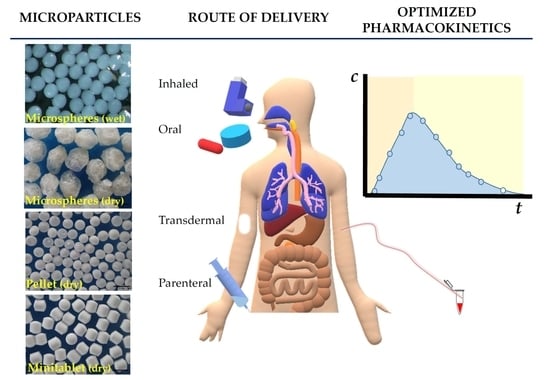
Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery
Abstract
1. Introduction
- -
- choice of dosage form for the desired drug delivery route (peroral tablets, parenteral injections);
- -
- modified and targeted (even site-specific) drug release and delivery;
- -
- more expectable pharmacokinetics with reduced intra- or inter-subject variability;
- -
- more homogenous distribution in the physiological environment;
- -
- stable fixed-dose combinations of drugs;
- -
- dose titration and less dose-dumping;
- -
- patient centricity through better compliance (e.g., patients with dysphagia) and adherence;
- -
- individual therapy (e.g., for pediatric or geriatric population);
- -
- improving stability of the medicinal preparations;
- -
- isolating the constituents to ensure better compatibility;
- -
- innovative products with a prolonged life cycle through patent protection.
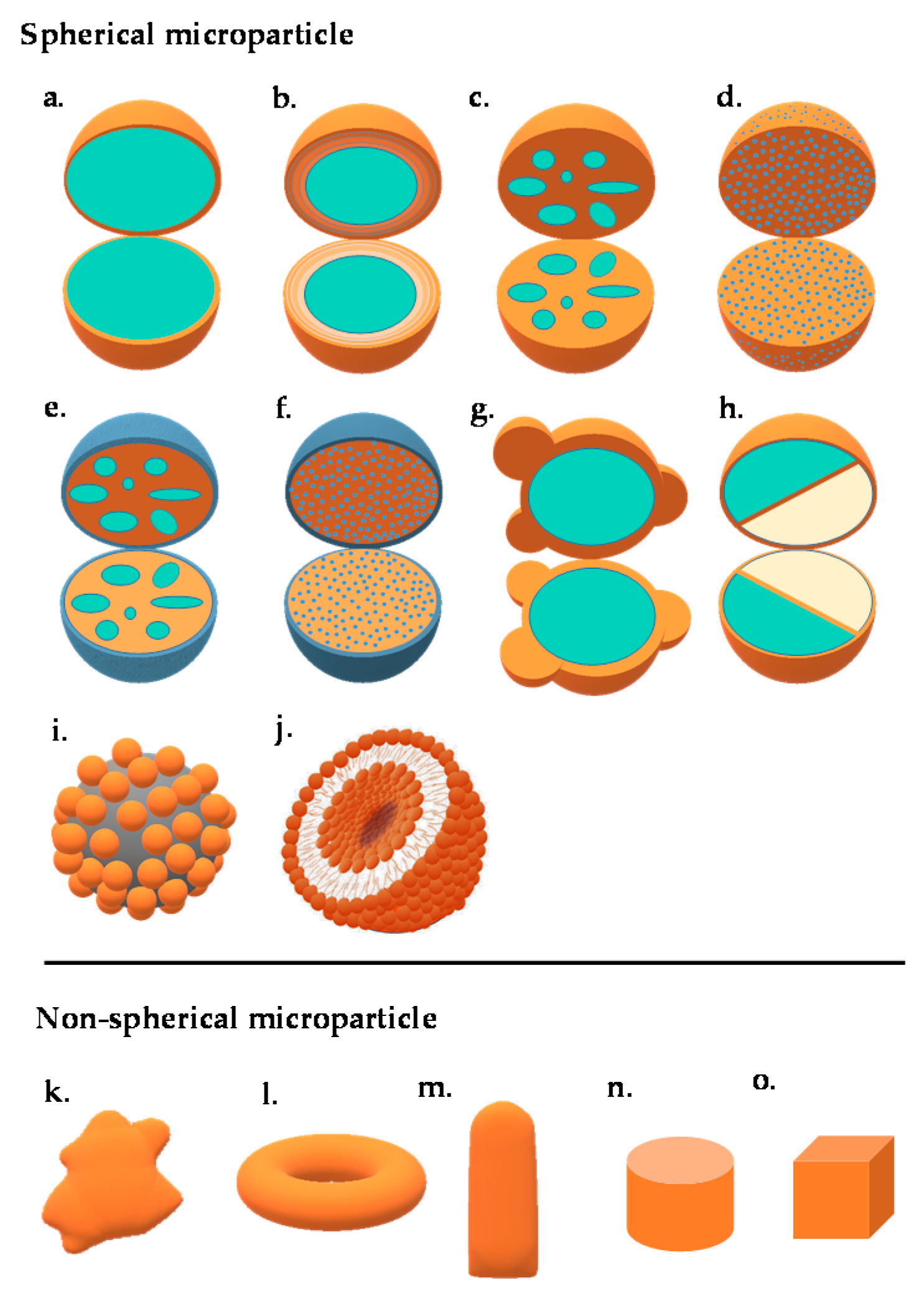
2. Construction and Structure
2.1. Microspheres and Microcapsules
2.1.1. Janus Particles
2.1.2. Patchy Particles
2.2. Liposomes
2.3. Colloidosomes
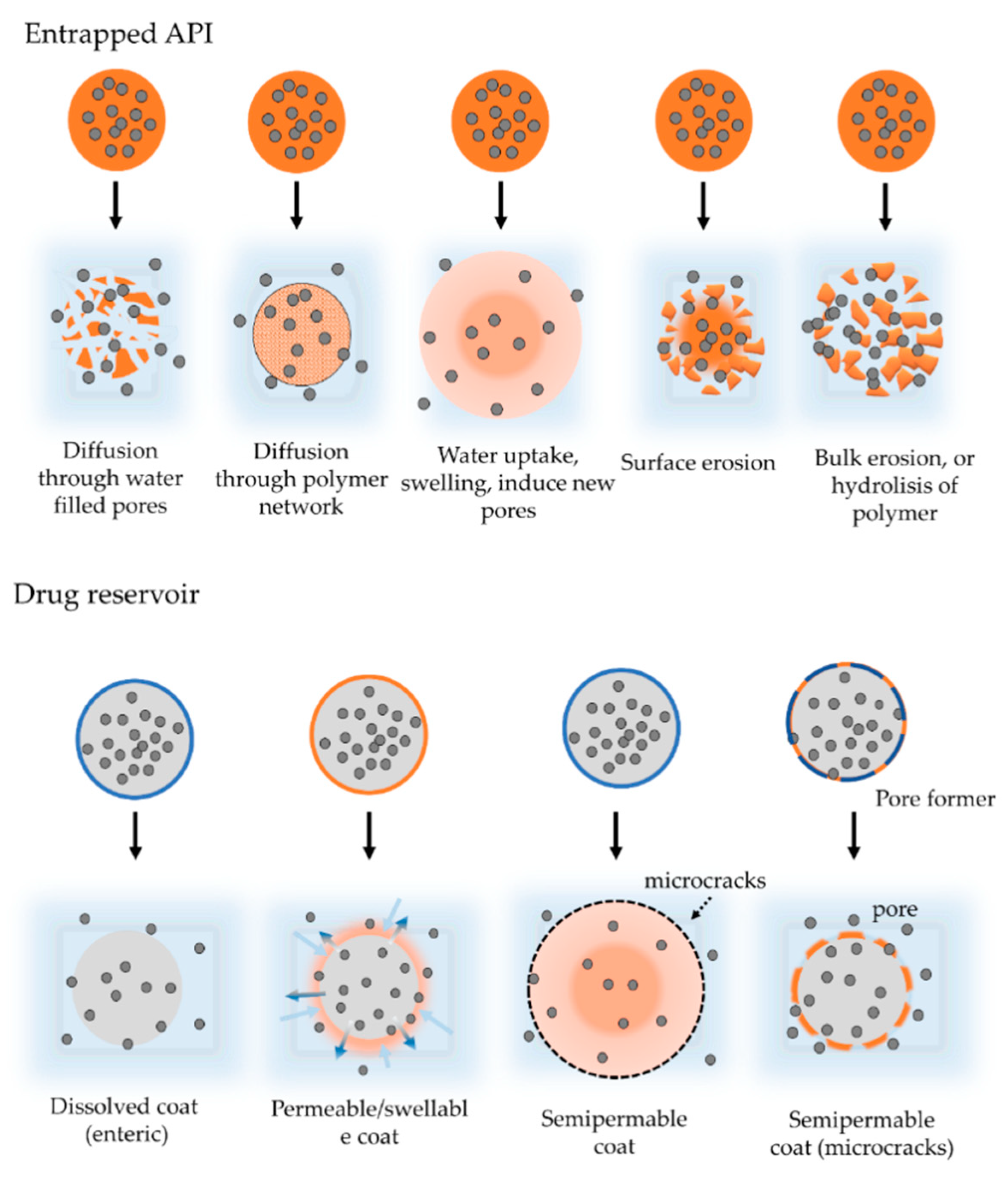
3. Types and Mechanism of Drug Release
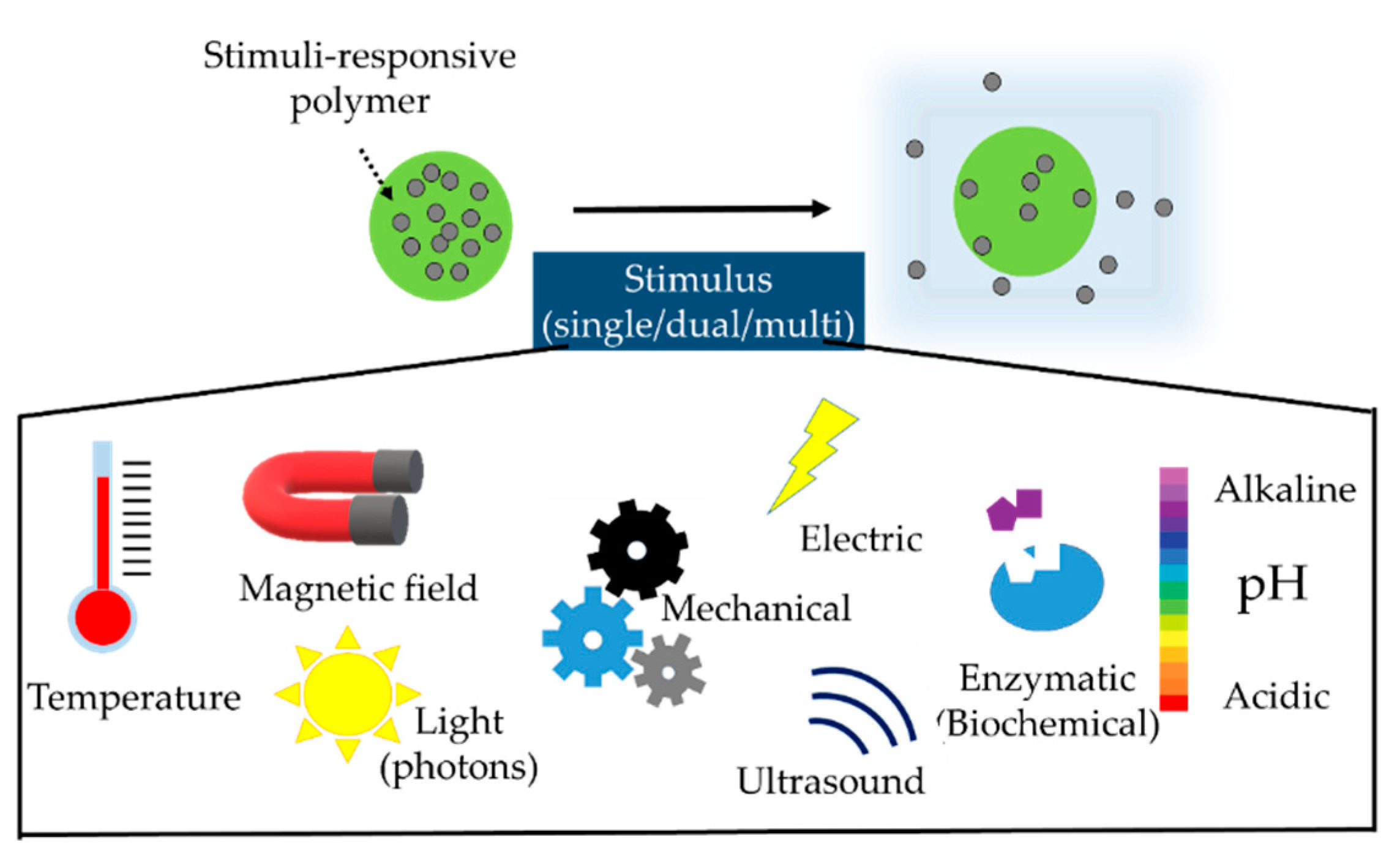
Magnetic Microcapsules
4. Formulation and Manufacturing
4.1. Composition and Excipients
4.2. Processes for the Particle Formation
4.2.1. Coacervation
4.2.2. Air Suspension Method/Fluid-Bed Coating
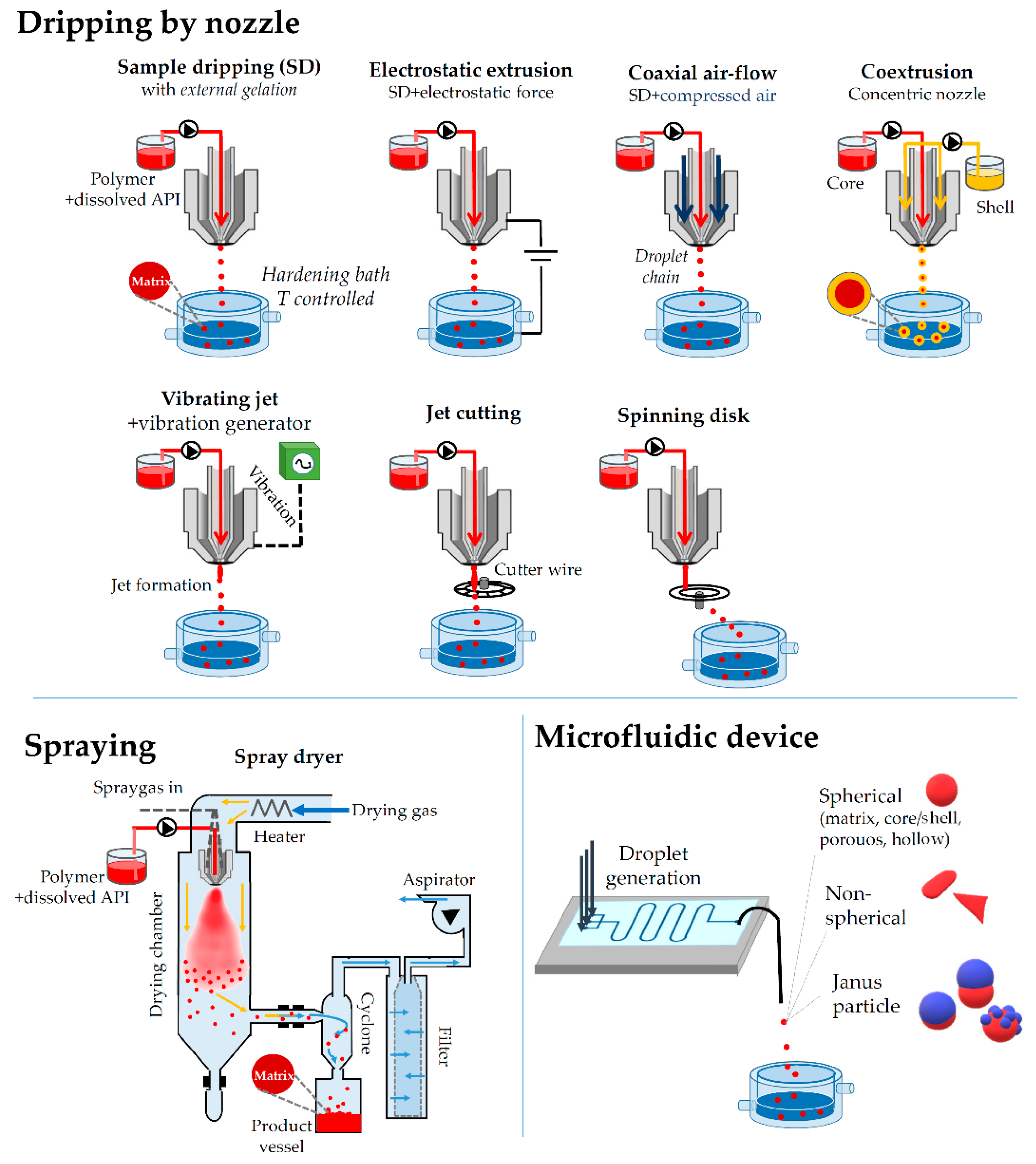
4.2.3. Extrusion through a Nozzle
4.2.4. Vibrational Jet/Electrostatic Extrusion
4.2.5. Spinning Disk and Cutting Wire
4.2.6. Spray Drying
4.2.7. Supercritical Fluid Precipitation
4.2.8. Freeze-Drying
4.2.9. Microfluidics in Microparticle Fabrication
Microfluidic Droplet Generators (MFDG)
4.2.10. Lithography
5. Characterization
5.1. Morphology
5.1.1. Particle Size Analysis
5.1.2. AFM (Atomic Force Microscopy)
5.1.3. Coulter Counter
5.1.4. Image Analysis
5.2. Physicochemical Properties
Zeta-Potential Analysis
5.3. Physical Properties
5.3.1. Density
5.3.2. Porosity
5.3.3. Microcomputed Tomography
5.3.4. Flowability and Compressibility Studies
5.3.5. Mechanical Test
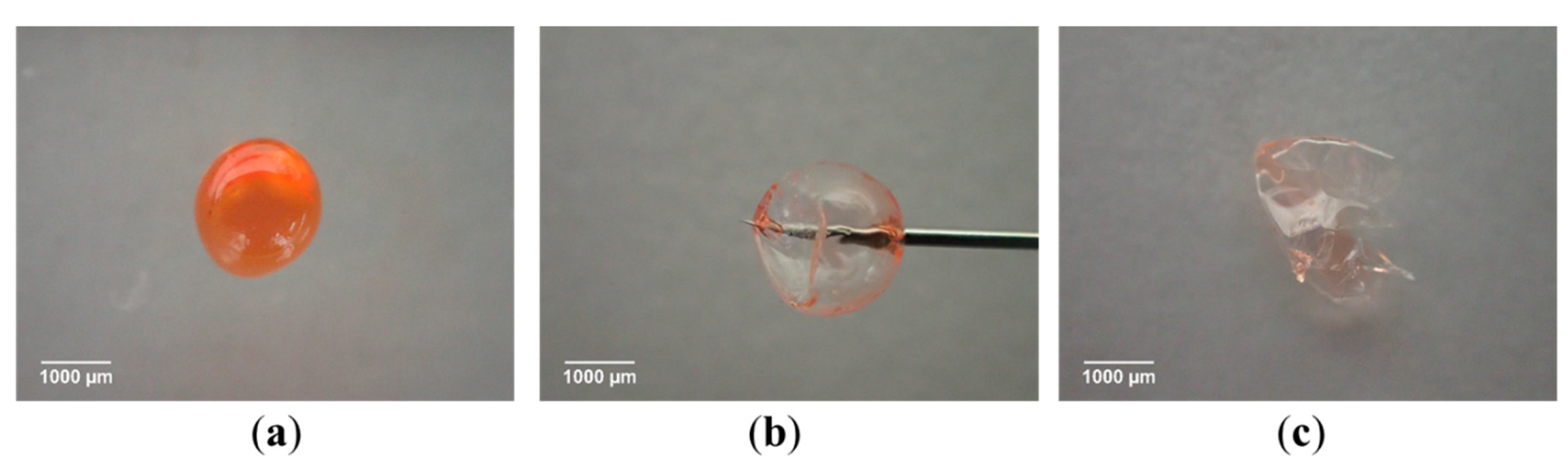
5.3.6. Swelling
5.3.7. Wetting Property
5.4. Drug Entrapment Efficiency
5.5. Drug Release
5.5.1. In Vitro Dissolution Test for Multiparticulates
5.5.2. Dissolution Test for Inhaled Particles
5.5.3. In Vitro Performance of Intramuscular Injections
5.5.4. In Vitro Dissolution Test for Topical Microsponge Preparations
6. Other Studies
6.1. Spectrometry
6.2. Thermoanalytical Methods
6.3. Biocompatibility
7. Applications in the Therapeutical Practice
7.1. Insulin for Inhalation (Technosphere®)
7.2. Depocyte® (Parenteral Suspension)
7.3. DepoDur™
7.4. Microsponge Delivery System (MDS)
7.5. Microbubbles
8. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bale, S.; Khurana, A.; Reddy, A.S.S.; Singh, M.; Godugu, C. Overview on Therapeutic Applications of Microparticle Drug Delivery Overview on Therapeutic Applications of Microparticulate Drug Delivery Systems. Crit. Rev. Ther. Drug Carr. Syst. 2016, 4, 309–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.H.; Longquin Hu, T.J.S. Drug Delivery to the Lymphatic System. In Drug Delivery Principles and Applications; Wang, B., Longquin Hu, T.J.S., Eds.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2016; p. 509. ISBN 9781118833230. [Google Scholar]
- Whelehan, M.; Marison, I.W. Microencapsulation using vibrating technology. J. Microencapsul. 2011, 28, 669–688. [Google Scholar] [CrossRef] [PubMed]
- Peanparkdee, M.; Iwamoto, S.; Yamauchi, R. Microencapsulation: A Review of Applications in the Food and Pharmaceutical Industries. Rev. Agric. Sci. 2016, 4, 56–65. [Google Scholar] [CrossRef]
- Desai, T.; Shea, L.D. Advances in islet encapsulation technologies. Nat. Publ. Gr. 2016, 16, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Sanchez, L.; Gao, Y.; Yu, Y. Janus particles for biological imaging and sensing. Analyst 2016, 141, 3526–3539. [Google Scholar] [CrossRef] [PubMed]
- Acter, S.; Cho, J.; Kim, J.W.; Byun, A.; Park, K.H.; Kim, J.W. Synthesis and shape control of uniform polymer microparticles by tailored adsorption of poly(ethylene oxide)-b-poly(ε-caprolactone) copolymer. Bull. Korean Chem. Soc. 2015, 36, 1467–1473. [Google Scholar] [CrossRef]
- Gong, Z.; Hueckel, T.; Yi, G.R.; Sacanna, S. Patchy particles made by colloidal fusion. Nature 2017, 550, 234–238. [Google Scholar] [CrossRef]
- Choi, K.; Salehizadeh, M.; Da Silva, R.B.; Hakimi, N.; Diller, E.; Hwang, D.K. 3D shape evolution of microparticles and 3D enabled applications using non-uniform UV flow lithography (NUFL). Soft Matter 2017, 13, 7255–7263. [Google Scholar] [CrossRef]
- Karami, N.; Moghimipour, E.; Salimi, A. Liposomes as a Novel Drug Delivery System: Fundamental and Pharmaceutical Application. Asian J. Pharm. 2018, 12, S31–S41. [Google Scholar]
- Rosenberg, R.T.; Dan, N.R. Diffusion through colloidosome shells. J. Colloid Interface Sci. 2011, 354, 478–482. [Google Scholar] [CrossRef]
- Yuan, Q.; Cayre, O.J.; Fujii, S.; Armes, S.P.; Williams, R.A.; Biggs, S. Responsive core-shell latex particles as colloidosome microcapsule membranes. Langmuir 2010, 26, 18408–18414. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, A.G.; Ozturk, S.S.; Palsson, B.O.; Wheatley, T.A.; Dressman, J.B. Mechanism of release from pellets coated with an ethylcellulose-based film. J. Control. Release 1990, 14, 203–213. [Google Scholar] [CrossRef]
- Tang, E.S.K.; Chan, L.W.; Heng, P.W.S. Coating of multiparticulates for sustained release. Am. J. Drug Deliv. 2005, 3, 17–28. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Jani, G.K.; Kapadia, J.R. Current knowledge on biodegradable microspheres in drug delivery. Expert Opin. Drug Deliv. 2015, 12, 1283–1299. [Google Scholar] [CrossRef] [PubMed]
- Doty, A.C. Mechanistic Analysis of In Vivo and In Vitro Drug Release From PLGA Microspheres. Ph.D. Thesis, University of Michigan, Ann Arbor, ML, USA, 2015. [Google Scholar]
- Bennet, D.; Kim, S.E.I. Application of Nanotechnology in Drug Delivery. In Polymer Nanoparticles for Smart Drug Delivery; Sezer, A.D., Ed.; InTechOpen: London, UK, 2014; ISBN 978-953-51-1628-8 b. [Google Scholar]
- Senyei, A.; Widder, K.; Czerlinski, G. Magnetic guidance of drug-carrying microspheres. J. Appl. Phys. 1978, 49, 3578–3583. [Google Scholar] [CrossRef]
- Farah, F.H. Magnetic Microspheres: A Novel Drug Delivery System. World, J. Pharm. Pharm. Sci. 2017, 3, 93–112. [Google Scholar] [CrossRef][Green Version]
- Foox, M.; Zilberman, M. Drug delivery from gelatin-based systems. Expert Opin. Drug Deliv. 2015, 12, 1547–1563. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.; Quinn, M. Handbook of Pharmaceutical Excipients, 7th ed.; Rowe, R.C., Sheskey, S.J., Cook, W.G.F.M., Eds.; Pharmaceutical Press: London, UK, 2009; ISBN 978-0857110275. [Google Scholar]
- Inada, A.; Oue, T.; Yamashita, S.; Yamasaki, M.; Oshima, T.; Matsuyama, H. Development of highly water-dispersible complexes between coenzyme Q10 and protein hydrolysates. Eur. J. Pharm. Sci. 2019, 136. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Kilara, A. Gelation of pH-Aggregated Whey Protein Isolate Solution Induced by Heat, Protease, Calcium Salt, and Acidulant. J. Agric. Food Chem. 1998, 8561, 1830–1835. [Google Scholar] [CrossRef]
- Peng, Z.G.; Hidajat, K.; Uddin, M.S. Adsorption of bovine serum albumin on nanosized magnetic particles. J. Colloid Interface Sci. 2004, 271, 277–283. [Google Scholar] [CrossRef]
- Bouman, J. Controlled Release from Zein Matrices: Interplay of Drug Hydrophobicity and pH. Pharm. Res. 2016, 33, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Yong, Z.; Lili, C.; Feng, L.; Nianqiu, S.; Chunlei, L.; Xianghui, Y.; Yan, C.; Wei, K. Design, fabrication and biomedical applications of zein-based nano/micro-carrier systems. Int. J. Pharm. 2016, 512, 191–210. [Google Scholar]
- Lopes-da-Silva, J.A.; Monteiro, S.R. Gelling and emulsifying properties of soy protein hydrolysates in the presence of a neutral polysaccharide. Food Chem. 2019, 294, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.V.; Serajuddin, A.T.M.; Mangione, R.A. Making All Medications Gluten Free. J. Pharm. Sci. 2018, 107, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Ranjha, N.M.; Khan, H. Encapsulation and characterization of controlled release flurbiprofen loaded microspheres using beeswax as an encapsulating agent. J. Mater. Sci. Mater. Med. 2010, 21, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Milanovic, J.; Manojlovic, V.; Levic, S.; Rajic, N.; Nedovic, V.; Bugarski, B. Microencapsulation of Flavors in Carnauba Wax. Sensors 2010, 10, 901–912. [Google Scholar] [CrossRef]
- Khor, C.M.; Ng, W.K.; Kanaujia, P.; Chan, K.P. Hot-melt extrusion microencapsulation of quercetin for taste-masking. J. Microencapsul. 2017, 34, 2048. [Google Scholar] [CrossRef]
- Gopal, K.; Singh, K.; Halder, S.; Pati, S.; Wang, J. Microencapsulation of Paraffin Wax Microspheres with Silver. Def. Sci. J. 2018, 68, 218–224. [Google Scholar]
- Rabani, F.; Aziz, A.; Jai, J.; Raslan, R.; Subuki, I. Microencapsulation of citronella oil by complex coacervation using chitosan-gelatin (b) system: Operating design, preparation and characterization. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2016. [Google Scholar]
- Hussain, S.A.; Abdelkader, H.; Abdullah, N.; Kmaruddin, S. Review on micro-encapsulation with Chitosan for pharmaceuticals applications. MOJ Curr. Res. Rev. 2018, 1, 77–84. [Google Scholar] [CrossRef]
- Cunha, L.; Rodrigues, S.; Rosa, A.M.; Faleiro, L.; Buttini, F.; Grenha, A. Inhalable chitosan microparticles for simultaneous delivery of isoniazid and rifabutin in lung tuberculosis treatment. Drug Dev. Ind. Pharm. 2019, 45, 1313–1320. [Google Scholar] [CrossRef]
- Lim, S.T.; Martin, G.P.; Berry, D.J.; Brown, M.B. Preparation and evaluation of the in vitro drug release properties and mucoadhesion of novel microspheres of hyaluronic acid and chitosan. J. Control. Release 2000, 66, 281–292. [Google Scholar] [CrossRef]
- Jia, X.; Yeo, Y.; Clifton, R.J.; Jiao, T.; Kohane, D.S.; Kobler, J.B.; Zeitels, S.M.; Langer, R. Hyaluronic Acid-Based Microgels and Microgel Networks for Vocal Fold Regeneration. Biomacromolecules 2006, 7, 3336–3344. [Google Scholar] [CrossRef] [PubMed]
- Eraso, M.O.; Aníbal, H. Use of Starches and Milk Proteins in Microencapsulation. Int. J. Veg. Sci. 2014, 20, 289–304. [Google Scholar] [CrossRef]
- Prabaharan, M. Prospective of guar gum and its derivatives as controlled drug delivery systems. Int. J. Biol. Macromol. 2011, 49, 117–124. [Google Scholar] [CrossRef]
- Kaity, S.; Isaac, J.; Ghosh, A. Interpenetrating polymer network of locust bean gum-poly (vinyl alcohol) for controlled release drug delivery. Carbohydr. Polym. 2013, 94, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Street, K.; Davé, V.; McCarthy, S. Review of konjac glucomannan. J. Polym. Environ. 1997, 5, 237–241. [Google Scholar]
- Liu, J.; Xu, Q.; Zhang, J.; Zhou, X.; Lyu, F.; Zhao, P. Preparation, composition analysis and antioxidant activities of konjac. Carbohydr. Polym. 2015, 130, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef]
- Sharratt, M.; Grasso, P.; Carpanini, F.; Gangolli, S.D. Carrageenan ulceration as a model for human ulcerative colitis. Lancet 1971, 297, 192–193. [Google Scholar] [CrossRef]
- Mano, F.; Reis, R.L.; Gasperini, L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar]
- Kim, D.Y.; Kwon, D.Y.; Kwon, J.S.; Kim, J.H.; Min, B.H.; Kim, M.S. Stimuli-responsive injectable in situ-forming hydrogels for regenerative medicines. Polym. Rev. 2015, 55, 407–452. [Google Scholar] [CrossRef]
- Asghari-varzaneh, E.; Shahedi, M.; Shekarchizadeh, H. Iron microencapsulation in gum tragacanth using solvent evaporation method. Int. J. Biol. Macromol. 2017, 103, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Edris, A.E.; Kalemba, D.; Adamiec, J.; Piaotkowski, M. Microencapsulation of Nigella sativa oleoresin by spray drying for food and nutraceutical applications. Food Chem. 2016, 204, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.A.; Phillips, G.O. Handbook of Hydrocolloids; CRC Press: Boca Raton, FL, USA, 2009; ISBN 9781845694142. [Google Scholar]
- Sammour, A. Optimization of the MIcroencapsulation of Lavender Oil by Spray Drying. J. Microencapsul. 2019, 17, 1–42. [Google Scholar]
- Endress, H. Nonfood Uses of Pectin; Academic Press, Inc.: Cambridge, MA, USA, 1991. [Google Scholar]
- Wong, T.W.; Colombo, G.; Sonvico, F. Pectin Matrix as Oral Drug Delivery Vehicle for Colon Cancer Treatment. AAPS PharmSciTech 2011, 12, 201–214. [Google Scholar] [CrossRef]
- Petri, D.F.S. Xanthan gum: A versatile biopolymer for biomedical and technological applications. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Mahdi, M.H.; Conway, B.R.; Smith, A.M. Development of mucoadhesive sprayable gellan gum fluid gels. Int. J. Pharm. 2015, 488, 12–19. [Google Scholar] [CrossRef]
- Varshosaz, J.; Ahmadi, F.; Emami, J.; Tavakoli, N.; Minaiyan, M.; Mahzouni, P.; Dorkoosh, F. Microencapsulation of budesonide with dextran by spray drying technique for colon-targeted delivery: An in vitro/in vivo evaluation in induced colitis in rat. J. Microencapsul. 2011, 28, 62–73. [Google Scholar] [CrossRef]
- Kadota, K.; Yanagawa, Y.; Tachikawa, T.; Deki, Y.; Uchiyama, H.; Shirakawa, Y.; Tozuka, Y. Osaka University of Pharmaceutical Sciences, 4-20-1 Nasahara, Takatsuki, Osaka 569-Department of Chemical Engineering and Material Science, Doshisha University, 1-3. Int. J. Pharm. 2018. [Google Scholar] [CrossRef]
- Kshirsagar, A.C.; Yenge, V.B.; Sarkar, A.; Singhal, R.S. Efficacy of pullulan in emulsification of turmeric oleoresin and its subsequent microencapsulation. Food Chem. 2009, 113, 1139–1145. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Jani, G.K.; Khanda, S.M. Pullulan: An exopolysaccharide and its various applications. Carbohydr. Polym. 2013, 95, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Chowdary, K.P.R.; Rao, Y.S. Design and in vitro and in vivo evaluation of mucoadhesive microcapsules of glipizide for oral controlled release: A technical note. AAPS PharmSciTech 2004, 4, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Bailon, F.; Osorio-Revilla, G.; Gallardo-Velazquez, T. Microencapsulation of insulin using a W/O/W double emulsion followed by complex coacervation to provide protection in the gastrointestinal tract. J. Microencapsul. 2015, 32, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Mansuri, S.; Kesharwani, P.; Jain, K.; Tekade, R.K.; Jain, N.K. Mucoadhesion: A promising approach in drug delivery system. React. Funct. Polym. 2016, 100, 151–172. [Google Scholar] [CrossRef]
- Karewicz, A.; Zasada, K.; Bielska, D.; Douglas, T.E.L.; Jansen, J.A.; Leeuwenburgh, S.C.G.; Nowakowska, M. Alginate-hydroxypropylcellulose hydrogel microbeads for alkaline phosphatase encapsulation. J. Microencapsul. 2014, 2048, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Katona, J.M.; Sovilj, V.J.; Petrović, L.B. Microencapsulation of oil by polymer mixture-ionic surfactant interaction induced coacervation. Carbohydr. Polym. 2010, 79, 563–570. [Google Scholar] [CrossRef]
- Jelvehgari, M.; Siahi-Shadbad, M.R.; Azarmi, S.; Martin, G.P.; Nokhodchi, A. The microsponge delivery system of benzoyl peroxide: Preparation, characterization and release studies. Int. J. Pharm. 2006, 308, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Fundueanu, G.; Constantin, M.; Esposito, E.; Cortesi, R.; Nastruzzi, C.; Menegatti, E. Cellulose acetate butyrate microcapsules containing dextran ion-exchange resins as self-propelled drug release system. Biomaterials 2005, 26, 4337–4347. [Google Scholar] [CrossRef] [PubMed]
- Koga, C.C.; Lee, S.; Lee, Y. Consumer Acceptance of Bars and Gummies with Unencapsulated and Encapsulated Resveratrol. J. Food Sci. 2016, 81, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Bilati, U.; Allémann, E.; Doelker, E. Poly(D,L-lactide-co-glycolide) protein-loaded nanoparticles prepared by the double emulsion method-Processing and formulation issues for enhanced entrapment efficiency. J. Microencapsul. 2005, 22, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Dai, C.; Yang, F.; Huang, T.; Hao, Z.; Tang, Q.; Wang, H. Cefquinome-Loaded Microsphere Formulations in Protection against Pneumonia with Klebsiella pneumonia Infection and Inflammatory Response in Rats. Pharm. Res. 2019, 36, 74. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Grishkewich, N.; Bromberg, L.; Hatton, T.A.; Tam, K.C. Cross-linked Pluronic-g-Polyacrylic acid Microgel system for the Controlled Release of Doxorubicin in Pharmaceutical Formulations. Eur. J. Pharm. Biopharm. 2017, 114, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Al-kasmi, B.; Alsirawan, M.H.D.B.; Bashimam, M.; El-zein, H. Mechanical microencapsulation: The best technique in taste masking for the manufacturing scale-Effect of polymer encapsulation on drug targeting. J. Control. Release 2017, 260, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, L.; Ju, X.; Zerrouki, D.; Xie, R.; Yang, L.; Chu, L. A Novel Thermo-Induced Self-Bursting Microcapsule with Magnetic—Targeting Property. Chem. Phys. Chem. 2009, 10, 2405–2409. [Google Scholar] [CrossRef] [PubMed]
- Li, X. Synthesis and properties of thermo-responsive guar gum/poly (N-isopropylacrylamide) interpenetrating polymer network hydrogels. Carbohydr. Polym. 2008, 71, 394–402. [Google Scholar] [CrossRef]
- Wattendorf, U.T.A.; Merkle, H.P. PEGylation as a Tool for the Biomedical Engineering of Surface Modified Microparticles. J. Pharm. Sci. 2008, 97, 4655–4669. [Google Scholar] [CrossRef]
- Heinemann, L.; Baughman, R.; Boss, A.; Hompesch, M. Pharmacokinetic and Pharmacodynamic Properties of a Novel Inhaled Insulin. J. Diabetes Sci. Technol. 2017, 11, 148–156. [Google Scholar] [CrossRef]
- Sæther, H.V.; Holme, H.K.; Maurstad, G.; Smidsrød, O.; Stokke, B.T. Polyelectrolyte complex formation using alginate and chitosan. Carbohydr. Polym. 2008, 74, 813–821. [Google Scholar] [CrossRef]
- Batubara, I.; Rahayu, D.; Mohamad, K.; Prasetyaningtyas, W.E. Leydig Cells Encapsulation with Alginate-Chitosan: Optimization of Microcapsule Formation. J. Encapsulation Adsorpt. Sci. 2012, 2, 15–20. [Google Scholar] [CrossRef][Green Version]
- Capretto, L.; Mazzitelli, S.; Luca, G.; Nastruzzi, C. Preparation and characterization of polysaccharidic microbeads by a microfluidic technique: Application to the encapsulation of Sertoli cells. Acta Biomater. 2010, 6, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.G.; Bozza, F.T.; Thomazini, M.; Favaro-Trindade, C.S. Microencapsulation of xylitol by double emulsion followed by complex coacervation. Food Chem. 2015, 171, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Shaddel, R.; Hesari, J.; Azadmard-damirchi, S.; Hamishehkar, H. Use of gelatin and gum Arabic for encapsulation of black raspberry anthocyanins by complex coacervation. Int. J. Biol. Macromol. 2017, 107, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.S.; Shukla, S.; Banerjee, S.; Chowdhury, P.; Chakraborty, P.; Ghosh, A. Tailored IPN Hydrogel Bead of Sodium Carboxymethyl Cellulose and Sodium Carboxymethyl Xanthan Gum for Controlled Delivery of Diclofenac Sodium. Polym. Plast. Technol. Eng. 2013, 52, 795–805. [Google Scholar] [CrossRef]
- Maciel, V.B.V.; Yoshida, C.M.P.; Pereira, S.M.S.S.; Goycoolea, F.M.; Franco, T.T. Nano- and Microparticles for Insulin Delivery. Molecules 2017, 22, 1707. [Google Scholar] [CrossRef]
- Rocha-Selmi, G.A.; Theodoro, A.C.; Thomazini, M.; Bolini, H.M.A.; Favaro-Trindade, C.S. Double emulsion stage prior to complex coacervation process for microencapsulation of sweetener sucralose. J. Food Eng. 2013, 119, 28–32. [Google Scholar] [CrossRef]
- Carneiro, H.C.F.; Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef]
- Fioramonti, S.A.; Stepanic, E.M.; Tibaldo, A.M.; Pavón, Y.L.; Santiago, L.G. Spray dried flaxseed oil powdered microcapsules obtained using milk whey proteins-alginate double layer emulsions. Food Res. Int. 2019, 119, 931–940. [Google Scholar] [CrossRef]
- Alvarado, Y.; Muro, C.; Illescas, J.; Díaz, M.D.C.; Riera, F. Encapsulation of Antihypertensive Peptides from Whey Proteins and Their Releasing in Gastrointestinal Conditions. Biomolecules 2019, 9, 164. [Google Scholar] [CrossRef]
- Riaz, T.; Iqbal, M.W.; Saeed, M.; Yasmin, I.; Hassanin, H.A.; Mahmood, S.; Rehman, A. In-vitro survival of Bifidobacterium bifidum microencapsulated in zein coated alginate hydrogel microbeads. J. Microencapsul. 2019, 36, 192–203. [Google Scholar] [CrossRef]
- Farris, E.; Brown, D.M.; Ramer-Tait, A.E.; Pannier, A.K. Chitosan-zein nano-in-microparticles capable of mediating in vivo transgene expression following oral delivery. J. Control. Release 2017, 249, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.M.; Burke, N.A.D.; Stöver, H.D.H. Cross-linked microcapsules formed from self-deactivating reactive polyelectrolytes. Langmuir 2010, 26, 4916–4924. [Google Scholar] [CrossRef] [PubMed]
- Giovagnoli, S.; Blasi, P.; Luca, G.; Fallarino, F.; Calvitti, M.; Mancuso, F.; Ricci, M.; Basta, G.; Becchetti, E.; Rossi, C.; et al. Bioactive long-term release from biodegradable microspheres preserves implanted ALG-PLO-ALG microcapsules from in vivo response to purified alginate. Pharm. Res. 2010, 27, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Al-Salami, H.; Morahan, G.; Jamieson, E. Designing anti-diabetic β-cells microcapsules using polystyrenic sulfonate, polyallylamine, and a tertiary bile acid: Morphology, bioenergetics, and cytokine analysis. Biotechnol. Prog. 2016, 32, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Wells, L.A.; Sheardown, H. Photosensitive controlled release with polyethylene glycol-anthracene modified alginate. Eur. J. Pharm. Biopharm. 2011, 79, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Mahou, R.; Meier, R.R.H.; Bühler, L.H.; Wandrey, C. Alginate-poly(ethylene glycol) hybrid microspheres for primary cell microencapsulation. Materials 2014, 7, 275–286. [Google Scholar] [CrossRef]
- Colinet, I.; Dulong, V.; Mocanu, G.; Picton, L.; Le Cerf, D. New amphiphilic and pH-sensitive hydrogel for controlled release of a model poorly water-soluble drug. Eur. J. Pharm. Biopharm. 2009, 73, 345–350. [Google Scholar] [CrossRef]
- Streubel, A.; Siepmann, J.; Bodmeier, R. Floating microparticles based on low density foam powder. Int. J. Pharm. 2002, 241, 279–292. [Google Scholar] [CrossRef]
- Wu, J.; Kong, T.; Yeung, K.W.K.; Shum, H.C.; Cheung, K.M.C.; Wang, L.; To, M.K.T. Fabrication and characterization of monodisperse PLGA-alginate core-shell microspheres with monodisperse size and homogeneous shells for controlled drug release. Acta Biomater. 2013, 9, 7410–7419. [Google Scholar] [CrossRef]
- Funami, T.; Kataoka, Y.; Omoto, T.; Goto, Y. Food hydrocolloids control the gelatinization and retrogradation behavior of starch. 2a. Functions of guar gums with different molecular weights on the gelatinization behavior of corn starch q. Food Hydrocoll. 2005, 19, 15–24. [Google Scholar] [CrossRef]
- Papa, S.; Veglianese, P. Improving the Pharmacodynamic and Pharmacological Profile of Bioactive Molecules Using Biopolymers; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; ISBN 9780081002667. [Google Scholar]
- Dalpiaz, A.; Fogagnolo, M.; Ferraro, L.; Capuzzo, A.; Pavan, B.; Rassu, G.; Salis, A.; Giunchedi, P.; Gavini, E. Nasal chitosan microparticles target a zidovudine prodrug to brain HIV sanctuaries. Antiviral Res. 2015, 123, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Haque, T.P.S. Superior Cell Delivery Features of Poly(ethylene glycol) Incorporated Alginate, Chitosan, and Poly-L-lysine Microcapsules. Mol. Pharm. 2004, 2, 29–36. [Google Scholar] [CrossRef] [PubMed]
- McNaught, A.W.A. International Union of Pure and Applied Chemistry Compendium of Chemical Terminology Gold Book; Version 2; Pergamon Press: Oxford, UK, 1978. [Google Scholar]
- Chuang, J.J.; Huang, Y.Y.; Lo, S.H.; Hsu, T.F.; Huang, W.Y.; Huang, S.L.; Lin, Y.S. Effects of pH on the Shape of Alginate Particles and Its Release Behavior. Int. J. Polym. Sci. 2017, 2017, 9. [Google Scholar] [CrossRef]
- Nemethova, V.; Lacik, I.; Razga, F. Vibration Technology for Microencapsulation: The Restrictive Role of Viscosity. J. Bioprocess. Biotech. 2015, 5. [Google Scholar] [CrossRef]
- Wurster, D.E.; Bhattacharjya, S.; Flanagan, D.R. Effect of Curing on Water Diffusivities in Acrylate Free Films as Measured via a Sorption Technique. AAPS PharmSciTech 2007, 8, 1–6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Das, S.K.; David, S.R.; Rajabalaya, R.; Halder, T. Microencapsulation techniques and its practices. Int. J. Pharm. Sci. Tech. 2011, 6, 1. [Google Scholar]
- Poncelet, D. Microencapsulation: Fundamentals, methods and applications. In Surface Chemistry in Biomed Environmental Science; Springer: Dordrecht, The Netherlands, 2006; pp. 26–34. ISBN 140204741X. [Google Scholar]
- Klokk, T.I.; Melvik, J.E. Controlling the size of alginate gel beads by use of a high electrostatic potential. J. Microencapsul. 2002, 19, 415–424. [Google Scholar] [CrossRef]
- Watanabe, A.; Hanawa, T.; Sugihara, M.; Yamamoto, K. Development and pharmaceutical properties of a new oral dosage form of theophylline using sodium caseinate for the possible use in elderly patients. Int. J. Pharm. 1995, 117, 23–30. [Google Scholar] [CrossRef]
- Manojlovic, V.; Djonlagic, J.; Obradovic, B.; Nedovic, V.; Bugarski, B. Immobilization of cells by electrostatic droplet generation: A model system for potential application in medicine. Int. J. Nanomed. 2006, 1, 163–171. [Google Scholar] [CrossRef]
- Haeberle, S.; Naegele, L.; Burger, R.; Zengerle, R.; Ducrée, J. Alginate Micro-Bead Fabrication on a Centrifugal Microfluidics Platform. In Proceedings of the 2007 IEEE 20th International Conference on Micro Electro Mechanical Systems (MEMS), Hyogo, Japan, 21–25 January 2007. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation Techniques for Food Bioactive Components: A Review. Food Bioprocess Technol. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- Emami, F.; Vatanara, A.; Park, E.J.; Na, D.H. Drying technologies for the stability and bioavailability of biopharmaceuticals. Pharmaceutics 2018, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, P.S.; Manz, A. Lab-on-a-chip: Microfluidics in drug discovery. Nat. Rev. Drug Discov. 2006, 5, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Damiati, S. Microfluidic Devices for Drug Delivery Systems and Drug Screening. Genes 2018, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Wang, L. Lab on a Chip Passive and active droplet generation with microfluidics: A review. Lab Chip 2017, 17, 34–75. [Google Scholar] [CrossRef] [PubMed]
- Nisisako, T. Recent advances in microfluidic production of Janus droplets and particles. Curr. Opin. Colloid Interface Sci. 2016, 25, 1–12. [Google Scholar] [CrossRef]
- Fang, Y.; Li, L.; Vreeker, R.; Yao, X.; Wang, J.; Ma, Q.; Jiang, F.; Phillips, G.O. Rehydration of dried alginate gel beads: Effect of the presence of gelatin and gum arabic. Carbohydr. Polym. 2011, 86, 1145–1150. [Google Scholar] [CrossRef]
- Hakimi, N.; Tsai, S.S.H.; Cheng, C.H.; Hwang, D.K. One-step two-dimensional microfluidics-based synthesis of three-dimensional particles. Adv. Mater. 2014, 26, 1393–1398. [Google Scholar] [CrossRef]
- Le Goff, G.C.; Lee, J.; Gupta, A.; Hill, W.A.; Doyle, P.S. High-Throughput Contact Flow Lithography. Adv. Sci. 2015, 2, 1500149. [Google Scholar] [CrossRef]
- Shekunov, B.Y.; Chattopadhyay, P.; Tong, H.H.Y.; Chow, A.H.L. Particle size analysis in pharmaceutics: Principles, methods and applications. Pharm. Res. 2007, 24, 203–227. [Google Scholar] [CrossRef]
- Shahi, S.; Pharmaceuticals, L. Micro particles: An approach for betterment of drug delivery system. Int. J. Pharm. Res. Dev. 2014, 1, 99–115. [Google Scholar]
- Hasirci, V.; Hasirci, N. Fundamentals of Biomaterials; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 9788847011946. [Google Scholar]
- Chou, D.K.; Krishnamurthy, R.; Randolph, T.W.; Carpenter, J.F.; Manning, M.C. Effects of Tween 20® and Tween 80® on the stability of Albutropin during agitation. J. Pharm. Sci. 2005, 94, 1368–1381. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.A.; AbouGhaly, M.H.H.; Schryer-Praga, J.V.; Chadwick, K. The effect of ionotropic gelation residence time on alginate cross-linking and properties. Carbohydr. Polym. 2017, 155, 362–371. [Google Scholar] [CrossRef]
- Song, M.; Li, N.; Tiedt, L.R.; Degennaro, M.D.; de Villiers, M.M. Preparation and Characterization of Highly Porous Direct Compression Carrier Particles with Improved Drug Loading During an Interactive Mixing Process. AAPS PharmSciTech 2010, 11, 698–707. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lengyel, M.; Balogh, E.; Szerőczei, D.; Dobó-Nagy, C.; Pápay, Z.; Stömmer, V.; Klebovich, I.; Antal, I. Study on process parameters and optimization of microencapsulation based on phase separation. Eur. J. Pharm. Sci. 2018, 122, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.Y.; Maincent, P.; Bodmeier, R. In vitro and in vivo evaluation of carbamazepine-loaded enteric microparticles. Int. J. Pharm. 2007, 331, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Adebisi, A.O.; Conway, B.R. Preparation and characterisation of gastroretentive alginate beads for targeting H. pylori. J. Microencapsul. 2014, 31, 58–67. [Google Scholar] [CrossRef] [PubMed]
- El-Say, K.M. Maximizing the encapsulation efficiency and the bioavailability of controlled-release cetirizine microspheres using Draper–Lin small composite design. Drug Des. Dev. Ther. 2016, 10, 825–839. [Google Scholar] [CrossRef]
- Dredán, J.; Antal, I.; Rácz, I. Evaluation of mathematical models describing drug release from lipophilic matrices. Int. J. Pharm. 1996, 145, 61–64. [Google Scholar] [CrossRef]
- Tiǧli Aydin, R.S.; Pulat, M. 5-fluorouracil encapsulated chitosan nanoparticles for pH-stimulated drug delivery: Evaluation of controlled release kinetics. J. Nanomater. 2012, 2012, 42. [Google Scholar] [CrossRef]
- Son, Y.J.; McConville, J.T. Development of a standardized dissolution test method for inhaled pharmaceutical formulations. Int. J. Pharm. 2009, 382, 15–22. [Google Scholar] [CrossRef]
- Rawat, A.; Bhardwaj, U.; Burgess, D.J. Comparison of in vitro-in vivo release of Risperdal® Consta® microspheres. Int. J. Pharm. 2012, 434, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Salama, R.O.; Traini, D.; Chan, H.; Young, P.M. Preparation and characterisation of controlled release co-spray dried drug–polymer microparticles for inhalation 2: Evaluation of in vitro release profiling methodologies for controlled release respiratory aerosols. Eur. J. Pharm. Biopharm. 2008, 70, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Shah, K.A.; Halquist, M.S.; Sakagami, M. In Vitro aqueous fluid-capacity-limited dissolution testing of respirable aerosol drug particles generated from inhaler products. Pharm. Res. 2010, 27, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Gerde, P.; Malmlöf, M.; Havsborn, L.; Sjöberg, C.-O.; Ewing, P.; Eirefelt, S.; Ekelund, K. Dissolv It: An In Vitro Method for Simulating the Dissolution and Absorption of Inhaled Dry Powder Drugs in the Lungs. Assay Drug Dev. Technol. 2017, 15, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Suo, Z. Strengthening Alginate/Polyacrylamide Hydrogels Using Various Multivalent Cations. ACS Appl. Mater. Interfaces 2013, 5, 10418–10422. [Google Scholar]
- Sgouras, D.; Duncan, R. Methods for the evaluation of biocompatibility of soluble synthetic polymers which have potential for biomedical use: 1-Use of the tetrazolium-based colorimetric assay (MTT) as a preliminary screen for evaluation of in vitro cytotoxicity. J. Mater. Sci. Mater. Med. 1990, 1, 61–68. [Google Scholar] [CrossRef]
- Leone-Bay, A.; Baughman, R.; Smutney, C.; Kocinsky, J. Innovations in Drug Delivery by Inhalation. OnDrugDelivery Magazine. November 2010, pp. 4–8. Available online: https://www.ondrugdelivery.com/wp-content/uploads/2018/11/Nov2010.pdf (accessed on 27 June 2019).
- Murry, D.J.; Blaney, S.M. Clinical pharmacology of encapsulated sustained-release cytarabine. Ann. Pharmacother. 2000, 34, 1173–1178. [Google Scholar] [CrossRef]
- Singhvi, G.; Manchanda, P.; Hans, N.; Dubey, S.K.; Gupta, G. Microsponge: An emerging drug delivery strategy. Drug Dev. Res. 2019, 80, 200–208. [Google Scholar] [CrossRef]
- Marquet, F.; Teichert, T.; Wu, S.Y.; Tung, Y.S.; Downs, M.; Wang, S.; Chen, C.; Ferrera, V.; Konofagou, E.E. Real-time, transcranial monitoring of safe blood-brain barrier opening in non-human primates. PLoS ONE 2014, 9, 1–11. [Google Scholar] [CrossRef]
- Upadhyay, A.; Dalvi, S.V.; Gupta, G.; Khanna, N. Effect of PEGylation on performance of protein microbubbles and its comparison with lipid microbubbles. Mater. Sci. Eng. C 2017, 71, 425–430. [Google Scholar] [CrossRef]
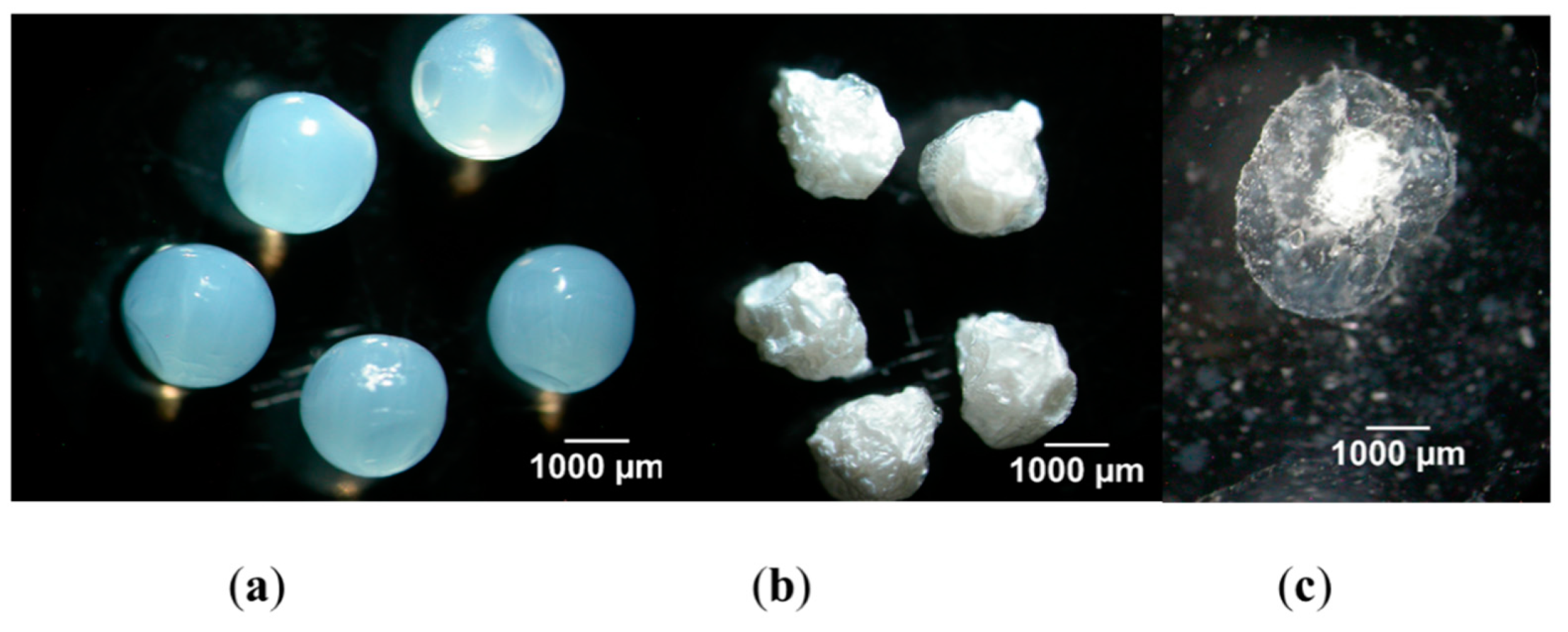
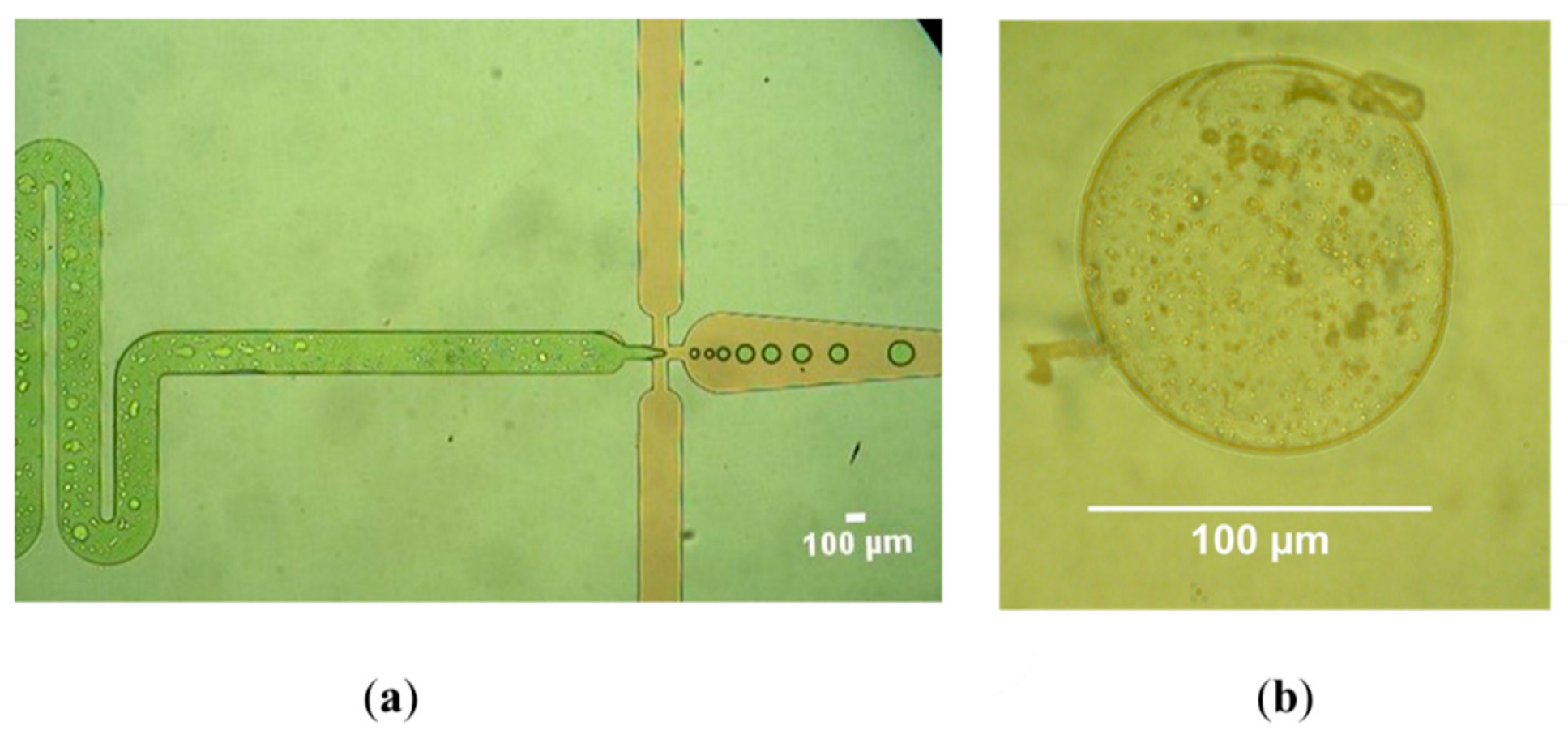
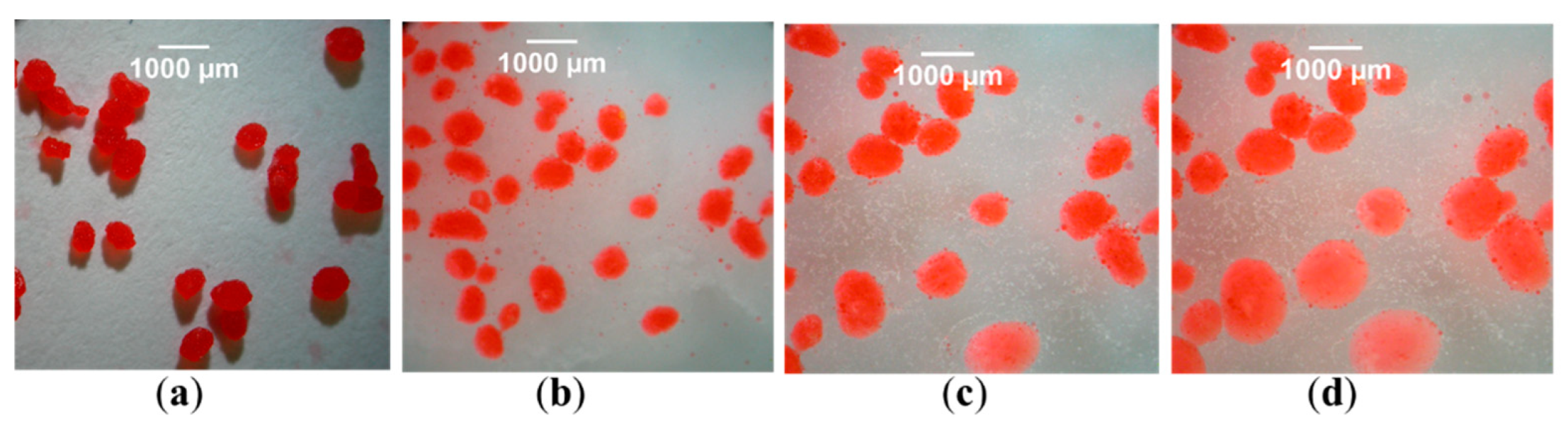
| Excipient | Physicochemical Properties | Application and Benefits | Limitations | Ref. |
|---|---|---|---|---|
| Gelatine | Amphoteric gelatin A (isoelectric point (IEP) pH: 7–9.4 gelatin B (IEP: pH 4.8–5.5) Swells, then dissolves in water | At low pH coacervation with negatively charged polymers, high potential of crosslinking emulsifier, stabilizer (high viscosity), binder Thermoreversible gelling, implantable pulmonary delivery pH-dependent, swelling, dissolution, erosion | Influence of pH and ionic strength on behavior Need for preservation against possible prion (BSE) contamination | [20,21] |
| Casein | Hydrophilic, metal binding Insoluble in water at its IEP (pH 4.6) | calcium caseinate –reversible thermal gelation solubility increase (coenzyme Q10) | Anaphylactic reactions | [22] |
| Whey protein | Insoluble at its IEP (pH 5.2) | Thermal gelation, encapsulation of oils film formation: gas barrier, good tensile strength | Thermally irreversible gel formation above 70 °C Denatures at higher salt conc. Anaphylactic reactions | [23] |
| Albumin | IEP: 4.7 Freely soluble in water negative charge at pH 7.4 | Pulmonary delivery Alginate-albumin | Chemical degradation, denaturation at high salt conc., enzymes, heat | [24] |
| Zein | α, β, γ, δ, zein with different Mw amphiphilic character IEP: 6.8 hydrophobic | Oral controlled release matrix and wall | Brittle, rigid wall, complex with a gelling component to plasticize | [25,26] |
| Soy protein | Partly soluble in water, depending on the extraction process; IEP 4.5 | Oral controlled release matrix and wall Emulsifier, foaming agent | Sensitivity | [27] |
| Gluten | Water-insoluble; IEP:7.5 | Wall material, good elastic, good thermoplastic properties | Gluten sensitivity | [28] |
| Bees-wax (Apis mellifera) | mp (melting point): 62–64 °C HLBrequired = 12 | Edible, easy use, smooth surface, hot melt extrusion prolonged release for hydrophilic substances, protection from chemical degradation | Oxidation | [29] |
| Carnauba wax (Copernica cerifera) | mp: 78–85 °C HLBrequired = 12 | Good compatibility hot melt extrusion, embedding water soluble components, taste masking | Oxidation | [30,31] |
| Paraffin (hard) (mineral) | mp: 50–61 °C | Embedding water soluble components liquid paraffin in the emulsification process | Sensitivity | [32] |
| Excipient | Physicochemical Properties | Applications and Benefits | Limitations | Ref. |
|---|---|---|---|---|
| Chitosan (deacylated chitin) | Soluble in weak acids Mucoadhesive reacts with negatively charged surfaces | Antifungal, antibacterial, reduces LDL (low-density lipoprotein), tissue regenerative, pulmonary delivery Ionotropic gelation, coacervation with anions, modified emulsification | pH dependence (insoluble above pH 6.5) Addition of electrolytes precipitates chitosan in solution, hygroscopic | [33,34,35] |
| Sodium hyaluronate | Anionic character, soluble in water, high viscosity at low concentration | In microspheres nasal, vaginal, ophthalmic delivery systems | Very hygroscopic, when heated, emits Na2O | [36,37] |
| Starch (wheat, corn, potato, rice, tapioca) | Starch from different origins Differ in particle size and shape Soluble in hot water after a time of gelatinization | Spray drying, extrusion, molecular inclusion, coacervation with proteins, hydrocolloid-forming, release via swelling, diffusion, erosion | Hygroscopic | [38] |
| Guar gum | Water soluble, nonionic, galactomannan forms a thixotropic solution, stable at pH 4–10.5 | Controlled-release, colon-targeted release, appetite suppressant, thermoreversible | Needs preservation, borate hinders swelling | [39] |
| Locust bean gum (LBG)/carob, ceratonia/ | Nonionic, galactomannan, dispersible in hot water, soluble at higher temperature | Controlled release in combination pseudoplastic, gelling with the addition of borate, solubility not affected by pH or ionic concentration | Low water solubility, hypersensitivity | [40] |
| Konjac gum | Water soluble | Elevation of temperature increases gelation, antioxidant properties | Indigestable | [41,42] |
| Κ, ι, λ−, Carrageenan | Anionic polymer, ι−Carrageenan: shear thinning thixotropic gel forming | λ−Carrageenan: highest anionic charge, high solubility, but no gelling, κ, ι−Carrageenan: elastic gel is formed with K+, Ca2+, thermoreversible gel forming Release by erosion (physical contact-Scentcaps®), effective against HPV (human papilloma virus) | Acid-catalyzed hydrolyses, especially at higher temperature and pH < 3 Administration of >2 g/kg orally Induces intestinal ulcers, not biodegradable | [43,44] |
| Agarose | Soluble in hot water | swelling, Thermoreversible gelation (at ≈37 °C) with hysteresis | Poor biodegradability | [45] |
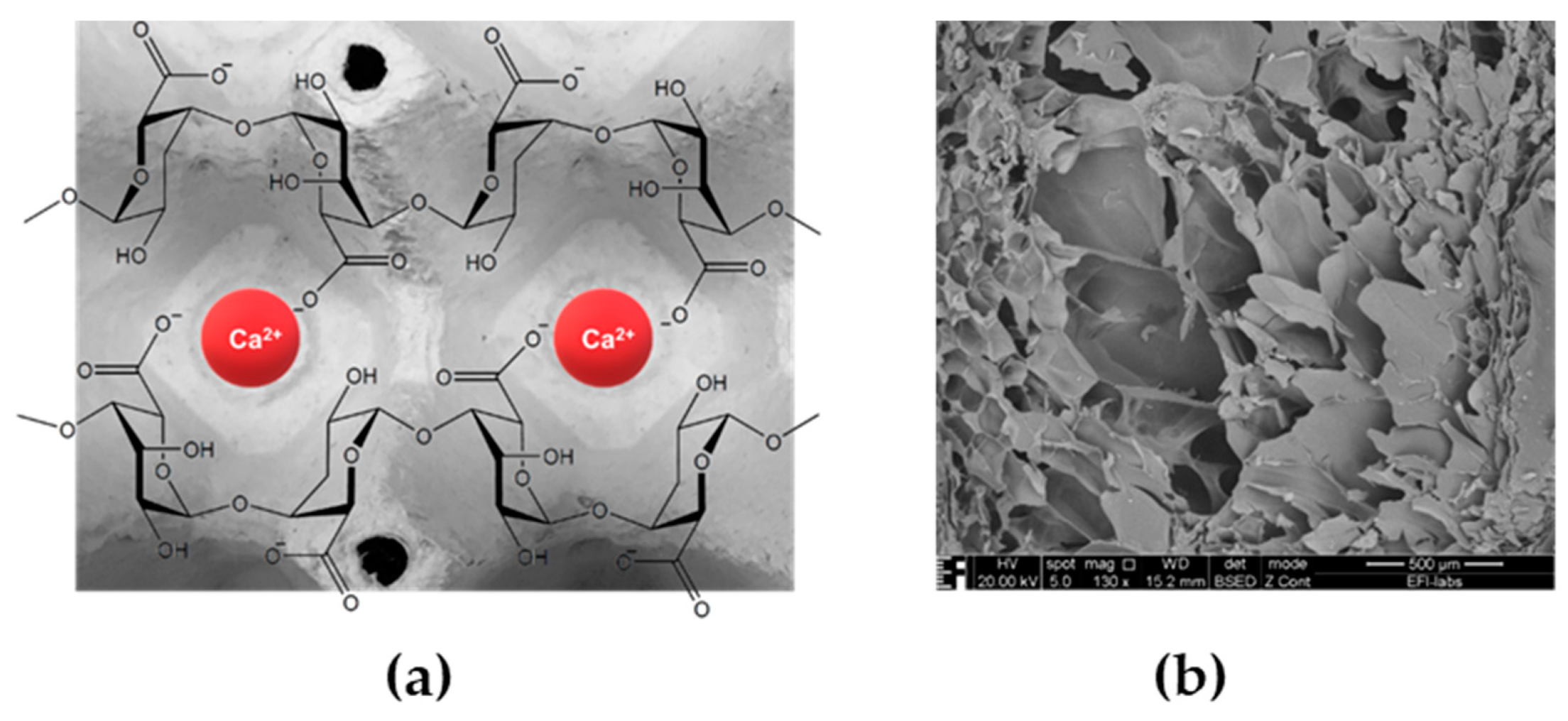
| Sodium alginate | Water soluble, anionic coacervation with ions (Ca2+, Sr2+, Ba2+), polycations (chitosan) or poly-l-lysine disintegrant, binder, viscosity increasing | swelling ability (200–300× of its own weight from water) pH-dependent swelling nontoxic, nonirritant low density (capable of floating in gastric juice) Diffusion, erosion, in situ forming hydrogels | pH dependence (insoluble in acidic medium) heat-sensitivity (hydrolysis) microbial spoilage on storage (depolymerization) | [46] |
| Tragacanth | Water soluble arabinogalactan and swellable bassorin components, swells quickly in water | Acid-resistance emulsifier, encapsulation of oils, stabilizer in emulsions | Di/trivalent cations cause drop in viscosity because of precipitation | [47] |
| Gum arabic/ Acacia gummi (Ph. Eur.) | Water soluble, clear solution of pH 4.5, protective colloid | Emulsifier, spray-dry encapsulation of flavor and of essential oils, 30% solution has a relatively low viscosity, Newtonian flow permeable coating dietary fiber | Variation dependent on source pH, ionic strength influences viscosity (max at ≈pH 6–7) | [48,49,50] |
| Pectin (low or high methoxylated) (apple, citrus peel, beet) | Negatively charged molecule | Gelation depends on the degree of esterification, cation (Ca,Zn) concentration in solution, temperature and pH In situ gelling, sustained delivery, dietary fiber, drug delivery in colorectal carcinoma (5-FU), antiviral activity | Gelling occurs at low pH (<3.5), presence of sugars necessary for gelation of HM pectin | [51,52] |
| Excipient | Physicochemical Properties | Applications and Benefits | Limitations | Ref. |
|---|---|---|---|---|
| Xanthan gum | Soluble in warm and cold water, not affected by pH anionic polyelectrolyte | No gelation at room temp, cryogelation possible, stable viscosity over wide pH range, surface activity, emulsion stabilizer, controlled-release, colon-targeted swelling, diffusion, matrix erosion | Hydrolysis Acceptable daily intake (WHO) 10 mg/kg BW Contains cellulase | [53] |
| Gellan gum | Anionic polyelectrolyte soluble in water | Thermoreversible gelation with cations sol-gel transition under physiological conditions bioadhesive nasal administration | Poor biodegragability | [54] |
| Dextran | Neutral polymer, solubility depends on degree of polymerization | Colon-targeted delivery, formation of porous particle pulmonary delivery | At parenteral adiminitration: possible platelet adhesiveness | [55,56] |
| Pullulan | Neutral polymer underivarized pullulan has high water solubility | Emulsifier sustained-release preparations | Relatively high price | [57,58] |
| Excipient | Physicochemical Properties | Applications and Benefits | Limitations | Ref. |
|---|---|---|---|---|
| Methylcellulose (MC) | Soluble in cold water Tsol-gel: 80 °C amphiphilic | Emulsifier, pseudoplastic solution, pH-independent gel formation above 50 °C, high variety in Mw | Complexation with surface-active components, laxative | [59] |
| Carboxymethyl-cellulose sodium (CMC-Na) | Anionic cellulose ether, dispersible in water (forms a colloidal solution) | Injectable thermoreversible gel-forming, mucoadhesive | Microbial instability hygroscopicity | [60,61] |
| Hydroxypropyl-cellulose (HPC) | Soluble in cold water, compatible with waxes, oils, Tsol-gel: 55 °C | High surface activity film-forming ability | Incompatible with alkaline substances, substituted phenol derivatives, anionic polymers increase viscosity | [62] |
| Hydroxypropyl-methylcellulose (HPMC) | Water soluble, nonionic | Reversible thermal gelation surface activity, emulsion stabilizer, film-forming ability | No complex with metallic salts or ionic organics | [63] |
| Ethylcellulose | Water-insoluble, hydrophobic coating | Film forming ability, membrane-controlled diffusion, modified release, floating, gastroretentive systems | Organic solvent residuals | [64] |
| Cellulose acetate butyrate | Insoluble in water, soluble in aceton-water blends | Semipermeable coating, extended release formulations, diffusion, matrix erosion | Organic solvent residuals | [65] |
| Excipient | Physicochemical Properties | Applications and Benefits | Limitations | Ref. |
|---|---|---|---|---|
| Poly (lactic acid) (PLA) | Insoluble in water degrades to CO2 and H2O over 12–24 months | Biodegradability, prolonged-release in im or sc injections, implants, oral solid dispersions | Digestive tract influences degradation, parenteral administration is favorable, initial burst release may occur | [64,65] |
| Polylactic acid-glycolic acid copolymer (PLGA) | Insoluble in water lactic acid-glycolic acid ratio influences degradation ability to thermoplastic gel forming | Injectable or implantable systems (microparticles, gels) for human and veterinary use, pH-responsive/non-pH-responsive polymer degradation, bone tissue engineering | Degrades into by-products that can induce inflammation | [66,67,68,69] |
| Polyacrylic acid (Carbopol) | Neutralization with alkaline chemicals for gelling | Bioadhesive, targeted delivery, intranasal administration with microencapsulation | Neutralization at preparation, preservative needed | [70] |
| Polymethacrylates | Soluble in organic solvents, most of them are miscible with water | pH-dependent solubility, permeability gastric or enteric targeted delivery possible | Water insolubility | [71] |
| Poly-(N-isopropylacrylamide) | LCST: 35–40 °C | Thermoresponsive | Not biodegradable | [72,73] |
| Polyethylene glycols | Liquid or solid grades depending on the Mw, PEG (polyethylene glycol) 1500 freezing point at 37–41 °C | Plasticizer in wall of compressible microcapsules, oral insulin delivery cell delivery in combination | Not biodegradable | [74] |
| Fumaryl diketopiperazine (FDKP) | High solubility at pH ≥ 6, no biological activity | Self-assembled highly porous microparticles, Technosphere® carrier | Not biodegradable, excreted via urine | [75] |
| Polymer | Active Ingredient | Particle Size | Physicochemical Mechanisms | Ref |
|---|---|---|---|---|
| Carbohydrate-Carbohydrate | ||||
| Chitosan-alginate | Leydig cells | 230–370 µm | Complex coacervation | [77] |
| Agarose-alginate (CaCl2) | Sertoli cells | 250 µm | Microfluidics ionic gelation, | [78] |
| Gum arabic | Xylitol | 100 µm | double emulsion/coacervation | [79] |
| Cellulose acetate butyrate (CAB) | Sulfopropylated dextran microspheres | 40–120 µm | O/W emulsification, solvent evaporation | [65] |
| Gelatin + gum arabic | Raspberry anthocyanins | 150 µm | Emulsification, coacervation | [80] |
| Maltodextrin + gum arabic | Lavender oil | 10–20 µm | S/O/W emulsification, spray-drying | [50] |
| CMC-Na + xanthan gum | Diclofenac sodium | 1000–1500 µm | Emulsification, ionic gelation, Interpenetrating network | [81] |
| LBG + PVA | Buflomedil hydrochloride (BH) | 350–750 µm | emulsification, Interpenetrating network | [40] |
| Chitosan + pectin | Insulin | 0.24–2 µm | electrostatic self-assembly | [82] |
| Carbohydrate-Protein | ||||
| Gelatin + gum arabic | Aspartame | 100 µm | Double emulsion/complex coacervation | [83] |
| Gelatin + chitosan | Citronella oil | 100 µm | Coacervation | [33] |
| whey Protein + maltodextrin | Flaxseed oil | ≈10 µm | emulsification, spray drying | [84] |
| Whey protein + alginate | Flaxseed oil | <10 µm | Double layer emulsification spray drying | [85] |
| Alginate + gelatin | Whey peptides (antihypertensive activity | 1000 µm | Dripping, coacervation | [86] |
| Alginate + zein | Bifidobacterium bifidum | 1200–1700 µm | Extrusion coacervation shell-core | [87] |
| Chitosan-zein | Oral gene delivery | 10 µm | Zein-sodium tripolyphosphate W/O emulsion | [88] |
| Poly-l-lysine | Poly (methyl vinyl ether-alt-maleic anhydride) (PMM0) | 400 µm | prilling, coacervation | [89] |
| Poly (l-ornithine) + alginate + PLA, PLGA | Superoxide dismutase, ketoprofen | 500 µm | W/O/W, O/W, solvent evaporation | [90] |
| Poly (l-ornithine) + ursodeoxycholic acid, Polystyrene sulfonate, polyallilamine | Pancreatic ß-cells | 700 µm | Complex coacervation, vibrational jet | [91] |
| Poly (ethylene glycol) (PEG)-anthracene alginate | Coomassie blue | no data | Coacervation | [92] |
| Vinyl-sulfone terminated PEG + alginate | Human foreskin fibroblast | 550 µm | Simple coacervation | [93] |
| Alginate + Poly-ε-caprolactone | Theophylline | 800 µm | Complex coacervation | [94] |
| Polypropylene + PMMA + ethylcellulose | Verapamil | 150–200 µm | O/W solvent evaporation | [95] |
| PLGA-alginate | Rifampicin | 15–50 µm | Microfluidics | [96] |
| Method | Typical Initial Form of the Core | Approximate Size (µm) | Structure | Final State | ||
|---|---|---|---|---|---|---|
| Matrix | Core/Shell | |||||
| Chemical | Interfacial polymerization | In emulsion or suspension | 0.1–500 | X | wet (slurry), can be dried | |
| In situ polymerization | In emulsion or suspension | 1–1000 | X | wet (slurry), can be dried | ||
| Physicochemical | left | In emulsion or suspension | 2–5000 | X | X | wet, can be dried |
| Dripping- (Vibrating nozzle-single; multiple) | Droplet formulation/extrusion; coextrusion | 10–5000 | X | X | wet, can be dried | |
| Solvent evaporation | In emulsion or suspension | 0.1–5000 | X | X | wet, can be dried | |
| Spray drying (from solution) | Droplet formulation/spraying | 1–100 | X | dry | ||
| Spray chilling | Droplet formulation/spraying | 1–100 | X | X | dry | |
| Fluid bed coating/drum coating | Solid core particle | 100–5000 | X | dry | ||
| Type | Dosage Form | Key Excipient | Drug | Indication | Product Example |
|---|---|---|---|---|---|
| Micropellets | Peroral pellets in capsule | HPC | Lansoprazole | Proton pump inhibitor | Lansoprazole® |
| Enteric-coated microgranules | Delayed-release orally disintegrating tablets | Methacrylic acid, polyacrylate | Lansoprazole | Proton pump inhibitor | Prevacid® SoluTab™ |
| Coated pellets | Compressing pellets into an extended release tablet | HPC, EC | Metoprolol succinate | Cardioselective beta blockers | Betaloc® ZOK |
| Microtablets | Peroral minitablets in capsule | Methacrylic acid-ethyl acrylate, MCC | Lipase, amylase, | Enzyme supply | Pangrol® |
| Dry powder (Technospheres®) | inhalation | Fumaryl diketopiperazine | Insulin | Diabetes | AfrezzaTM |
| Microspheres | Im suspension injection | Poly-d, l-lactid-co-glycolide | Risperidone | Schizophrenia | Risperdal® consta |
| Microspheres | Powder for injection | Poly-d, l-lactide-co-glycolide | Bromocriptine | Acromegaly, parkinsonism | Parlodel® LAR |
| Microspheres | Powder and solvent for suspension and injection | Poly-d, l-lactide-co-glycolide | Octreotide | Acromegaly pancreatic tumors | Sandostatin LAR® |
| Microspheres | Prolonged-release suspension for injection | Poly-d, l-lactide-co-glycolide | Exenatide | Diabetes type 2 | Bydureon® |
| Lyophilized microspheres | Suspension depot injection | Poly-d, l-lactid-co-glycolide | Leuprolide acetate | Endometriosis | Lupron® depot |
| Liposomes | Liposome inhalation suspension | Cholesterol, dipalmitoylphosphatidylcholine | Amikacin | Antibacterial | Arikayce® |
| Liposomes (DepoFoam™) | Powder for suspension for injection | Cholesterol, DOPC, DPPG | Cytarabine | Neoplastic meningitis | Depocyte® |
| Liposomes (DepoFoam™) | Powder for suspension for injection | Cholesterol, DOPC, DPPG, tricaprylin, triolein | Morphine | Epidural analgesia | DepoDur® |
| Microbubbles | Intravenous injection | Albumin | Perflutren | Ultrasound contrast agent | Albunex® |
| Microbubbles | Intravenous injection | PEG 4000, DSPC, DPPG-Na, palmitic acid | Sulfur hexafluoride | Ultrasound contrast agent | Lumason/SonoVue® |
| Microbubbles | Intravenous injection | DPPA, DPPC, MPEG5000 DPPE | Perflutren | Ultrasound contrast agent | Definity® |
| Microsponge | Topical gel | Methyl methacrylate/glycol dimethacrylate crosspolymer | Tretinoin | Acne vulgaris | Retin A® micro gel |
| Microsponge | Topical cream | Methyl methacrylate glycol dimethacrylate crosspolymer, dimethicone. | 5-fluorouracil | Multiple acne/solar keratoses | Carac® cream 0.5% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. https://doi.org/10.3390/scipharm87030020
Lengyel M, Kállai-Szabó N, Antal V, Laki AJ, Antal I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Scientia Pharmaceutica. 2019; 87(3):20. https://doi.org/10.3390/scipharm87030020
Chicago/Turabian StyleLengyel, Miléna, Nikolett Kállai-Szabó, Vince Antal, András József Laki, and István Antal. 2019. "Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery" Scientia Pharmaceutica 87, no. 3: 20. https://doi.org/10.3390/scipharm87030020
APA StyleLengyel, M., Kállai-Szabó, N., Antal, V., Laki, A. J., & Antal, I. (2019). Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Scientia Pharmaceutica, 87(3), 20. https://doi.org/10.3390/scipharm87030020