, S.; Asghar, W.; Jamali, F. Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J. Pharm. Pharm. Sci. 2013, 16, 821–847. [Google Scholar] [CrossRef]
- Sevilla, L.M.; Pérez, P. Roles of the glucocorticoid and mineralocorticoid receptors in skin pathophysiology. Int. J. Mol. Sci. 2018, 19, 1906. [Google Scholar] [CrossRef]
- Landriscina, A.; Rosen, J.; Friedman, A.J. Nanotechnology, inflammation and the skin barrier: Innovative approaches for skin health and cosmesis. Cosmetics 2015, 2, 177–186. [Google Scholar] [CrossRef]
- Barnes, P.J.; Adcock, I.M. Glucocorticoid resistance in inflammatory diseases. Lancet 2009, 373, 1905–1917. [Google Scholar] [CrossRef]
- Derry, S.; Conaghan, P.; Jap, D.S.; Pj, W.; Ra, M. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst. Rev. 2016, 2016, CD007400. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Wei, J.; Persson, M.S.M.; Sarmanova, A.; Doherty, M.; Xie, D.; Wang, Y.; Li, X.; Li, J.; Long, H.; et al. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: A systematic review and network meta-analysis of randomised controlled trials and observational studies. Br. J. Sports Med. 2018, 52, 642–650. [Google Scholar] [CrossRef]
- Kumar, A.H.S. Rediscovering the Drug Discovery with Natural Products as Therapeutic Tools. J. Nat. Sci. Biol. Med. 2018, 9, 1. [Google Scholar] [CrossRef]
- McClements, D.J. Future Foods; Springer Nature, 2019; Available online: https://www.springer.com/gp/book/9783030129941 (accessed on 24 May 2020).
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.; Lightfoot, D. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Xiao, J.; Bai, W. Bioactive phytochemicals. Crit. Rev. Food Sci. Nutr. 2019, 59, 827–829. [Google Scholar] [CrossRef]
- Daliu, P.; Santini, A.; Novellino, E. From pharmaceuticals to nutraceuticals: Bridging disease prevention and management. Expert Rev. Clin. Pharmacol. 2019, 12, 1–7. [Google Scholar] [CrossRef]
- Howes, M.J.R.; Perry, N.S.L.; Vásquez-Londoño, C.; Perry, E.K. Role of phytochemicals as nutraceuticals for cognitive functions affected in ageing. Br. J. Pharmacol. 2019, 177, 1294–1315. [Google Scholar] [CrossRef]
- Applequist, W.L.; Moerman, D.E. Yarrow (Achillea millefolium L.): A neglected panacea? A review of ethnobotany. Econ. Bot. 2011, 65, 209–225. [Google Scholar] [CrossRef]
- Ali, S.I.; Gopalakrishnan, B.; Venkatesalu, V. Pharmacognosy, Phytochemistry and Pharmacological Properties of Achillea millefolium L.: A Review. Phyther. Res. 2017, 31, 1140–1161. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Peethambaran, B. Anti-Inflammatory and Anti-Microbial Properties of Achillea Millefolium in Acne Treatment; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Wilkinson, J.A.; Brown, A.M.G. Horse Chestnut-Aesculus Hippocastanum: Potential Applications in Cosmetic Skin-care Products. Int. J. Cosmet. Sci. 1999, 21, 437–447. [Google Scholar] [CrossRef]
- Küçükkurt, I.; Ince, S.; Keleş, H.; Küpeli Akkol, E.; Avcı, G.; Yeşilada, E.; Bacak, E. Beneficial effects of Aesculus hippocastanum L. seed extract on the body’s own antioxidant defense system on subacute administration. J. Ethnopharmacol. 2010, 129, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Varinská, L.; Fáber, L.; Kello, M.; Petrovová, E.; Balážová, L.; Solár, P.; Coma, M.; Urdzík, P.; Mojžiš, J.; Vajdlenka, E.; et al. β-escin effectively modulates HUVECs proliferation and tube formation. Molecules 2018, 23, 197. [Google Scholar] [CrossRef] [PubMed]
- Foca, G.; Ulrici, A.; Cocchi, M.; Durante, C.; Vigni, M.L.; Marchetti, A.; Sighinolfi, S.; Tassi, L. Seeds of Horse Chestnut (Aesculus hippocastanum L.) and Their Possible Utilization for Human Consumption. Nuts Seeds Heal. Dis. Prev. 2011. [Google Scholar] [CrossRef]
- Margină, D.; Olaru, O.T.; Ilie, M.; Grădinaru, D.; Guțu, C.; Voicu, S.; Dinischiotu, A.; Spandidos, D.A.; Tsatsakis, A.M. Assessment of the potential health benefits of certain total extracts from Vitis vinifera, Aesculus hyppocastanum and Curcuma longa. Exp. Ther. Med. 2015, 10, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Mihai, P.D.; Seremet, C.O.; Nitulescu, G.; Ivopol, M.; Sevastre, A.-S.; Negres, S.; Ivopol, G.; Nitulescu, M.G.; Olaru, T.O. Evaluation of Natural Extracts in Animal Models of Pain and Inflammation for a Potential Therapy of Hemorrhoidal Disease. Sci. Pharm. 2019, 87, 14. [Google Scholar] [CrossRef]
- Samuelsen, A.B. The traditional uses, chemical constituents and biological activities of Plantago major L. A review. J. Ethnopharmacol. 2000, 71, 1–21. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Beara, I.N.; Lesjak, M.M.; Orčić, D.Z.; Simin, N.D.; Četojević-Simin, D.D.; Božin, B.N.; Mimica-Dukić, N.M. Comparative analysis of phenolic profile, antioxidant, anti-inflammatory and cytotoxic activity of two closely-related Plantain species: Plantago altissima L. and Plantago lanceolata L. LWT Food Sci. Technol. 2012, 47, 64–70. [Google Scholar] [CrossRef]
- Najafian, Y.; Hamedi, S.S.; Kaboli Farshchi, M.; Feyzabadi, Z. Plantago major in Traditional Persian Medicine and modern phytotherapy: A narrative review. Electron. Physician 2018, 10, 6390–6399. [Google Scholar] [CrossRef] [PubMed]
- Genc, Y.; Harput, U.S.; Saracoglu, I. Cytotoxic and Antiinflammatory Activity Guided Studies on Plantago holosteum Scop. Proceedings 2017, 1, 997. [Google Scholar] [CrossRef]
- Zubair, M.; Widén, C.; Renvert, S.; Rumpunen, K. Water and ethanol extracts of Plantago major leaves show anti-inflammatory activity on oral epithelial cells. J. Tradit. Complement. Med. 2019, 9, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Thummasuwan, S.; Sehgal, S.K.; Chouvarine, P.; Peterson, D.G. Characterization of the genome of bald cypress. BMC Genom. 2011, 12, 553. [Google Scholar] [CrossRef]
- Cortés-Arroyo, A.R.; Domínguez-Ramírez, A.M.; Gómez-Hernández, M.; López, J.R.M.; de la Peña, M.H.; López-Muñoz, F.J. Antispasmodic and bronchodilator activities of Taxodium mucronatum ten leaf extract. Afr. J. Biotechnol. 2011, 10, 54–64. [Google Scholar]
- Li, S. Ethnobotany, Phytochemistry, and Biological Activities of Taxodium Rich. Pharm. Crop. 2013, 4, 1–14. [Google Scholar] [CrossRef][Green Version]
- El Tantawy, M.E.; El Sakhawy, F.S.; El Sohly, M.A.; Ross, S.A. Chemical composition and biological activity of the essential oil of the fruit of taxodium distichum L. Rich growing in egypt. J. Essent. Oil Res. 1999, 11, 386–392. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Savidov, N.; Poroikov, V.V.; Gloriozova, T.A.; Imbs, A.B. Naturally occurring aromatic steroids and their biological activities. Appl. Microbiol. Biotechnol. 2018, 102, 4663–4674. [Google Scholar] [CrossRef]
- Nitulescu, G.; Mihai, D.P.; Nicorescu, I.M.; Olaru, O.T.; Ungurianu, A.; Zanfirescu, A.; Nitulescu, G.M.; Margina, D. Discovery of natural naphthoquinones as sortase A inhibitors and potential anti-infective solutions against Staphylococcus aureus. Drug Dev. Res. 2019, 80, 1136–1145. [Google Scholar] [CrossRef]
- Seremet, O.C.; Olaru, O.T.; Gutu, C.M.; Nitulescu, G.M.; Ilie, M.; Negres, S.; Zbarcea, C.E.; Purdel, C.N.; Spandidos, D.A.; Tsatsakis, A.M.; et al. Toxicity of plant extracts containing pyrrolizidine alkaloids using alternative invertebrate models. Mol. Med. Rep. 2018, 17, 7757–7763. [Google Scholar] [CrossRef]
- Nitulescu, G.; Nicorescu, I.; Olaru, O.; Ungurianu, A.; Mihai, D.; Zanfirescu, A.; Nitulescu, G.; Margina, D. Molecular Docking and Screening Studies of New Natural Sortase A Inhibitors. Int. J. Mol. Sci. 2017, 18, 2217. [Google Scholar] [CrossRef] [PubMed]
- Candan, F.; Unlu, M.; Tepe, B.; Daferera, D.; Polissiou, M.; Sökmen, A.; Akpulat, H.A. Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan. (Asteraceae). J. Ethnopharmacol. 2003, 87, 215–220. [Google Scholar] [CrossRef]
- Ogunwande, I.A.; Olawore, N.O.; Ogunmola, O.O.; Walker, T.M.; Schmidt, J.M.; Setzer, W.N. Cytotoxic effects of Taxodium distichum oils. Pharm. Biol. 2007, 45, 106–110. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Koblin, D.D. Urethane: Help or hindrance? Anesth. Analg. 2002, 94, 241–242. [Google Scholar]
- Ukrainets, V.I.; Petrushova, A.L.; Fedosov, I.A.; Voloshchuk, I.N.; Bondarenko, S.P.; Shishkina, V.S.; Sidorenko, V.L.; Sim, G. Crystal Habits and Biological Properties of N-(4-Trifluoromethylphenyl)-4-Hydroxy-2,2-Dioxo-1H-2λ6,1-Benzothiazine-3-Carboxamide. Sci. Pharm. 2020, 88, 1. [Google Scholar] [CrossRef]
- Benbow, T.; Campbell, J. Comparison of the Topical Analgesic Effects of a Novel Diclofenac Microemulsion to a Marketed Diclofenac Macroemulsion Formulation in Rats Using the Tail Flick Test. J. Dev. Drugs 2018, 7, 1–6. [Google Scholar]
- Seo, S.M.; Park, H.M.; Park, I.K. Larvicidal activity of ajowan (Trachyspermum ammi) and Peru balsam (Myroxylon pereira) oils and blends of their constituents against mosquito, Aedes aegypti, acute toxicity on water flea, Daphnia magna, and aqueous residue. J. Agric. Food Chem. 2012, 60, 5909–5914. [Google Scholar] [CrossRef]
- Park, H.-M.; Kim, J.; Chang, K.-S.; Kim, B.-S.; Yang, Y.-J.; Kim, G.-H.; Shin, S.-C.; Park, I.-K. Larvicidal Activity of Myrtaceae Essential Oils and Their Components Against Aedes aegypti, Acute Toxicity on Daphnia magna, and Aqueous Residue. J. Med. Entomol. 2011, 48, 405–410. [Google Scholar] [CrossRef]
- Smelcerovic, A.; Lamshoeft, M.; Radulovic, N.; Ilic, D.; Palic, R. LC-MS analysis of the essential oils of Achillea millefolium and Achillea crithmifolia. Chromatographia 2010, 71, 113–116. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ribeiro, M.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Anti-inflammatory and Chondroprotective Activity of ( + )-α-Pinene: Structural and Enantiomeric Selectivity. J. Nat. Prod. 2014, 77, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Paun, G.; Neagu, E.; Moroeanu, V.; Albu, C.; Ursu, T.M.; Zanfirescu, A.; Negres, S.; Chirita, C.; Radu, G.L. Anti-inflammatory and antioxidant activities of the Impatiens noli-tangere and Stachys officinalis polyphenolic-rich extracts. Braz. J. Pharmacogn. 2018, 28, 57–64. [Google Scholar] [CrossRef]
- Gades, N.M.; Danneman, P.J.; Wixson, S.K.; Tolley, E.A. The Magnitude and Duration of the Analgesic Effect of Morphine, Butorphanol, and Buprenorphine in Rats and Mice. Contemp. Top. Lab. Anim. Sci. 2000, 39, 8–13. [Google Scholar] [PubMed]
- Arrau, S.; Delporte, C.; Cartagena, C.; Rodríguez-Díaz, M.; González, P.; Silva, X.; Cassels, B.K.; Miranda, H.F. Antinociceptive activity of Quillaja saponaria Mol. saponin extract, quillaic acid and derivatives in mice. J. Ethnopharmacol. 2011, 133, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Núñez Guillén, M.E.; Da Silva Emim, J.A.; Souccar, C.; Lapa, A.J. Analgesic and antiinflammatory activities of the aqueous extract of Plantago major L. Pharm. Biol. 1997, 35, 99–104. [Google Scholar]
- Türel, I.; Özbek, H.; Erten, R.; Öner, A.C.; Cengiz, N.; Yilmaz, O. Hepatoprotective and anti-inflammatory activities of Plantago major L. Indian J. Pharmacol. 2009, 41, 120–124. [Google Scholar]
- Palmeiro, N.S.; Esteves Almeida, C.; Ghedini, P.C.; Goulart, L.S.; Baldisserotto, B. Analgesic and anti-inflammatory properties of Plantago australis hydroalcoholic extract. Acta Farm. Bonaer. 2002, 21, 89–92. [Google Scholar]
- Oloumi, M.M.; Vosough, D.; Derakhshanfar, A.; Nematollahi, M.H. The Healing Potential of Plantago lanceolata Ointment on Collagenase-Induced Tendinitis in Burros (Equus asinus). J. Equine Vet. Sci. 2011, 31, 470–474. [Google Scholar] [CrossRef]
- Hussan, F.; Mansor, A.S.; Hassan, S.N.; Kamaruddin, T.N.E.; Budin, S.B.; Othman, F. Anti-Inflammatory Property of Plantago major Leaf Extract Reduces the Inflammatory Reaction in Experimental Acetaminophen-Induced Liver Injury. Evid. Based Complement. Altern. Med. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Kazemi, M. Chemical composition and antimicrobial, antioxidant activities and anti-inflammatory potential of Achillea millefolium L., Anethum graveolens L., and Carum copticum L. essential oils. J. Herb. Med. 2015, 5, 217–222. [Google Scholar] [CrossRef]
- Abdossi, V.; Kazemi, M. Bioactivities of Achillea millefolium essential oil and its main terpenes from Iran. Int. J. Food Prop. 2015, 19, 1798–1808. [Google Scholar] [CrossRef]
- Villalva, M.; Jaime, L.; Villanueva-Bermejo, D.; Lara, B.; Fornari, T.; Reglero, G.; Santoyo, S. Supercritical anti-solvent fractionation for improving antioxidant and anti-inflammatory activities of an Achillea millefolium L. extract. Food Res. Int. 2019, 115, 128–134. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).