GaN Single Crystalline Substrates by Ammonothermal and HVPE Methods for Electronic Devices
Abstract
:1. Introduction
2. Requirements for GaN Substrates
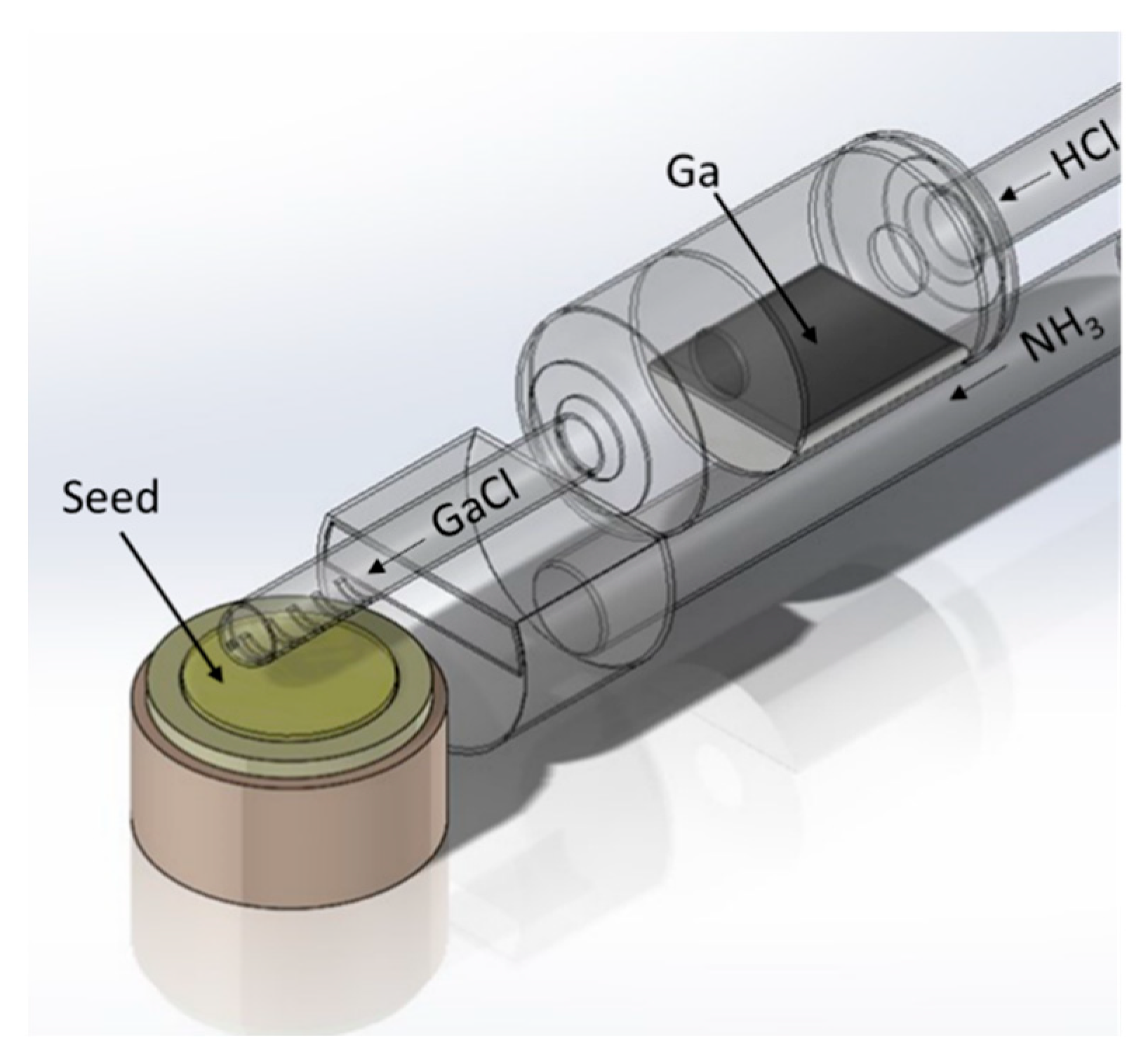
3. HVPE-GaN Wafers—Crystallization on Foreign Seeds
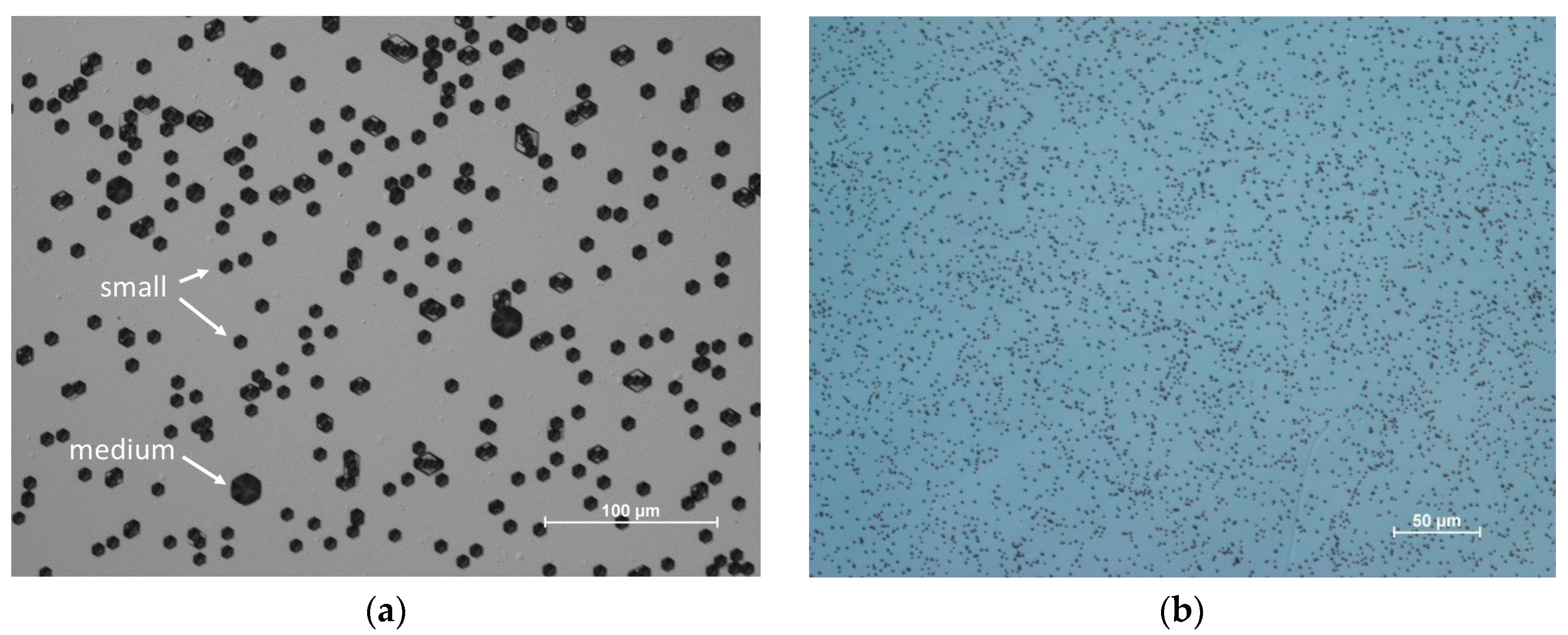
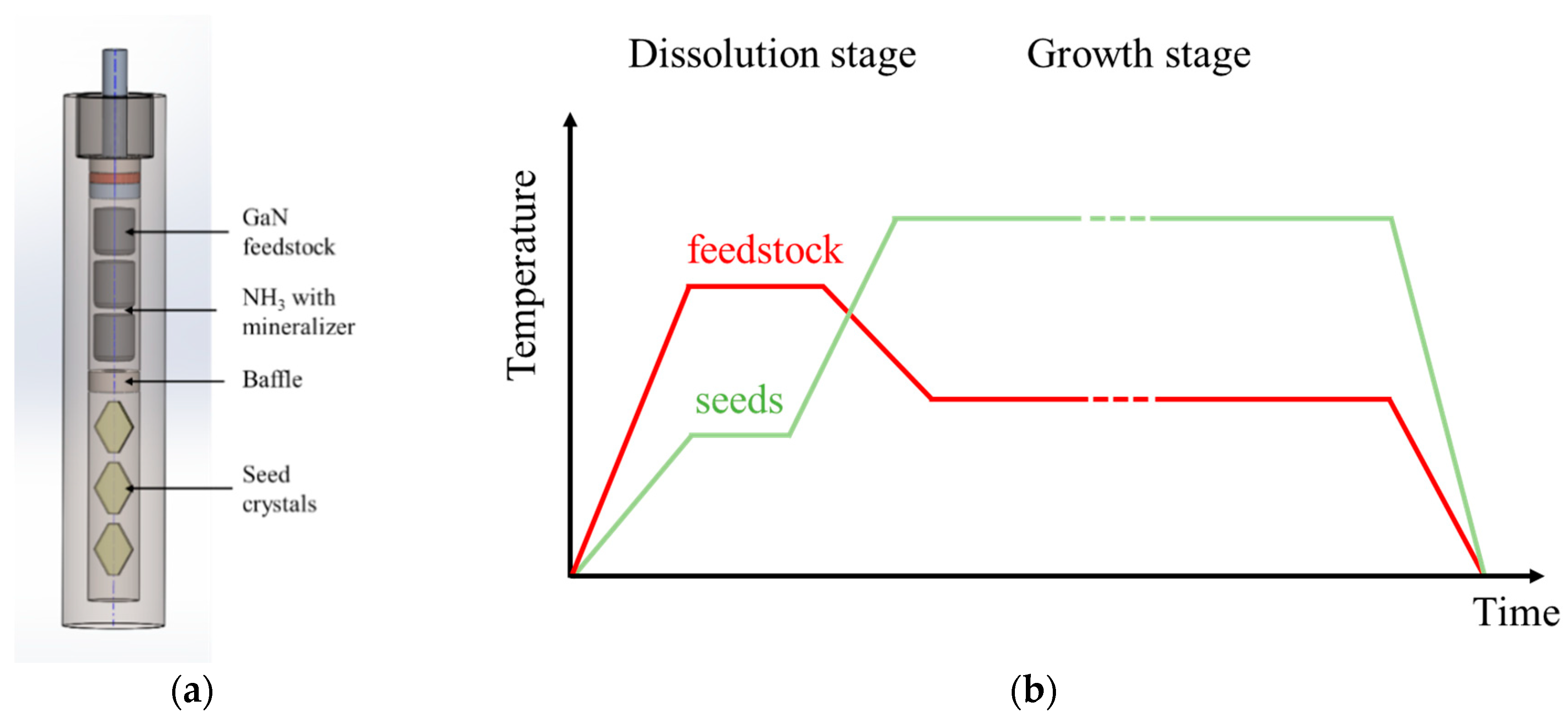
4. Ammonothermal Crystal Growth of GaN
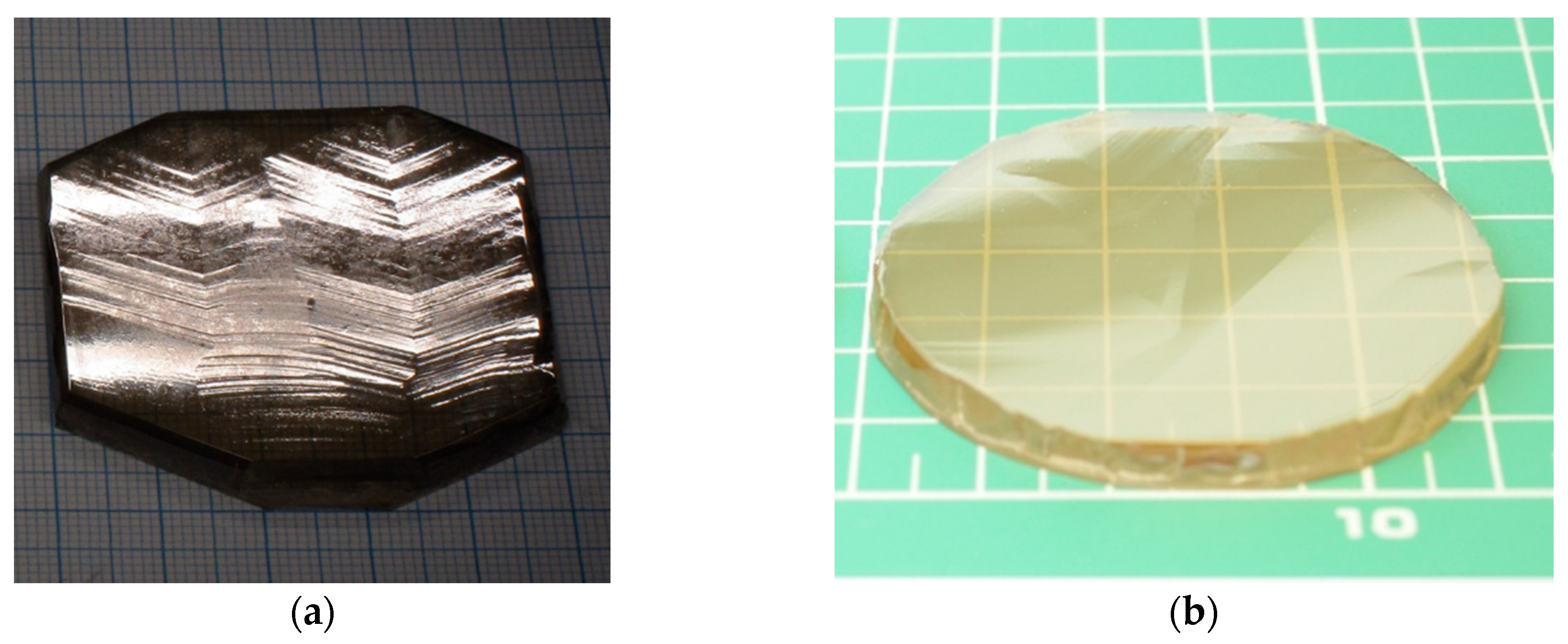
5. HVPE-GaN on Native Ammonothermal GaN Seeds
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Utsumi, W.; Saitoh, H.; Kaneko, H.; Watanuki, T.; Aoki, K.; Shimomura, O. Congruent melting of gallium nitride at 6 GPa and its application to single-crystal growth. Nat. Mater. 2003, 2, 735. [Google Scholar] [CrossRef] [PubMed]
- Porowski, S.; Sadovyi, B.; Gierlotka, S.; Rzoska, S.J.; Grzegory, I.; Petrusha, I.; Turkevich, V.; Stratiichuk, D. The challenge of decomposition and melting of gallium nitride under high pressure and high temperature. J. Phys. Chem. Solids 2015, 85, 138–143. [Google Scholar] [CrossRef]
- Porowski, S.; Sadovyi, B.; Karbovnyk, I.; Gierlotka, S.; Rzoska, S.J.; Petrusha, I.; Startiichuk, D.; Turkievich, V.; Grzegory, I. Melting of tetrahedrally bonded semiconductors:“anomaly” of the phase diagram of GaN? J. Cryst. Growth 2019, 505, 5–9. [Google Scholar] [CrossRef]
- GaN Substrates. Available online: https://www.sciocs.com/english/products/GaN_substrate.html (accessed on 18 August 2020).
- GaN Substrates. Available online: https://global-sei.com/sc/products_e/gan/ (accessed on 18 August 2020).
- GaN Wafer (GaN substrate). Available online: https://www.m-chemical.co.jp/en/products/departments/mcc/nes/product/1201029_9004.html (accessed on 18 August 2020).
- Electronic Materials. Available online: https://www.furukawakk.co.jp/e/business/material/ (accessed on 18 August 2020).
- Suzhou Nanowin Science and Technology. Available online: http://shopen.nanowin.com.cn (accessed on 18 August 2020).
- Eta Research. Available online: http://www.etaresearch.com/ (accessed on 18 August 2020).
- Free Standing GaN Substrates. Available online: https://www.lumilog.com/products/free-standing (accessed on 18 August 2020).
- Zajac, M.; Kucharski, R.; Grabianska, K.; Gwardys-Bak, A.; Puchalski, A.; Wasik, D.; Litwin-Staszewska, E.; Piotrzkowski, R.; Domagala, J.Z.; Bockowski, M. Basic ammonothermal growth of Gallium Nitride—State of the art, challenges, perspectives. Prog. Cryst. Growth Charact. Mater. 2018, 64, 63–74. [Google Scholar] [CrossRef]
- Hashimoto, T.; Letts, E.R.; Key, D.; Jordan, B. Growth of bulk GaN crystals. Jpn. J. Appl. Phys. 2019, 58, SC1005. [Google Scholar] [CrossRef]
- Pimputkar, S.; Kawabata, S.; Speck, J.S.; Nakamura, S. Improved growth rates and purity of basic ammonothermal. J. Cryst. Growth 2014, 403, 7. [Google Scholar] [CrossRef]
- Schimmel, S.; Duchstein, P.; Steigerwald, T.G.; Kimmel, A.-C.L.; Schlücker, E.; Zahn, D.; Niewa, R.; Wellmann, P. In situ X-ray monitoring of transport and chemistry of Ga-containing intermediates under ammonothermal growth conditions of GaN. J. Cryst. Growth 2018, 498, 214–223. [Google Scholar] [CrossRef]
- Mikawa, Y.; Ishinabe, T.; Kawabata, S.; Mochizuki, T.; Kojima, A.; Kagamitani, Y.; Fujisawa, H. Determination of absolute value of quantum efficiency of radiation in high quality GaN single crystals using an integrating sphere. Proc. SPIE 2015, 9363, 936302. [Google Scholar]
- Bao, Q.; Hashimoto, T.; Sato, F.; Hazu, K.; Saito, M.; Kagamitani, Y.; Ishinabe, T.; Kayano, R.; Tomida, D.; Qiao, K.; et al. Acidic ammonothermal growth of GaN crystals using GaN powder as a nutrient. CrystEngComm 2013, 15, 5382–5386. [Google Scholar] [CrossRef]
- Ehrentraut, D.; Pakalapati, R.T.; Kamber, D.S.; Jiang, W.; Pocius, D.W.; Downey, B.C.; McLaurin, M.; D’Evelyn, M.P. High quality, low cost ammonothermal bulk GaN substrates. Jpn. J. Appl. Phys. 2013, 52, 08JA01. [Google Scholar] [CrossRef]
- Institute of High Pressure Physics. Available online: www.unipress.waw.pl (accessed on 18 August 2020).
- Mori, Y.; Imade, M.; Maruyama, M.; Yoshimura, M.; Yamane, H.; Kawamura, F.; Kawamura, T. Handbook of Crystal Growth: Bulk Crystal Growth: Basic Techniques, and Growth Mechanisms and Dynamics, 2nd ed.; Rudolph, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 505–533. [Google Scholar]
- Mori, Y. Recent Advances of GaN Growth by Na-Flux Method. In Proceedings of the International Conference on Nitride Semiconductors (ICNS-13), Seattle, WA, USA, 7–12 July 2019. [Google Scholar]
- Mikawa, Y.; Ishinabe, T.; Kawabata, S.; Mochizuki, T.; Kojima, A.; Kagamitani, Y.; Fujisawa, H. Ammonothermal growth of polar and non-polar bulk GaN crystal. Proc. SPIE 2015, 9363, 936302. [Google Scholar]
- Mikawa, Y. Recent progress of large size and low dislocation bulk GaN growth. In Proceedings of the SPIE Photonics West 2020, San Francisco, CA, USA, 4–6 February 2020. [Google Scholar]
- Bockowski, M.; Iwinska, M.; Amilusik, M.; Fijalkowski, M.; Lucznik, B.; Sochacki, T. Challenges and future perspectives in HVPE-GaN growth on ammonothermal GaN seeds. Semicond. Sci. Technol. 2016, 31, 093002. [Google Scholar] [CrossRef]
- Weyher, J.L.; Lazar, S.; Macht, L.; Liliental-Weber, Z.; Molnar, R.J.; Muller, S.; Sivel, G.M.; Nowak, G.; Grzegory, I. Orthodox etching of HVPE-grown GaN. J. Cryst. Growth 2007, 305, 384. [Google Scholar] [CrossRef]
- Sochacki, T.; Amilusik, M.; Fijalkowski, M.; Iwinska, M.; Lucznik, B.; Weyher, J.L.; Kamler, G.; Kucharski, R.; Grzegory, I.; Bockowski, M. Examination of defects and the seed’s critical thickness in HVPE-GaN growth on ammonothermal GaN seed. Phys. Status Solidi B 2014, 1–8. [Google Scholar] [CrossRef]
- Keller, S.; Suh, C.S.; Fichtenbaum, N.A.; Furukawa, M.; Chu, R.; Chen, Z.; Vijayraghavan, K.; Rajan, S.; DenBaars, S.P.; Speck, J.S.; et al. Growth and characterization of In-polar and N-polar InAlN by metal organic chemical vapor deposition. J. Appl. Phys. 2008, 104, 093510. [Google Scholar] [CrossRef]
- Franssen, G.; Suski, T.; Krysko, M.; Lucznik, B.; Grzegory, I.; Krukowski, S.; Khachapuridze, A.; Czernecki, R.; Grzanka, S.; Mensz, P.; et al. Influence of substrate misorientation on properties of InGaN layers grown on freestanding GaN. Phys. Status Solidi C 2008, 5, 1485–1487. [Google Scholar] [CrossRef]
- Leach, J.H.; Biswas, N.; Paskova, T.; Preble, E.A.; Evans, K.R.; Wu, M.; Ni, X.; Li, X.; Özgür, Ü.; Morkoç, H. Effect of substrate offcut on AlGaN/GaN HFET structures on bulk GaN substrates. Proc. SPIE 2011, 7939, 79390E. [Google Scholar]
- Paskova, T.; Bickerman, M. Handbook of Crystal Growth, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015; p. 602. [Google Scholar]
- Fujito, K.; Kubo, S.; Nagaoka, H.; Mochizuki, T.; Namita, H.; Nagao, S. Bulk GaN crystals grown by HVPE. J. Cryst. Growth 2009, 311, 3011. [Google Scholar] [CrossRef]
- Cadoret, R. Growth mechanisms of (0 0. 1) GaN substrates in the hydride vapour-phase method: Surface diffusion, spiral growth, H2 and GaCl3 mechanisms. J. Cryst. Growth 1999, 205, 123–135. [Google Scholar] [CrossRef]
- Koukitu, A.; Kumagai, Y. Technology of Gallium Nitride Crystal Growth; Ehrentraut, D., Meissner, E., Bockowski, M., Eds.; Springer: Heidelberg, Germany, 2010; p. 31. [Google Scholar]
- Oshima, Y.; Yoshida, T.; Watanabe, K.; Shibata, M.; Mishima, T. Properties of Ge-doped, high-quality bulk GaN crystals fabricated by hydride vapor phase epitaxy. J. Cryst. Growth 2010, 312, 3569–3573. [Google Scholar] [CrossRef]
- Richter, E.; Gridneva, E.; Weyers, M.; Traenkle, G. Fe-doping in hydride vapor-phase epitaxy for semi-insulating gallium nitride. J. Cryst. Growth 2016, 456, 97–100. [Google Scholar] [CrossRef]
- Ke, X.; Jian-Feng, W.; Guo-Qiang, R. Progress in bulk GaN growth. Chin. Phys. B 2015, 24, 066105. [Google Scholar]
- Iwinska, M.; Piotrzkowski, R.; Litwin-Staszewska, E.; Sochacki, T.; Amilusik, M.; Fijalkowski, M.; Lucznik, B.; Bockowski, M. Highly resistive C-doped hydride vapor phase epitaxy-GaN grown on ammonothermally crystallized GaN seeds. Appl. Phys. Express 2017, 10, 011003. [Google Scholar] [CrossRef]
- Iwinska, M.; Zajac, M.; Lucznik, B.; Fijalkowski, M.; Amilusik, M.; Sochacki, T.; Litwin-Staszewska, E.; Piotrzkowski, R.; Grzegory, I.; Bockowski, M. Iron and manganese as dopants used in the crystallization of highly resistive HVPE-GaN on native seeds. Jpn. J. Appl. Phys. 2019, 58, SC1047. [Google Scholar] [CrossRef]
- Motoki, K. SEI Technical Review, Number 70; Sumitomo Electric Industries: Osaka, Japan, 2010; p. 28. [Google Scholar]
- Oshima, Y.; Yoshida, T.; Eri, T.; Watanabe, K.; Shibata, M.; Mishima, T. Technology of Gallium Nitride Crystal Growth; Ehrentraut, D., Meissner, E., Bockowski, M., Eds.; Springer: Heidelberg, Germany, 2010; p. 79. [Google Scholar]
- Motoki, K.; Okahisa, T.; Nakahata, S.; Matsumoto, N.; Kimura, H.; Kasai, H.; Takemoto, K.; Uematsu, K.; Ueno, M.; Kumagai, Y.; et al. Growth and characterization of freestanding GaN substrates. J. Cryst. Growth 2002, 237, 912. [Google Scholar] [CrossRef]
- Motoki, K.; Okahisa, T.; Hirota, R.; Nakahata, S.; Uematsu, K.; Matsumoto, N. Dislocation reduction in GaN crystal by advanced-DEEP. J. Cryst. Growth 2007, 305, 377. [Google Scholar] [CrossRef]
- Oshima, Y.; Eri, T.; Shibata, M.; Sunakawa, H.; Kobayashi, K.; Ichihashi, T.; Usui, A. Preparation of freestanding GaN wafers by hydride vapor phase epitaxy with void-assisted separation. Jpn. J. Appl. Phys. 2003, 42, L1. [Google Scholar] [CrossRef]
- Usui, A.; Ichihashi, T.; Kobayashi, K.; Sunakawa, H.; Oshima, Y.; Eri, T.; Shibata, M. Role of TiN film in the fabrication of freestanding GaN wafers using hydride vapor phase epitaxy with void-assisted separation. Phys. Status Solidi A 2002, 194, 572–575. [Google Scholar] [CrossRef]
- Kirste, L.; Danilewsky, A.N.; Sochacki, T.; Kohler, K.; Zajac, M.; Kucharski, R.; Bockowski, M.; McNally, P.J. Doping in bulk HVPE-GaN grown on native seeds–highly conductive and semi-insulating crystals. ECS J. Solid State Sci. Technol. 2015, 4, P324–P330. [Google Scholar] [CrossRef]
- Doradzinski, R.; Dwilinski, R.; Garczynski, J.; Sierzputowski, L.P.; Kanbara, Y. Technology of Gallium Nitride Crystal Growth; Ehrentraut, D., Meissner, E., Bockowski, M., Eds.; Springer: Heidelberg, Germany, 2010; pp. 137–158. [Google Scholar]
- Hashimoto, T.; Nakamura, S. Technology of Gallium Nitride Crystal Growth; Ehrentraut, D., Meissner, E., Bockowski, M., Eds.; Springer: Heidelberg, Germany, 2010; pp. 161–183. [Google Scholar]
- Ehrentraut, D.; Bockowski, M. Handbook of Crystal Growth: Bulk Crystal Growth: Basic Techniques, and Growth Mechanisms and Dynamics, 2nd ed.; Rudolph, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 577–619. [Google Scholar]
- Sochacki, T.; Amilusik, M.; Lucznik, B.; Bockowski, M.; Weyher, J.L.; Nowak, G.; Sadovyi, B.; Kamler, G.; Grzegory, I.; Kucharski, R.; et al. HVPE-GaN growth on ammonothermal GaN crystals. Proc. SPIE 2013, 8625, 86250B-1. [Google Scholar]
- Tsukada, Y.; Enatsu, Y.; Kubo, S.; Ikeda, H.; Kurihara, K.; Matsumoto, H.; Nagao, S.; Mikawa, Y.; Fujito, K. High-quality, 2-inch-diameter m-plane GaN substrates grown by hydride vapor phase epitaxy on acidic ammonothermal seeds. Jpn. J. Appl. Phys. 2016, 55, 05FC01. [Google Scholar] [CrossRef]
- Bockowski, M.; Iwinska, M.; Amilusik, M.; Lucznik, B.; Fijalkowski, M.; Litwin-Staszewska, E.; Piotrzkowski, R.; Sochacki, T. Doping in bulk HVPE-GaN grown on native seeds–highly conductive and semi-insulating crystals. J. Cryst. Growth 2018, 499, 1–7. [Google Scholar] [CrossRef]
- Domagala, J.Z.; Smalc-Koziorowska, J.; Iwinska, M.; Sochacki, T.; Amilusik, M.; Lucznik, B.; Fijalkowski, M.; Kamler, G.; Grzegory, I.; Kucharski, R.; et al. Influence of edge-grown HVPE GaN on the structural quality of c-plane oriented HVPE-GaN grown on ammonothermal GaN substrates. J. Cryst. Growth 2016, 456, 80–85. [Google Scholar] [CrossRef]
- Amilusik, M.; Wlodarczyk, D.; Suchocki, A.; Bockowski, M. Micro-Raman studies of strain in bulk GaN crystals grown by hydride vapor phase epitaxy on ammonothermal GaN seeds. Jpn. J. Appl. Phys. 2019, 58, SCCB32. [Google Scholar] [CrossRef]
- Sitar, Z.; North Carolina State University, Raleigh, NC, USA. Personal Communication, 2017.
- HexaTech, Inc. Available online: http://www.hexatechinc.com/company.html (accessed on 18 August 2020).
- Kucharski, R.; Sochacki, T.; Lucznik, B.; Amilusik, M.; Grabianska, K.; Iwinska, M.; Bockowski, M. Wide Bandgap Semiconductors for Power Electronics. Materials, Devices, Applications; Wellmann, P., Ohtani, N., Rupp, R., Eds.; Wiley-VCH: Hoboken, NJ, USA, 2020. [Google Scholar]




| HVPE | Ammonothermal | |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabianska, K.; Jaroszynska, A.; Sidor, A.; Bockowski, M.; Iwinska, M. GaN Single Crystalline Substrates by Ammonothermal and HVPE Methods for Electronic Devices. Electronics 2020, 9, 1342. https://doi.org/10.3390/electronics9091342
Grabianska K, Jaroszynska A, Sidor A, Bockowski M, Iwinska M. GaN Single Crystalline Substrates by Ammonothermal and HVPE Methods for Electronic Devices. Electronics. 2020; 9(9):1342. https://doi.org/10.3390/electronics9091342
Chicago/Turabian StyleGrabianska, Karolina, Arianna Jaroszynska, Aneta Sidor, Michal Bockowski, and Malgorzata Iwinska. 2020. "GaN Single Crystalline Substrates by Ammonothermal and HVPE Methods for Electronic Devices" Electronics 9, no. 9: 1342. https://doi.org/10.3390/electronics9091342
APA StyleGrabianska, K., Jaroszynska, A., Sidor, A., Bockowski, M., & Iwinska, M. (2020). GaN Single Crystalline Substrates by Ammonothermal and HVPE Methods for Electronic Devices. Electronics, 9(9), 1342. https://doi.org/10.3390/electronics9091342





