CMOS Interfaces for Internet-of-Wearables Electrochemical Sensors: Trends and Challenges
Abstract
1. Introduction
2. Smart Wearables and the IoT: Basic Requirements and Main Fields of Application
2.1. Wearables and Non-Invasive Monitoring
2.2. Wearable Sensors in Health
2.3. Wearable Sensors in Sport
2.4. Safety at Work
2.5. Defense and Law Enforcement
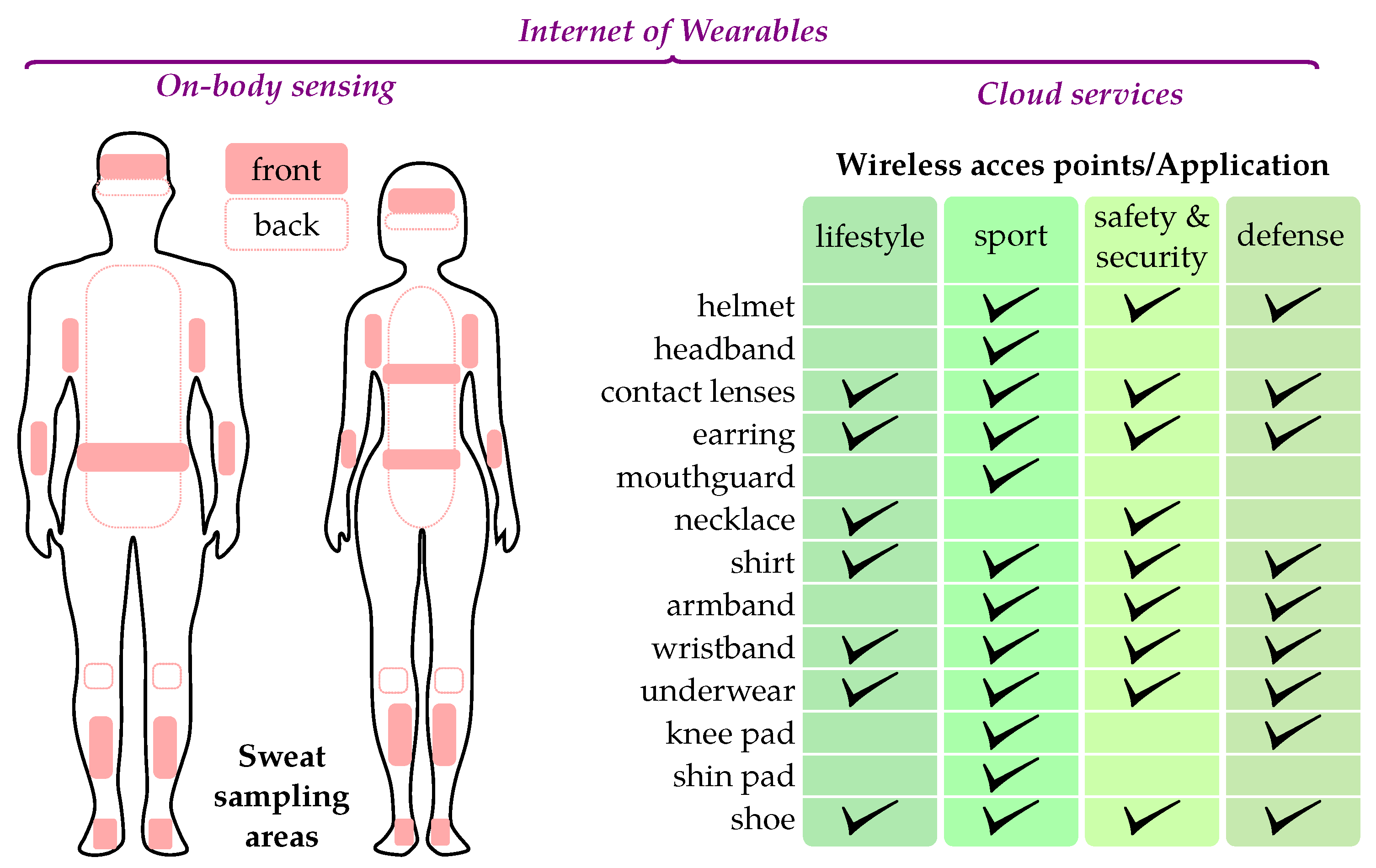
2.6. Design and Fabrication of Wearable Devices
2.7. Data-Access Points: Smartphone, Smartwatch and WPAN Radio
3. Electrochemical Sensing Overview
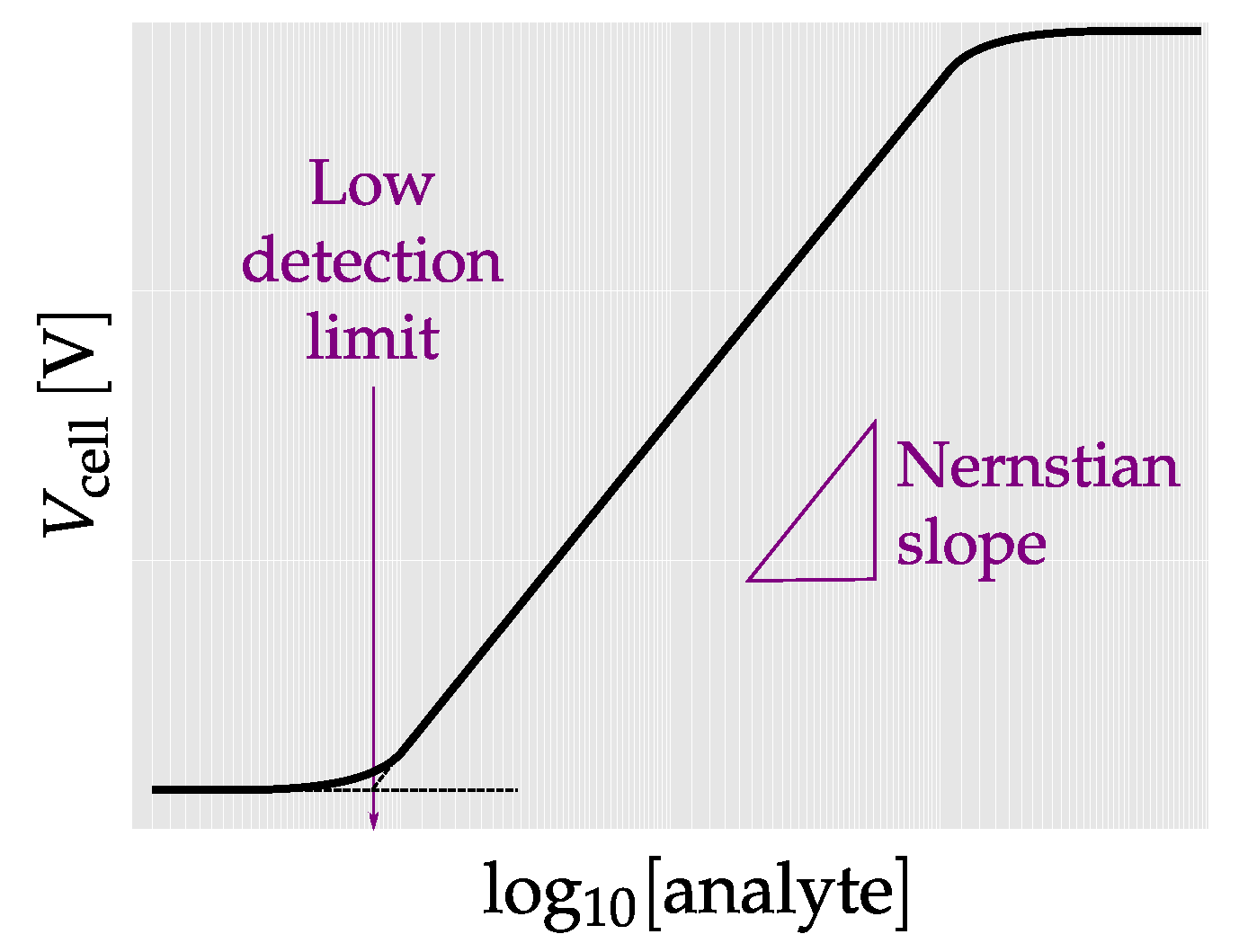
3.1. Potentiometric Sensing
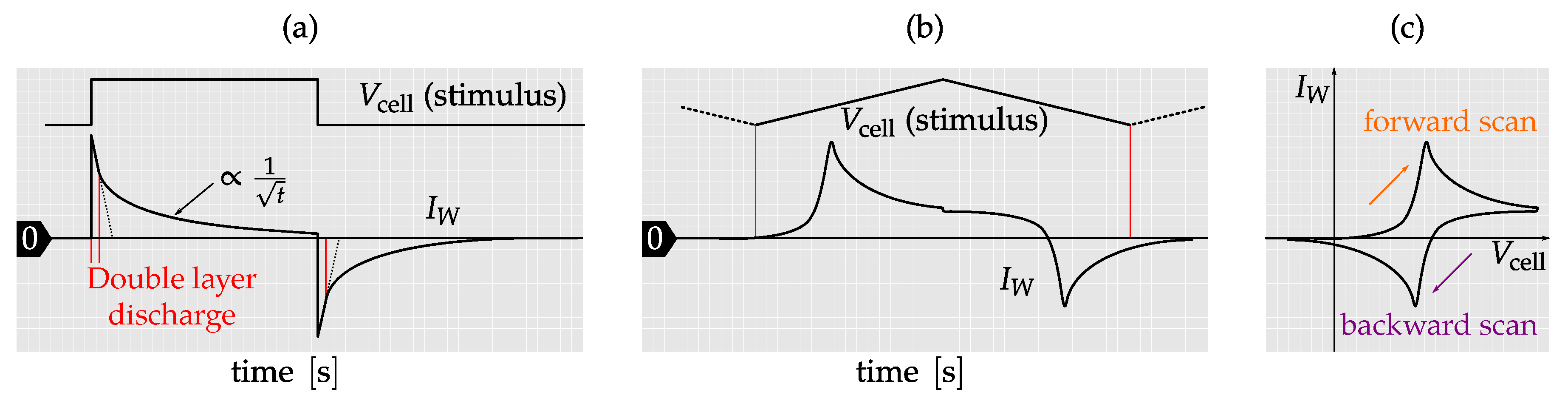
3.2. Amperometric Sensing
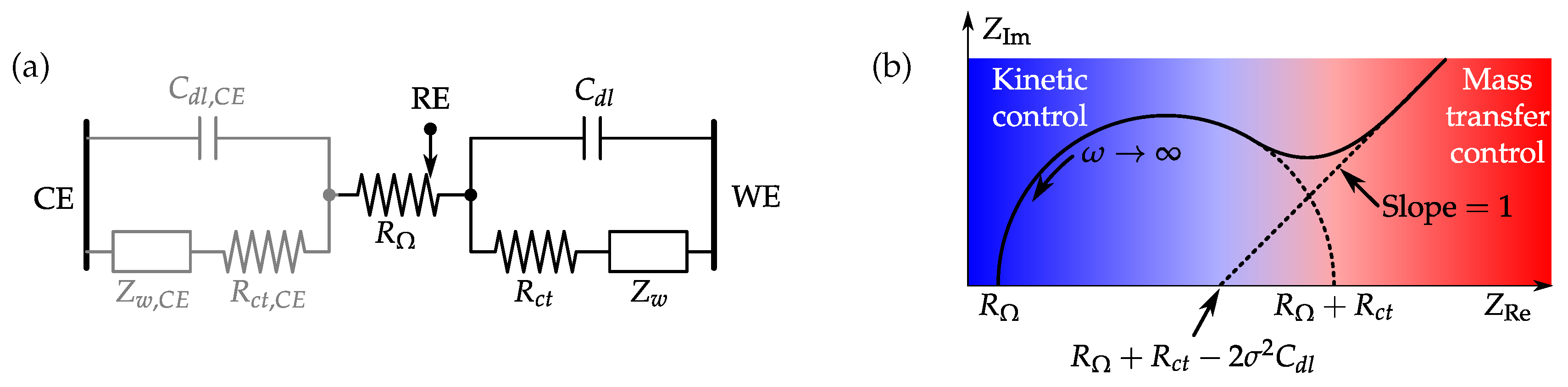
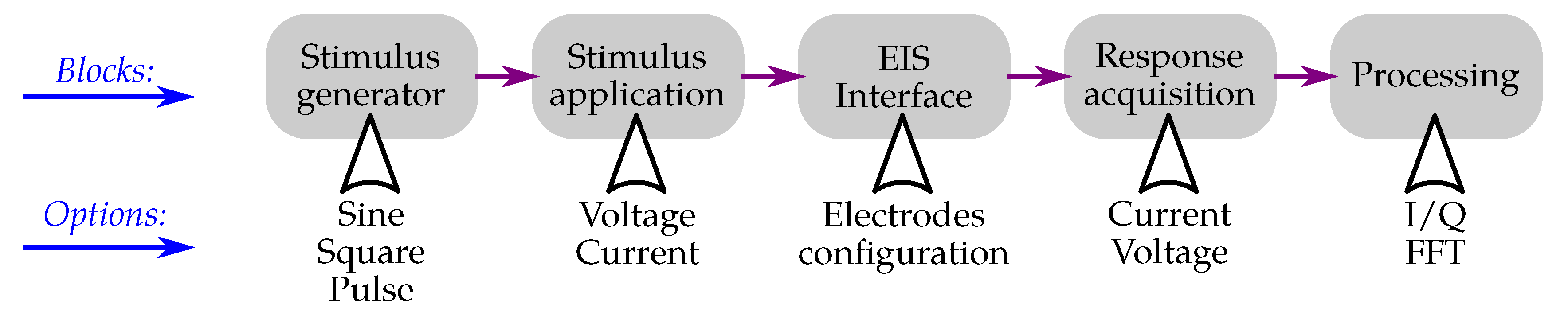
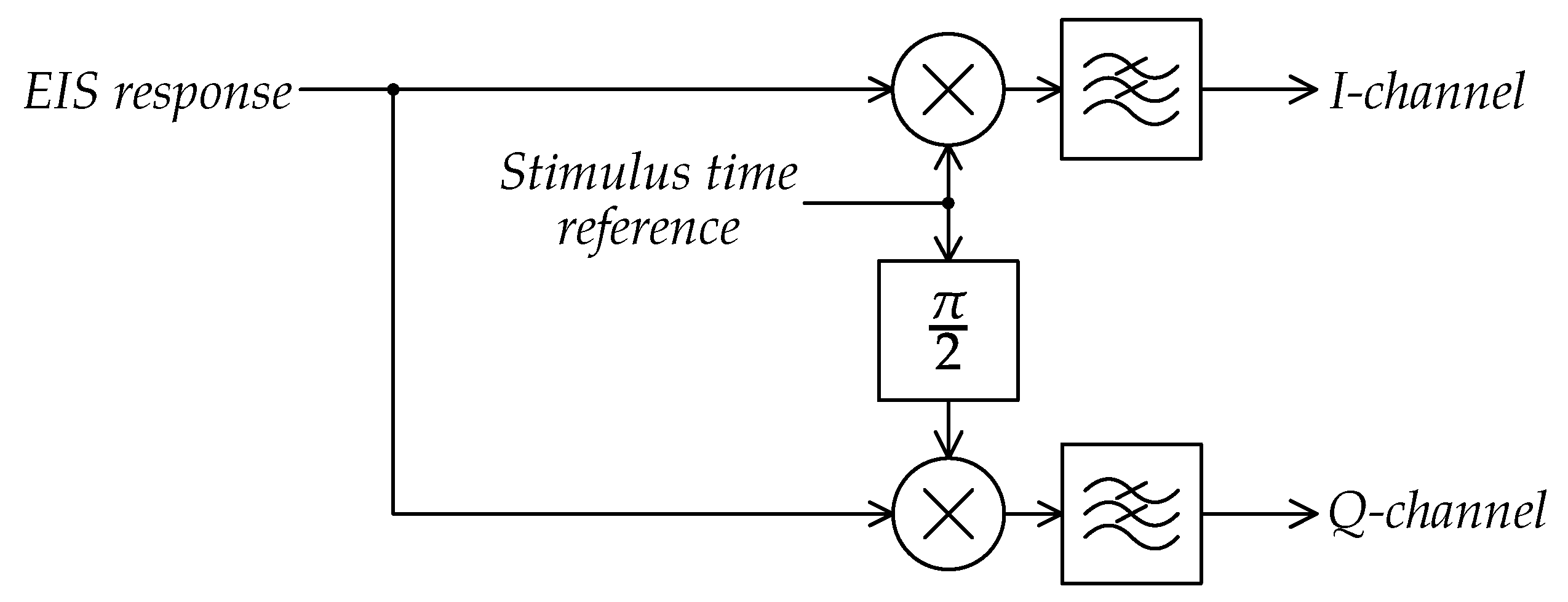
3.3. Electrochemical Impedance Spectroscopy
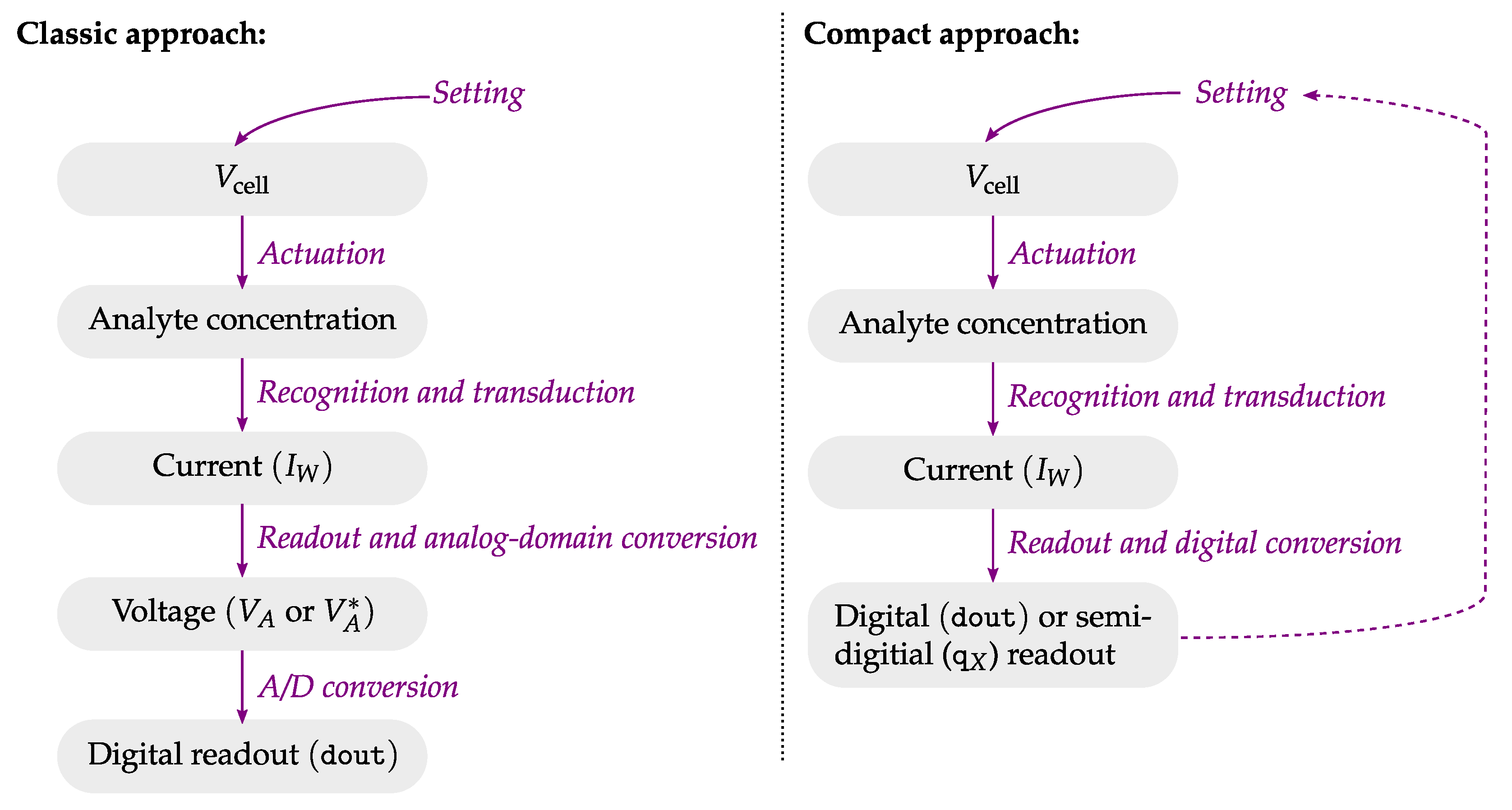
4. Advanced CMOS Interfaces for Electrochemical Sensors
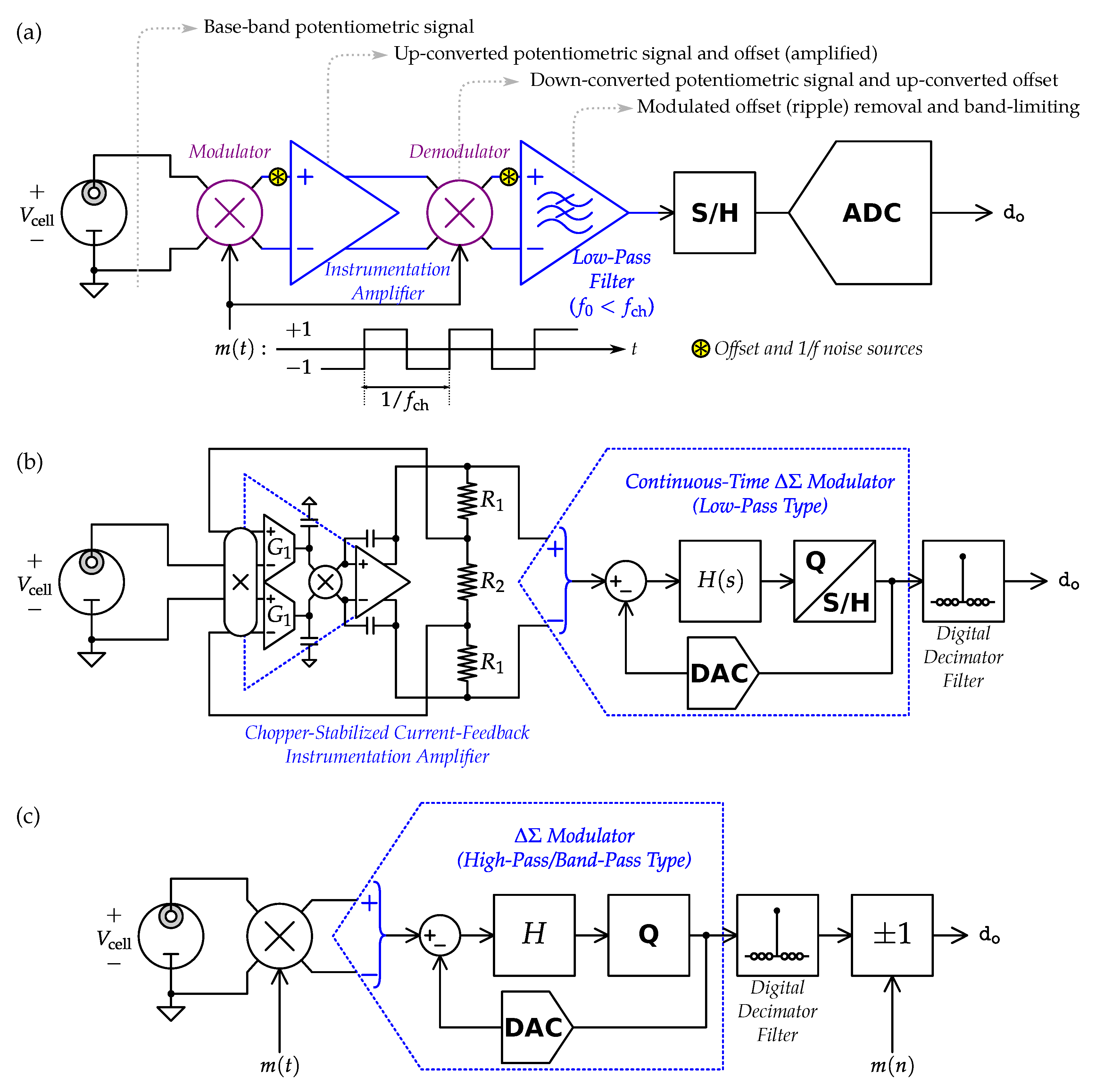
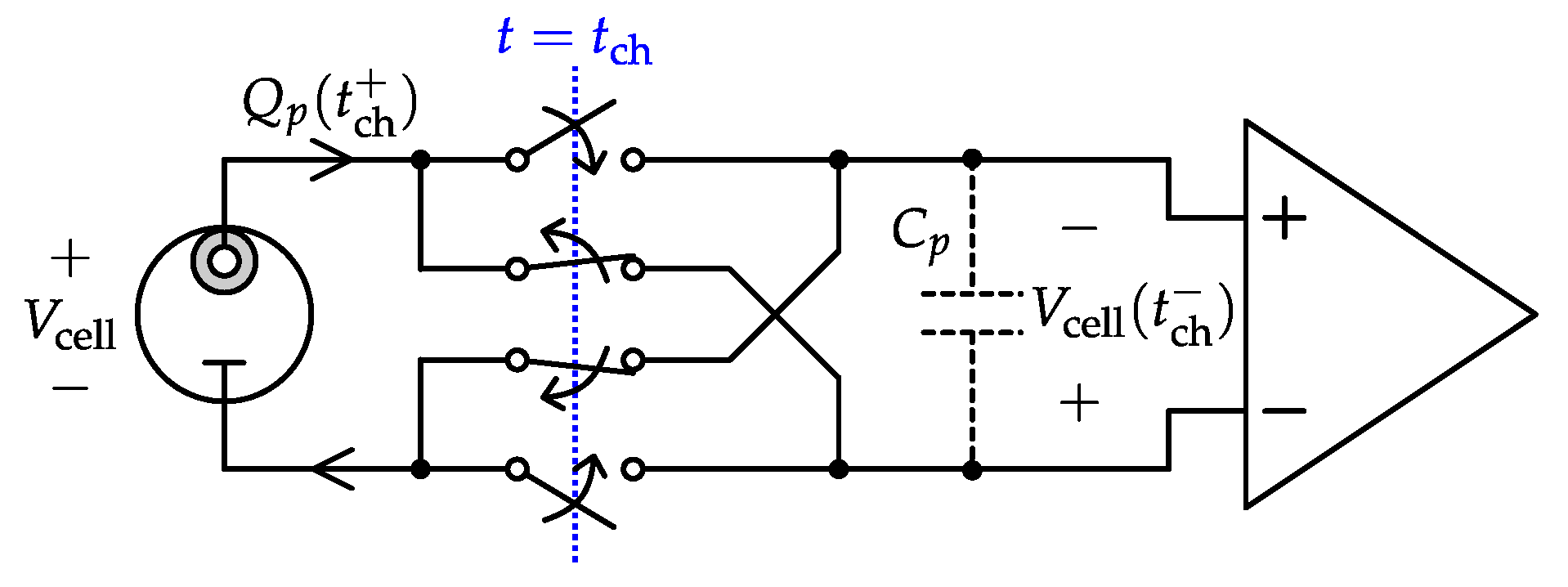
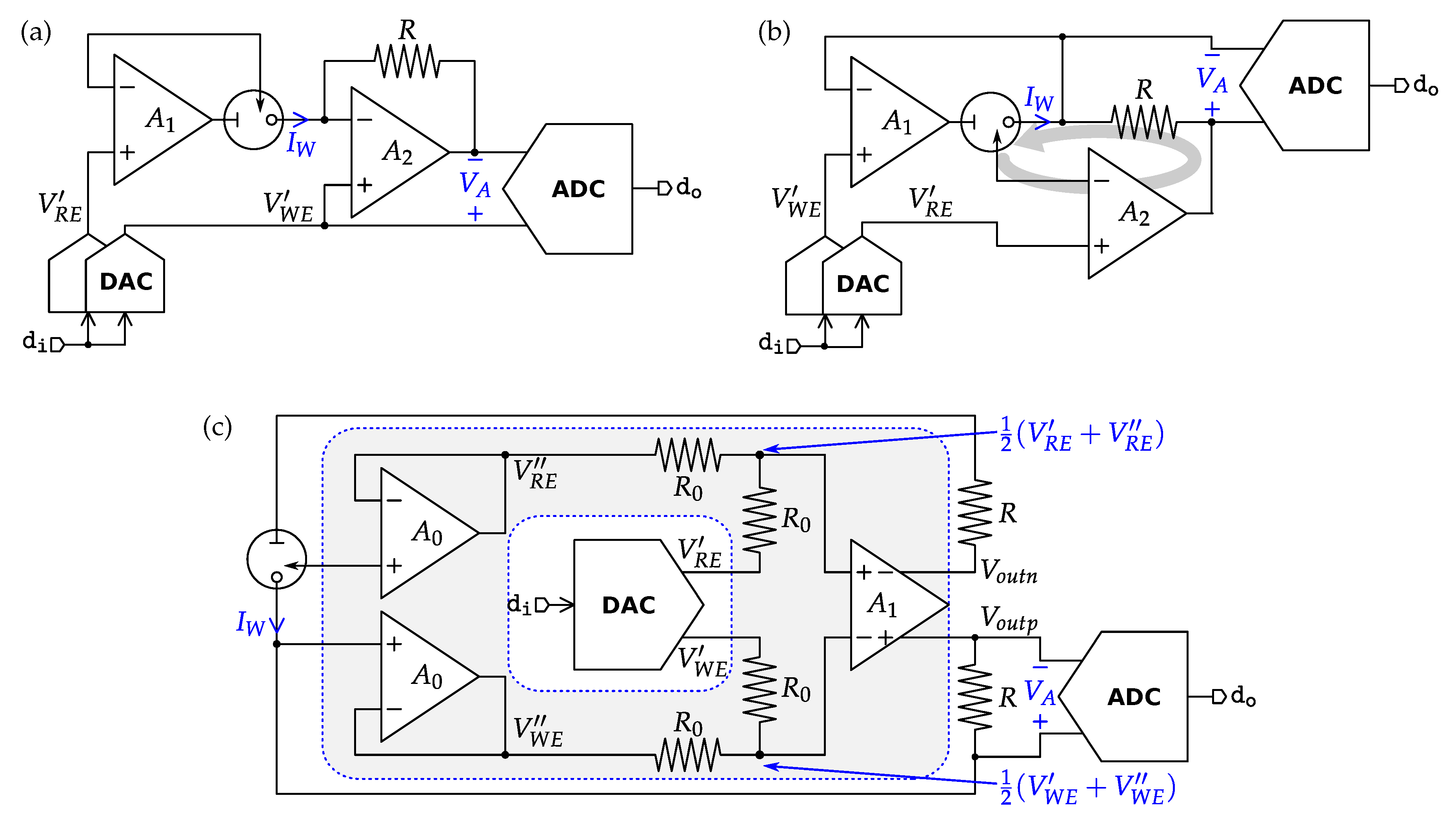
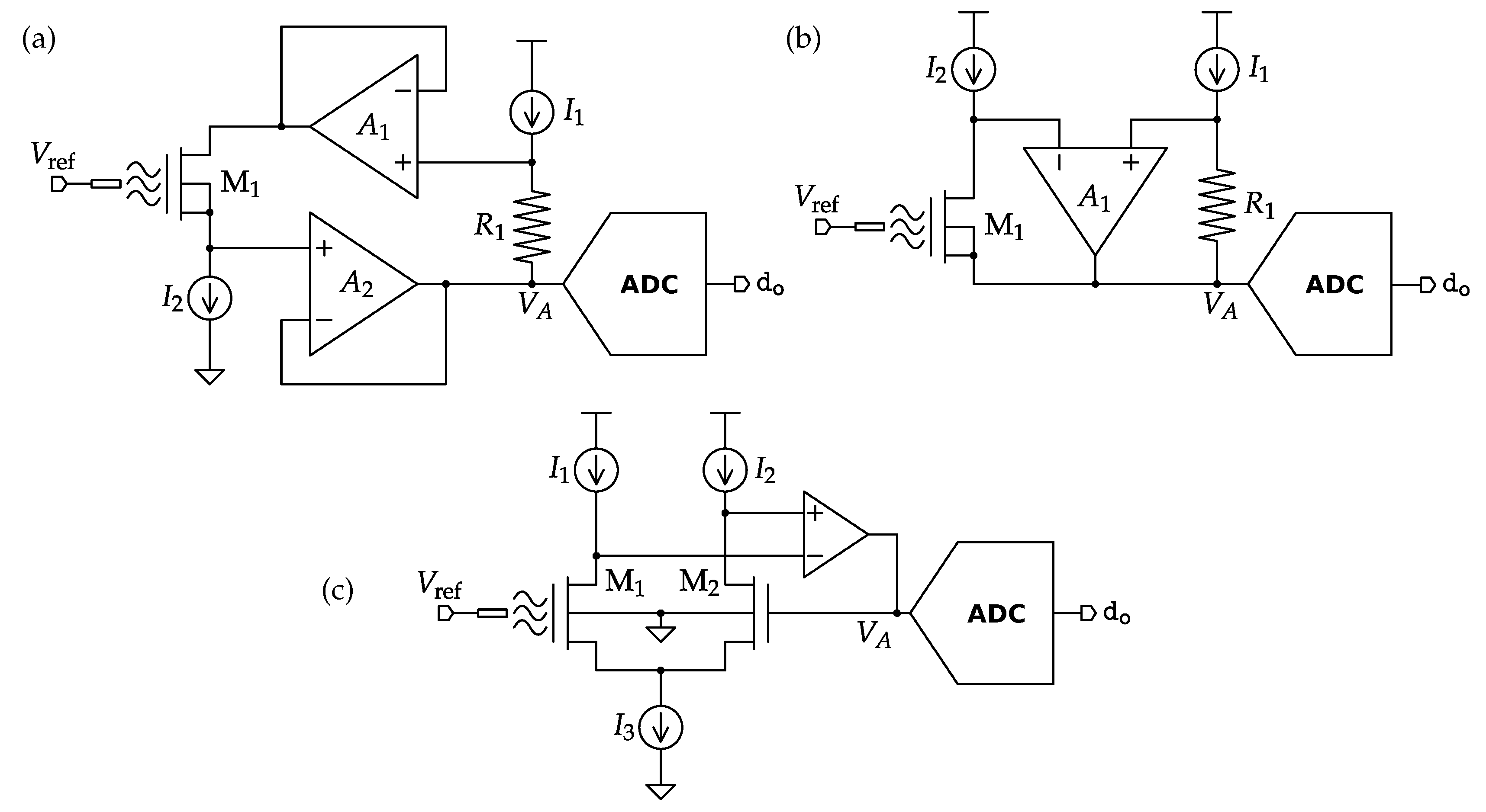
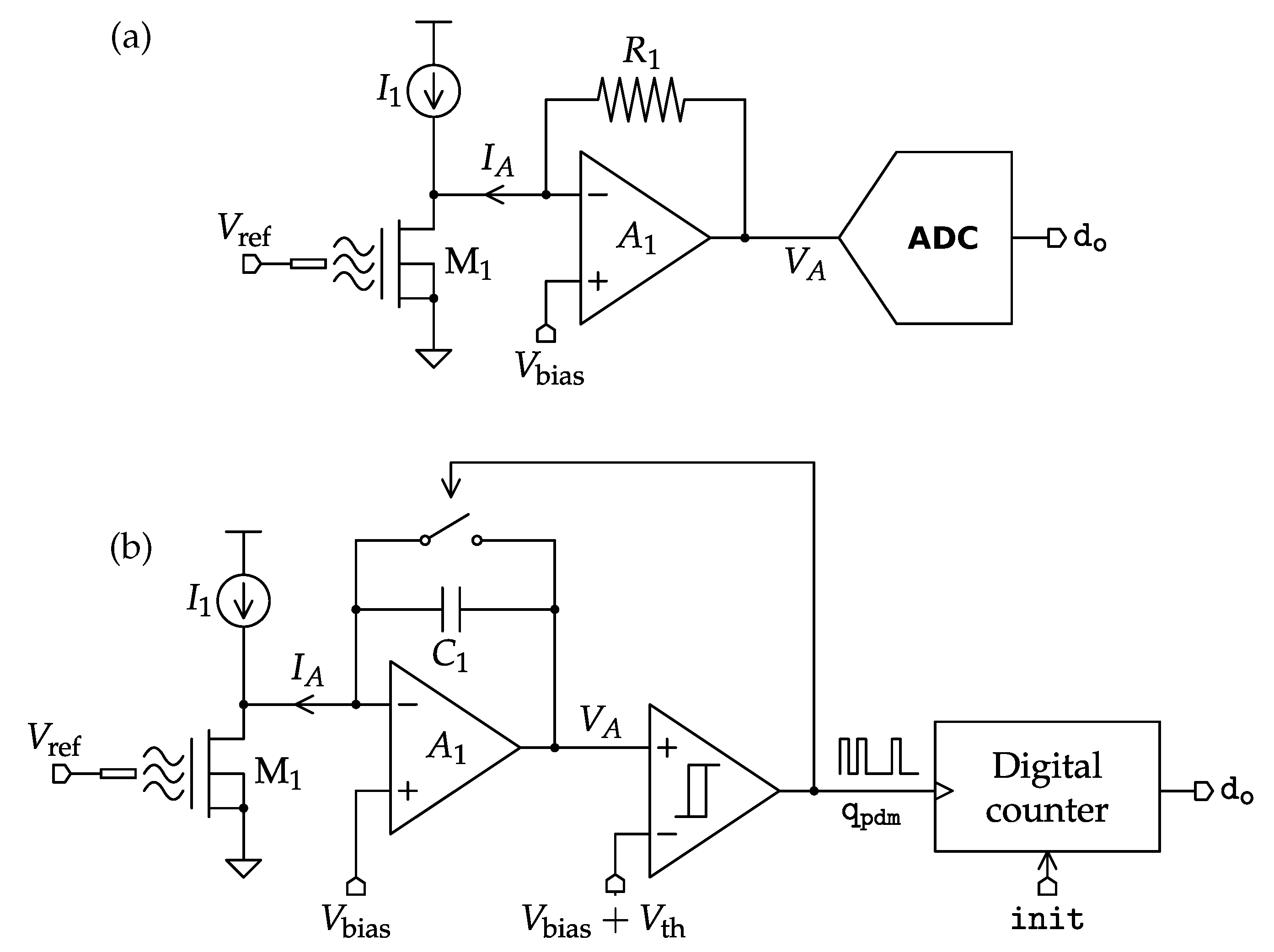
4.1. Potentiometric Interfaces
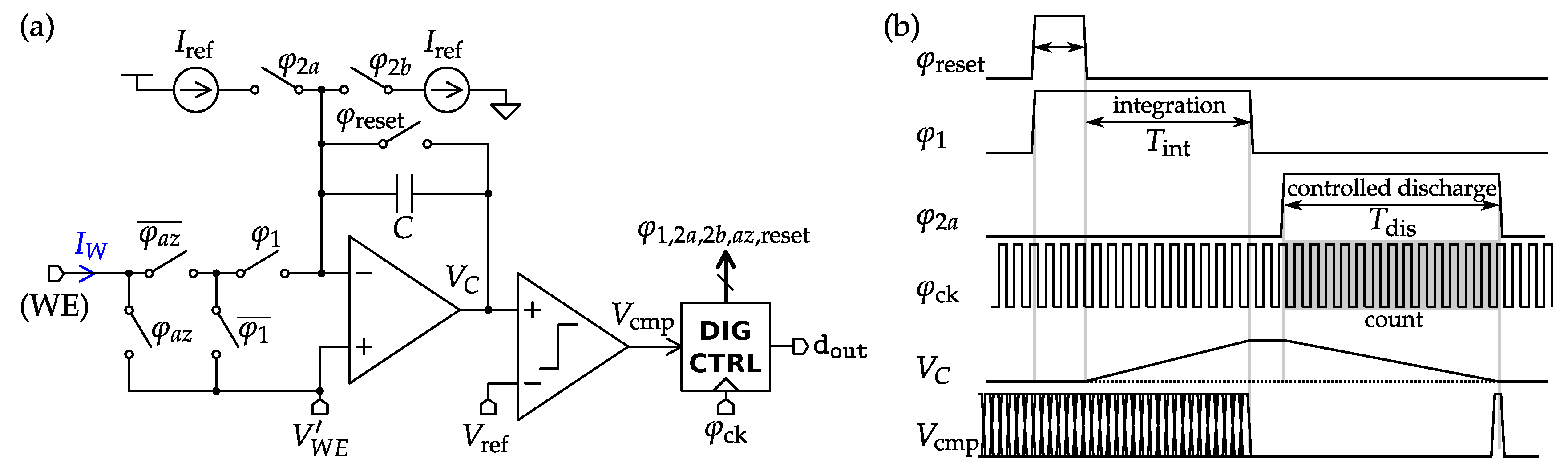
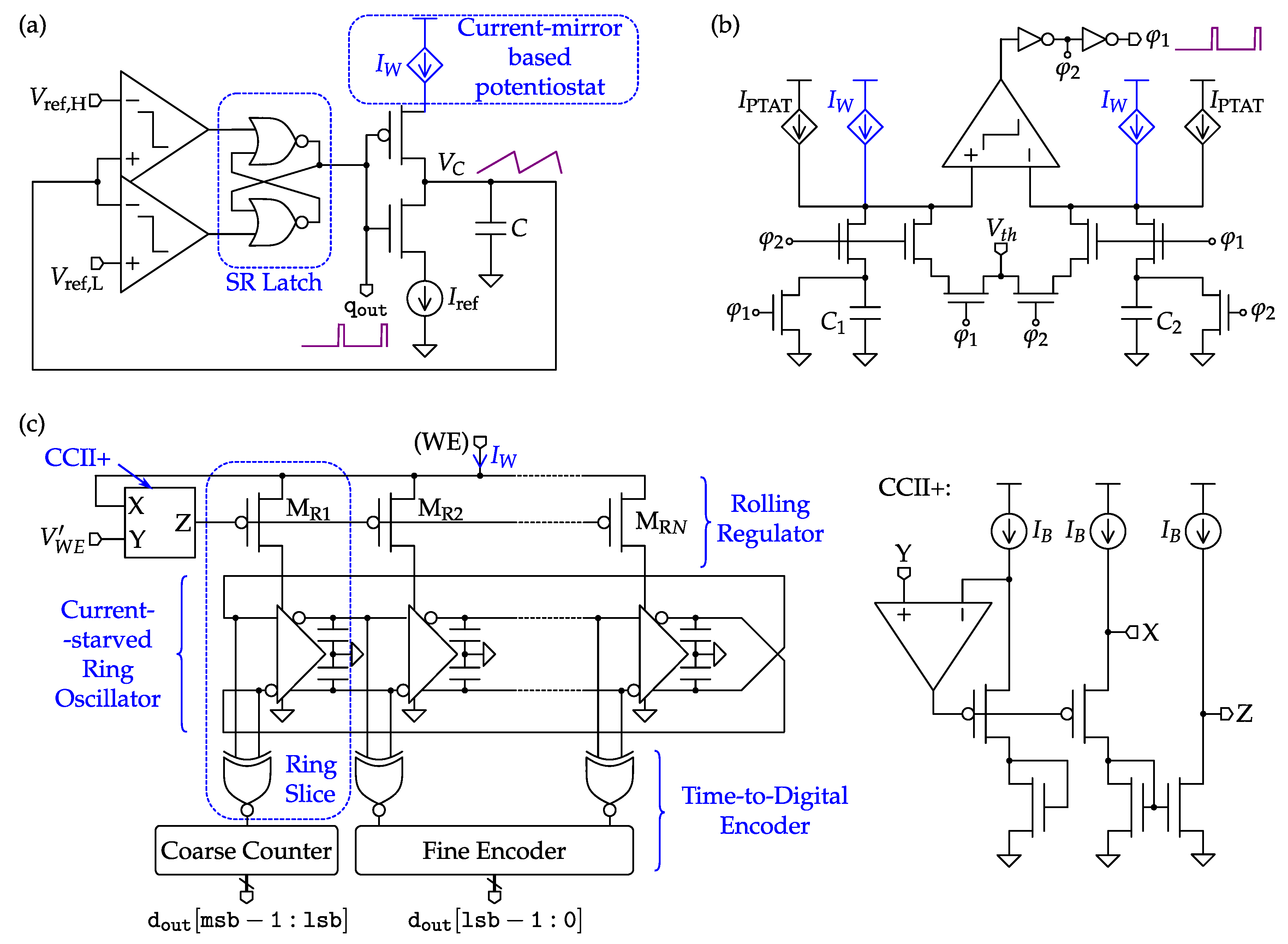
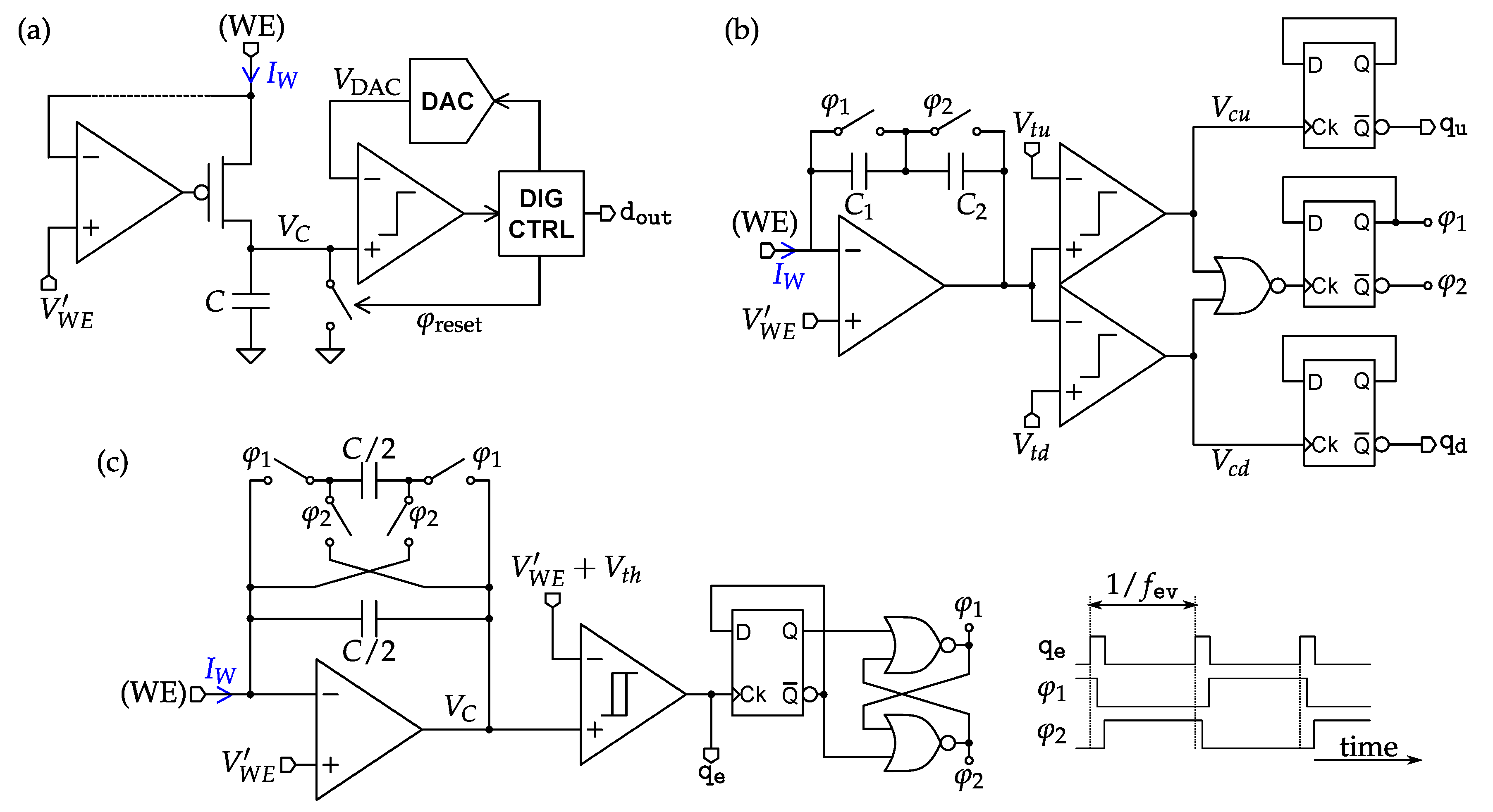
4.2. Amperometric Interfaces
4.3. Interfaces for Sensing FETs
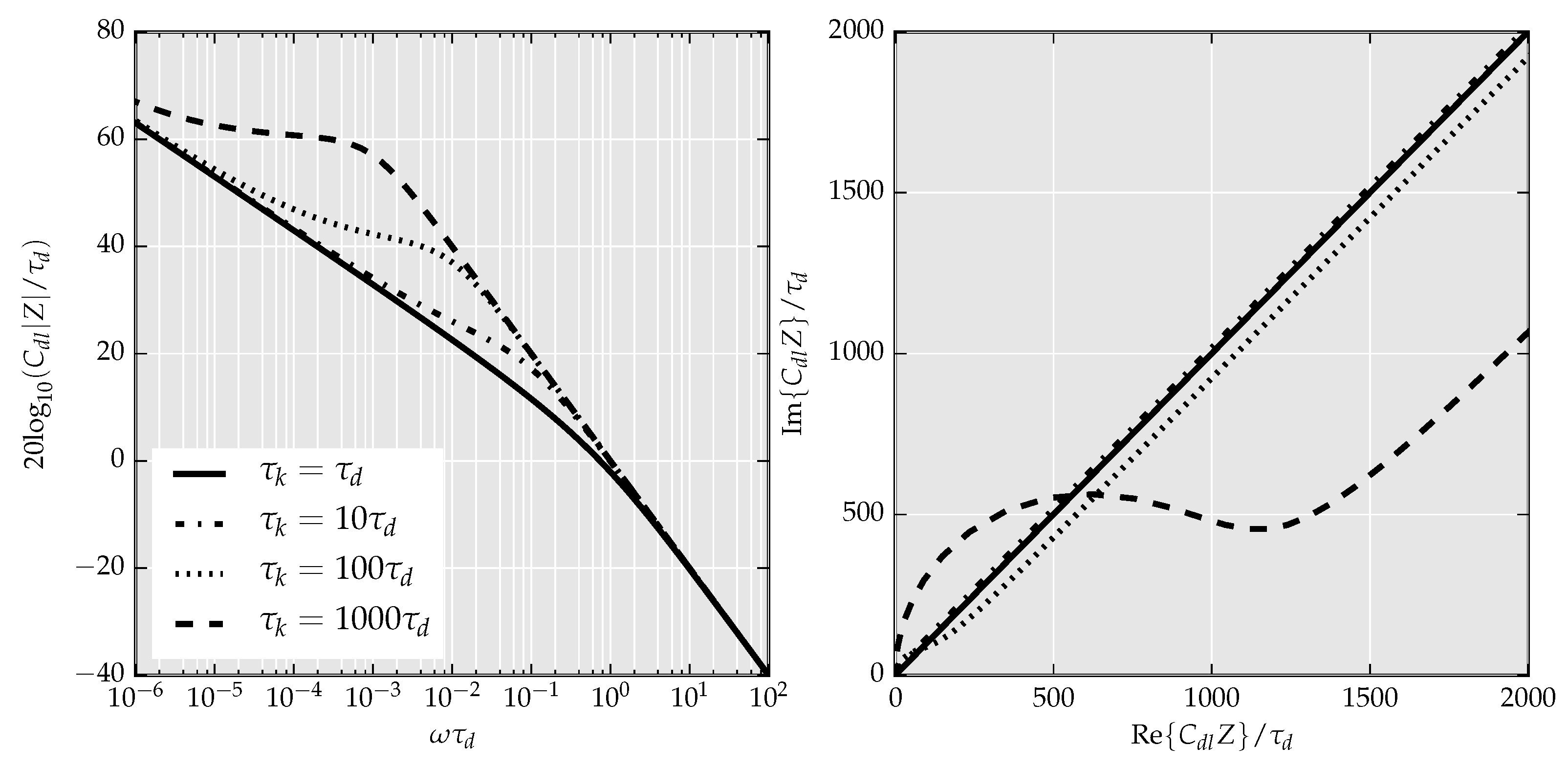
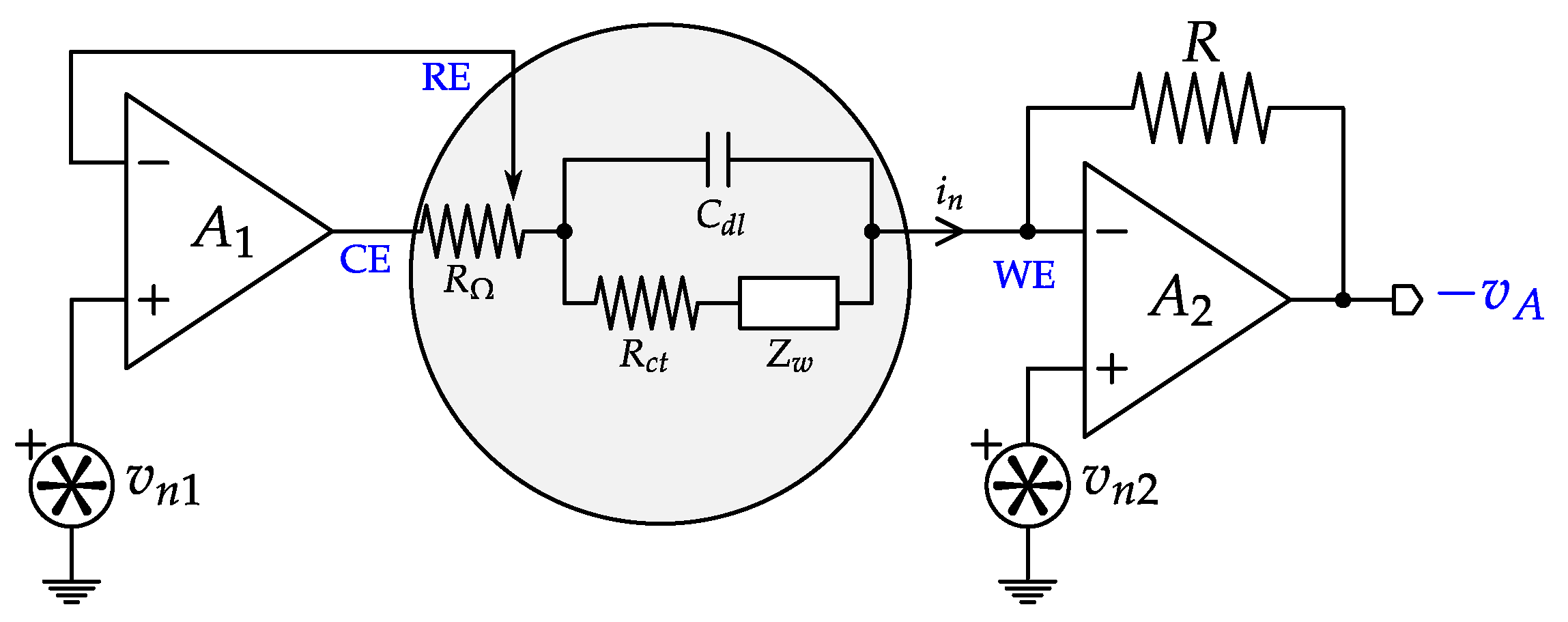
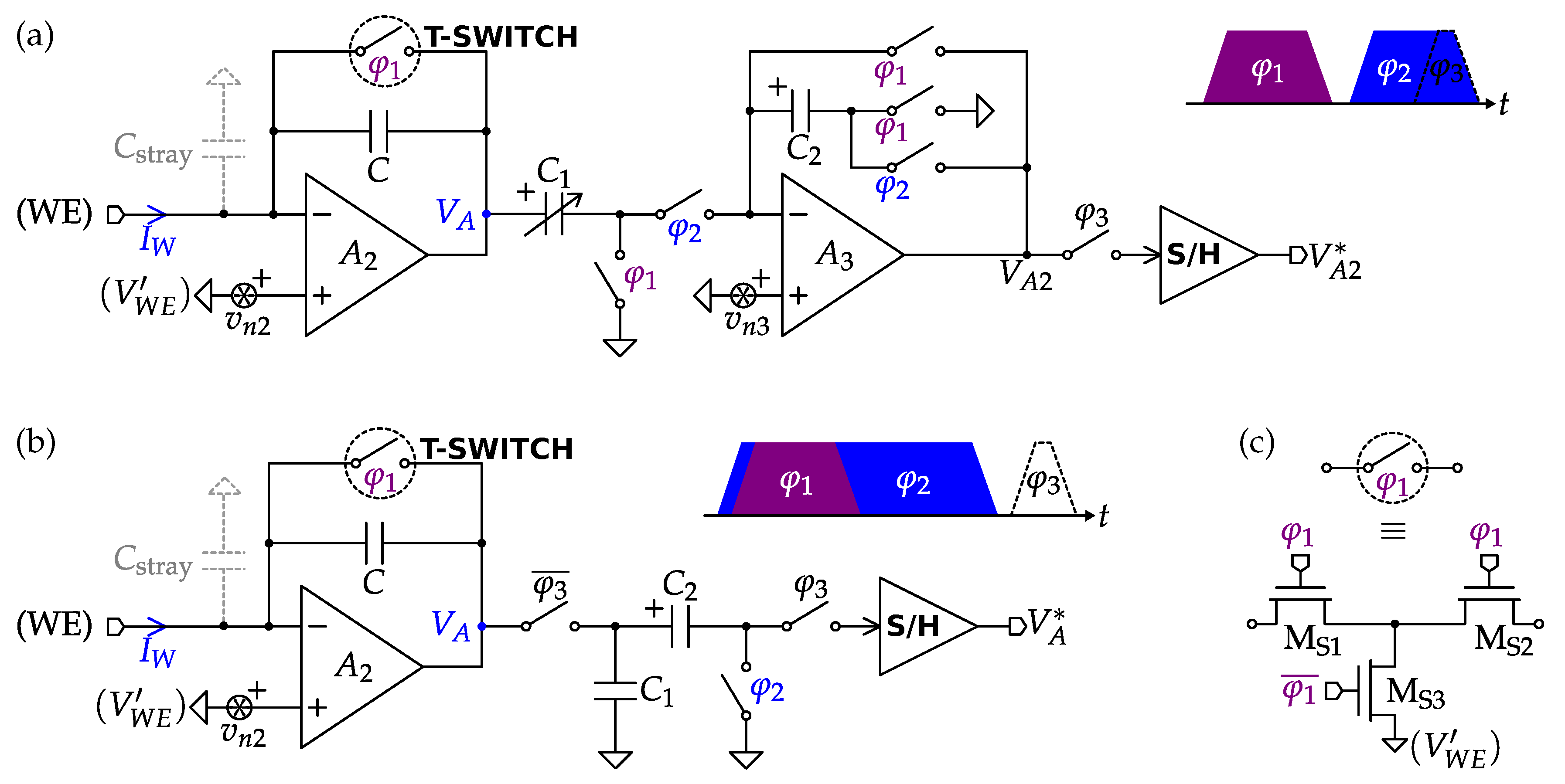
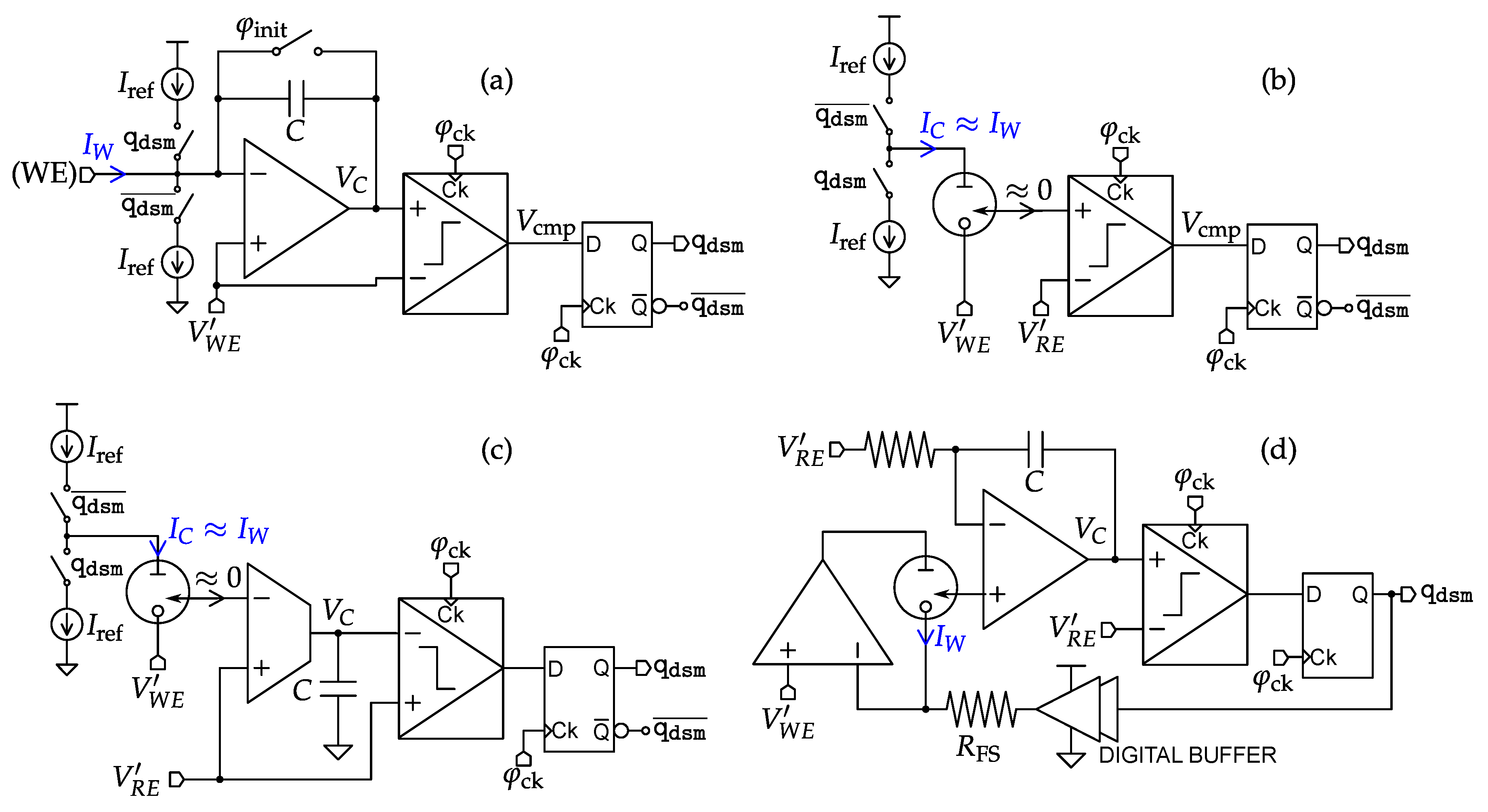
4.4. Impedance Spectroscopy Interfaces
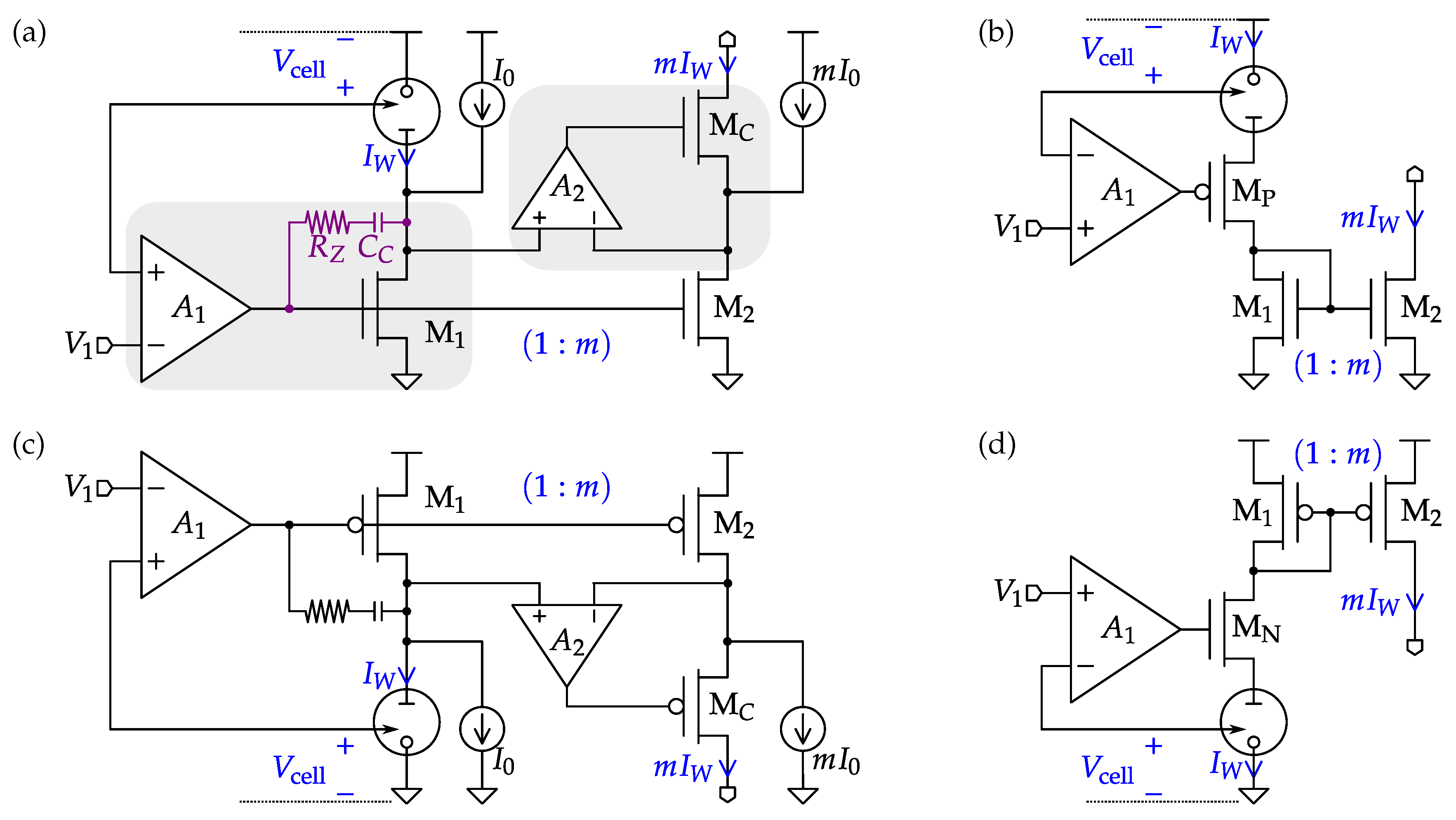
4.5. Readout Interfaces for Bio-Fuel Cells
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, X.; Li, L.; Mason, A.J. Handbook of Bioelectronics; Carrara, S., Iniewski, K., Eds.; Cambridge University Press: Cambridge, UK, 2015; ISBN 978-1-107-04083-0. [Google Scholar]
- Ghafar-Zadeh, E. Wireless Integrated Biosensors for Point-of-Care Diagnostic Applications. Sensors 2015, 15, 3236–3261. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, X.; Li, L.; Mu, X.; Genov, R.; Mason, A.J. CMOS Electrochemical Instrumentation for Biosensor Microsystems: A Review. Sensors 2017, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Market Research Engine. Available online: www.marketresearchengine.com/wearable-devices-market (accessed on 21 January 2019).
- Lymberis, A. Feedback from Stakeholders on the Smart Wearables Reflection and Orientation Paper. News Article. Available online: https://ec.europa.eu/digital-single-market/en/news/feedback-stakeholders-smart-wearables-reflection-and-orientation-paperec.europa.eu/digital-single-market/en/news/feedback-stakeholders-smart-wearables-reflection-and-orientation-paper (accessed on 21 January 2019).
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Kim, J.; Jeerapan, I.; Sempionatto, J.R.; Barfidokht, A.; Mishra, R.K.; Campbell, A.S.; Hubble, L.J.; Wang, J. Wearable Bioelectronics: Enzyme-Based Body-Worn Electronic Devices. Acc. Chem. Res. 2018, 51, 2820–2828. [Google Scholar] [CrossRef] [PubMed]
- Ngamchuea, K.; Chaisiwamongkhol, K.; Batchelor-McAuleya, C.; Compton, R.G. Chemical analysis in saliva and the search for salivary biomarkers—A tutorial review. Analyst 2018, 143, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Ngamchuea, K.; Chaisiwamongkhol, K.; Batchelor-McAuleya, C.; Compton, R.G. Correction: Chemical analysis in saliva and the search for salivary biomarkers—A tutorial review. Analyst 2018, 143, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Farandos, N.M.; Yetisen, A.K.; Monteiro, M.J.; Lowe, C.R.; Yun, S.H. Contact Lens Sensors in Ocular Diagnostics. Adv. Healthc. Mater. 2014, 4, 792–810. [Google Scholar] [CrossRef]
- Selvam, A.P.; Muthukumar, S.; Kamakoti, V.; Prasad, S. A wearable biochemical sensor for monitoring alcohol consumption lifestyle through Ethyl glucuronide (EtG) detection in human sweat. Sci. Rep. 2016, 6, 23111. [Google Scholar] [CrossRef]
- Kim, J.; Sempionatto, J.R.; Imani, S.; Hartel, M.C.; Barfidokht, A.; Tang, G.; Campbell, A.S.; Mercier, P.P.; Wang, J. Simultaneous Monitoring of Sweat and Interstitial Fluid Using a Single Wearable Biosensor Platform. Adv. Sci. 2018, 5, 1800880. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Martin, A.; García-Carmona, L.; Barfidokht, A.; Kurniawan, J.F.; Moreto, J.R.; Tang, G.; Shin, A.; Liu, X.; Escarpa, A.; et al. Skin-Worn Soft Microfluidic Potentiometric Detection System. Electroanalysis 2018. [Google Scholar] [CrossRef]
- Kim, S.B.; Lee, K.H.; Raj, M.S.; Lee, B.; Reeder, J.T.; Koo, J.; Hourlier-Fargette, A.; Bandodkar, A.J.; Won, S.M.; Sekine, Y.; et al. Soft, Skin-Interfaced Microfluidic Systems with Wireless, Battery-Free Electronics for Digital, Real-Time Tracking of Sweat Loss and Electrolyte Composition. Small 2018, 14, e1802876. [Google Scholar] [CrossRef] [PubMed]
- Anastasova, S.; Crewther, B.; Bembnowicz, P.; Curto, V.; Ip, H.M.; Rosa, B.; Yang, G.Z. A wearable multisensing patch for continuous sweat monitoring. Biosens. Bioelectron. 2017, 93, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Burtis, C.A.; Bruns, D.E. Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics, 7th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015; ISBN 978-1-4557-4165-6. [Google Scholar]
- Baker, L.B. Sweating Rate and Sweat Sodium Concentration in Athletes: A Review of Methodology and Intra/Interindividual Variability. Sports Med. 2017, 47, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Mitsubayashi, K.; Suzuki, M.; Tamiya, E.; Karube, I. Analysis of metabolites in sweat as a measure of physical condition. Anal. Chim. Acta 1994, 289, 27–34. [Google Scholar] [CrossRef]
- Sakharov, D.A.; Shkurnikov, M.U.; Vagin, M.Y.; Yashina, E.I.; Karyakin, A.A.; Tonevitsky, A.G. Relationship between lactate concentrations in active muscle sweat and whole blood. Bull. Exp. Biol. Med. 2010, 150, 83–85. [Google Scholar] [CrossRef]
- Pribil, M.M.; Laptev, G.U.; Karyakina, E.E.; Karyakin, A.A. Noninvasive Hypoxia Monitor Based on Gene-Free Engineering of Lactate Oxidase for Analysis of Undiluted Sweat. Anal. Chem. 2014, 86, 5215–5219. [Google Scholar] [CrossRef]
- Van Haeringen, N.J. Clinical biochemistry of tears. Surv. Ophthalmol. 1981, 26, 84–96. [Google Scholar] [CrossRef]
- Carreño, E.; Enríquez-de-Salamanca, A.; Tesón, M.; García-Vázquez, C.; Stern, M.E.; Whitcup, S.M.; Calonge, M. Cytokine and chemokine levels in tears from healthy subjects. Acta Ophthalmol. 2010, 88, e250–e258. [Google Scholar] [CrossRef]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose Sensing for Diabetes Monitoring: Recent Developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef]
- Abelson, M.B.; Udell, I.J.; Weston, J.H. Normal human tear pH by direct measurement. Arch. Ophthalmol. 1981, 99, 301. [Google Scholar] [CrossRef]
- Tékus, É.; Kaj, M.; Szabó, E.; Szénási, N.; Kerepesi, I.; Figler, M.; Gábriel, R.; Wilhelm, M. Comparison of blood and saliva lactate level after maximum intensity exercise. Acta Biol. Hung. 2012, 63, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Hagen, T.; Korson, M.; Wolfsdorf, J. Urinary lactate excretion to monitor the efficacy of treatment of type I glycogen storage disease. Mol. Genet. Metab. 2000, 70, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Steckl, A.J.; Ray, P. Stress Biomarkers in Biological Fluids and Their Point-of-Use Detection. ACS Sens. 2018, 3, 2025–2044. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, B.H. An overview of minimally invasive technologies. Clin. Chem. 1992, 38, 1596–1600. [Google Scholar] [PubMed]
- World Health Organization, Diabetes Programme. Available online: https://www.who.int/diabetes/en/ (accessed on 21 January 2019).
- Wilson, R.; Turner, A.P.F. Glucose oxidase: An ideal enzyme. Biosens. Bioelectron. 1992, 7, 165–185. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Toghill, K.E.; Compton, R.G. Electrochemical non-enzymatic glucose sensors: A perspective and an evaluation. Int. J. Electrochem. Sci. 2010, 5, 1246–1301. [Google Scholar]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Gifford, R. Continuous Glucose Monitoring: 40 Years, What We’ve Learned and What’s Next. ChemPhysChem 2013, 14, 2032–2044. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Tierney, M.J.; Tamada, J.A.; Potts, R.O.; Jovanovic, L.; Garg, S. Clinical evaluation of the GlucoWatch biographer: A continual, non-invasive glucose monitor for patients with diabetes. Biosens. Bioelectron. 2001, 16, 621–629. [Google Scholar] [CrossRef]
- FreeStyle Libre. Available online: https://www.freestylelibre.com (accessed on 21 January 2019).
- Hoss, U.; Budiman, E.S. Factory-Calibrated Continuous Glucose Sensors: The Science Behind the Technology. Diabetes Technol. Ther. 2017, 19, S-44–S-50. [Google Scholar] [CrossRef] [PubMed]
- James, D.A.; Petrone, N. Sensors and Wearable Technologies in Sport: Technologies, Trends and Approaches for Implementation; Future Direction Sereires; Springer: Singapore, 2016; p. 49. ISBN 978-981-10-0992-1. [Google Scholar]
- Rossi, A.; Pappalardo, L.; Cintia, P.; Iaia, F.M.; Fernàndez, J.; Medina, D. Effective injury forecasting in soccer with GPS training data and machine learning. PLoS ONE 2018, 13, e0201264. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, D.R.; Drummond, C.; Craker, J.; Rowbottom, J.R.; Voos, J.E. Wearable Devices for Sports: New Integrated Technologies Allow Coaches, Physicians, and Trainers to Better Understand the Physical Demands of Athletes in Real Time. IEEE Pulse 2017, 8, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Sonner, Z.; Hauke, A.; Wilder, E.; Kasting, J.; Gaillard, T.; Swaille, D.; Sherman, F.; Mao, X.; Hagen, J.; et al. A new oil/membrane approach for integrated sweat sampling and sensing: sample volumes reduced from μL‘s to nL’s and reduction of analyte contamination from skin. Lab Chip 2016, 16, 4415–4423. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Tai, L.C.; Ngo, Q.P.; Chao, M.; Zhang, G.B.; Gao, W.; Bariya, M.; Bullock, J.; Kim, H.; Fahad, H.M.; et al. A Wearable Microfluidic Sensing Patch for Dynamic Sweat Secretion Analysis. ACS Sens. 2018, 3, 944–952. [Google Scholar] [CrossRef]
- Choi, J.; Xue, Y.; Xia, W.; Ray, T.R.; Reeder, J.T.; Bandodkar, A.J.; Kang, D.; Xu, S.; Huang, Y.; Rogers, J.A. Soft, skin-mounted microfluidic systems for measuring secretory fluidic pressures generated at the surface of the skin by eccrine sweat glands. Lab Chip 2017, 17, 2572–2580. [Google Scholar] [CrossRef]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef]
- Garcia, S.O.; Ulyanovaa, Y.V.; Figueroa-Teranb, R.; Bhatta, K.H.; Singhala, S.; Atanassov, P. Wearable Sensor System Powered by a Biofuel Cell for Detection of Lactate Levels in Sweat. ECS J. Solid State Sci. Technol. 2016, 5, M3075–M3081. [Google Scholar] [CrossRef]
- Toft, A.D.; Jensen, L.B.; Bruunsgaard, H.; Ibfelt, T.; Halkjær-Kristensen, J.; Febbraio, M.; Pedersen, B.K. Cytokine response to eccentric exercise in young and elderly humans. Am. J. Physiol. Cell Physiol. 2002, 283, C289–C295. [Google Scholar] [CrossRef]
- Marques-Deak, A.; Marques-Deak, A.; Cizza, G.; Eskandari, F.; Torvik, S.; Christie, I.C.; Sternberg, E.M.; Phillips, T.M. Measurement of cytokines in sweat patches and plasma in healthy women: Validation in a controlled study. J. Immunol. Methods 2006, 315, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Willner, I.; Zayats, M. Electronic aptamer-based sensors. Angew. Chem. 2007, 46, 6408–6418. [Google Scholar] [CrossRef]
- Kumar, L.S.S.; Wang, X.; Hagen, J.; Naik, R.; Papautskya, I.; Heikenfeld, J. Label free nano-aptasensor for interleukin-6 in protein-dilute bio fluids such as sweat. Anal. Methods 2016, 8, 3440–3444. [Google Scholar] [CrossRef]
- Morgan, R.M.; Patterson, M.J.; Nimmo, M.A. Acute effects of dehydration on sweat composition in men during prolonged exercise in the heat. Acta Physiol. Scand. 2004, 182, 37–43. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Kenefick, R.W. Dehydration: Physiology, assessment, and performance effects. Compr. Physiol. 2014, 4, 257–285. [Google Scholar] [CrossRef] [PubMed]
- Moran, P.; Prichard, J.G.; Ansley, L.; Howatson, G. The influence of blood lactate sample site on exercise prescription. J. Strength Cond. Res. 2012, 26, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Sonner, Z.; Wilder, E.; Heikenfeld, J.; Kasting, G.; Beyette, F.; Swaile, D.; Sherman, F.; Joyce, J.; Hagen, J.; Kelley-Loughnane, N.; et al. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics 2015, 9, 031301. [Google Scholar] [CrossRef]
- Wearable Devices Used for Industrial Applications. Available online: https://vandrico.com/wearables/device-categories/application/industrial (accessed on 21 January 2019).
- Cone, E.J.; Hillsgrove, M.J.; Jenkins, A.J.; Keenan, R.M.; Darwin, W.D. Sweat testing for heroin, cocaine, and metabolites. J. Anal. Toxicol. 1994, 18, 298–305. [Google Scholar] [CrossRef]
- Mishra, R.K.; Hubble, L.J.; Martín, A.; Kumar, R.; Barfidokht, A.; Kim, K.; Musameh, M.M.; Kyratzis, I.L.; Wang, J. Wearable Flexible and Stretchable Glove Biosensor for On-Site Detection of Organophosphorus Chemical Threats. ACS Sens. 2017, 2, 553–561. [Google Scholar] [CrossRef]
- Mishra, R.K.; Barfidokht, A.; Karajic, A.; Sempionatto, J.R.; Wang, J.; Wang, J. Wearable potentiometric tattoo biosensor for on-body detection of G-type nerve agents simulants. Sens. Actuator B Chem. 2018, 273, 966–972. [Google Scholar] [CrossRef]
- Mishra, R.K.; Martín, A.; Nakagawa, T.; Barfidokht, A.; Lu, X.; Sempionatto, J.R.; Lyu, K.M.; Karajic, A.; Musameh, M.M.; Kyratzis, I.L.; et al. Detection of vapor-phase organophosphate threats using wearable conformable integrated epidermal and textile wireless biosensor systems. Biosens. Bioelectron. 2018, 101, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tzeng, T.; Lin, T.; Huang, C.; Yen, P.; Kuo, P.; Lin, C.; Lu, S. A Self-Powered CMOS Reconfigurable Multi-Sensor SoC for Biomedical Applications. IEEE J. Solid-State Circuits 2014, 49, 851–866. [Google Scholar] [CrossRef]
- Sun, A.; Venkatesh, A.G.; Hall, D.A. A Multi-Technique Reconfigurable Electrochemical Biosensor: Enabling Personal Health Monitoring in Mobile Devices. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.P.; Ratterman, M.E.; Griffin, D.K.; Hou, L.; Kelley-Loughnane, N.; Naik, R.R.; Hagen, J.A.; Papautsky, I.; Heikenfeld, J.C. Adhesive RFID Sensor Patch for Monitoring of Sweat Electrolytes. IEEE Trans. Biomed. Circuits Syst. 2015, 62, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Beni, V.; Nilsson, D.; Arven, P.; Norberg, P.; Gustafsson, G.; Turner, A.P.F. Printed Electrochemical Instruments for Biosensors. ECS J. Solid State Sci. Technol. 2015, 4, S3001–S3005. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, Y.Y.H.; Challa, S.; Chen, K.; Peck, A.; Hossain, M.F.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nat. Lett. 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Aller-Pellitero, M.; Guimerà, A.; Kitsara, M.; Villa, R.; Rubio, C.; Lakard, B.; Doche, M.L.; Hihnc, J.Y.; del Campo, F.J. Quantitative self-powered electrochromic biosensors. Chem. Sci. 2017, 8, 1995–2002. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Park, H.; Park, J.; Noh, J.; Choi, Y.; Jung, M.; Jung, K.; Pyo, M.; Chen, K.; Javey, A.; et al. Fully printed flexible and disposable wireless cyclic voltammetry tag. Sci. Rep. 2015, 5, 8105. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Facchetti, A.F.; Reuss, R. A Circuits and Systems Perspective of Organic/Printed Electronics: Review, Challenges, and Contemporary and Emerging Design Approaches. IEEE Trans. Emerg. Sel. Top. Circuits Syst. 2017, 7, 7–26. [Google Scholar] [CrossRef]
- Shiwaku, R.; Matsui, H.; Nagamine, K.; Uematsu, M.; Mano, T.; Maruyama, Y.; Nomura, A.; Tsuchiya, K.; Hayasaka, K.; Takeda, Y.; et al. A printed Organic Circuit System for Wearable Amperometric Electrochemical Sensors. Sci. Rep. 2018, 8, 6368. [Google Scholar] [CrossRef]
- Liao, Y.; Yao, H.; Lingley, A.; Parviz, B.; Otis, B.P. A 3-μW CMOS Glucose Sensor for Wireless Contact-Lens Tear Glucose Monitoring. IEEE J. Solid-State Circuits 2012, 47, 335–344. [Google Scholar] [CrossRef]
- Huang, W.; Deb, S.; Seo, Y.; Rao, S.; Chiao, M.; Chiao, J.C. A Passive Radio-Frequency pH-Sensing Tag for Wireless Food-Quality Monitoring. IEEE Sens. J. 2012, 12, 487–495. [Google Scholar] [CrossRef]
- Kassal, P.; Steinberg, M.D.; Steinberg, I.M. Wireless chemical sensors and biosensors: A review. Sens. Actuators B Chem. 2018, 266, 228–245. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Q. Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosens. Bioelectron. 2016, 75, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Michelini, E.; Zangheri, M.; Di Fusco, M.; Calabria, D.; Simoni, P. Smartphone-based biosensors: A critical review and perspectives. TrAC Trends Anal. Chem. 2016, 79, 317–325. [Google Scholar] [CrossRef]
- Quesada-González, D.; Merkoçi, A. Mobile phone-based biosensing: An emerging “diagnostic and communication” technology. Biosens. Bioelectron. 2017, 92, 549–562. [Google Scholar] [CrossRef]
- Grossi, M. A sensor-centric survey on the development of smartphone measurement and sensing systems. J. Meas. 2019, 135, 572–592. [Google Scholar] [CrossRef]
- Johnston, A.H.; Weiss, G.M. Smartwatch-based biometric gait recognition. In Proceedings of the IEEE International Conference on Biometrics Theory, Applications and Systems, Arlington, VA, USA, 8–11 September 2015. [Google Scholar] [CrossRef]
- Xu, W.; Shen, Y.; Zhang, Y.; Bergmann, N.; Hu, W. Gait-Watch: A Context-aware Authentication System for Smart Watch Based on Gait Recognition. In Proceedings of the IEEE/ACM International Conference on Internet-of-Things Design and Implementation, Pittsburgh, PA, USA, 18–21 April 2017; ISBN 978-1-4503-4966-6. [Google Scholar]
- Bandodkar, A.M.; You, J.M.; Kim, N.H.; Gu, Y.; Kumar, R.; Vinu Mohan, A.M.; Kurniawan, J.; Imani, S.; Nakagawa, T.; Parish, B.; et al. Soft, stretchable, high power density electronic skin-based biofuel cells for scavenging energy from human sweat. Energy Environ. Sci. 2017, 10, 1581–1589. [Google Scholar] [CrossRef]
- Lorwongtragool, P.; Sowade, E.; Watthanawisuth, N.; Baumann, R.R.; Kerdcharoen, T. A Novel Wearable Electronic Nose for Healthcare Based on Flexible Printed Chemical Sensor Array. Sensors 2014, 14, 19700–19712. [Google Scholar] [CrossRef]
- Mostafalu, P.; Lenk, W.; Dokmeci, M.R.; Ziaie, B.; Khademhosseini, A.; Sonkusale, S.R. Wireless Flexible Smart Bandage for Continuous Monitoring of Wound Oxygenation. IEEE Trans. Biomed. Circuits Syst. 2015, 9, 670–677. [Google Scholar] [CrossRef]
- Matzeu, G.; O’Quigley, C.; McNamara, E.; Zuliani, C.; Fay, C.; Glennon, T.; Diamond, D. An integrated sensing and wireless communications platform for sensing sodium in sweat. Anal. Methods 2016, 8, 64–71. [Google Scholar] [CrossRef]
- Farooqui, M.F.; Shamim, A. Low Cost Inkjet Printed Smart Bandage for Wireless Monitoring of Chronic Wounds. Sci. Rep. 2016, 6, 28949. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Imani, S.; de Araujo, W.R.; Warchall, J.; Valdés-Ramírez, G.; Paixão, T.R.; Mercier, P.P.; Wang, J. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.; Jeerapan, I.; Imani, S.; Cho, T.N.; Bandodkar, A.; Cinti, S.; Mercier, P.P.; Wang, J. Noninvasive Alcohol Monitoring Using a Wearable Tattoo-Based Iontophoretic-Biosensing System. ACS Sens. 2016, 1, 11–1019. [Google Scholar] [CrossRef]
- Yao, S.; Myers, A.; Malhotra, A.; Lin, F.; Bozkurt, A.; Muth, J.F.; Zhu, Y. A Wearable Hydration Sensor with Conformal Nanowire Electrodes. Adv. Healthc. Mater. 2017, 6, 1601159. [Google Scholar] [CrossRef] [PubMed]
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Nyein, H.Y.Y.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z.; et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. PNAS 2017, 114, 4625–4630. [Google Scholar] [CrossRef] [PubMed]
- Oletic, D.; Bilas, V. Design of Sensor Node for Air Quality Crowdsensing. In Proceedings of the IEEE Sensors Applications Symposium, Zadar, Croatia, 13–15 April 2015. [Google Scholar]
- Azzarelli, J.M.; Mirica, K.A.; Ravnsbæk, J.B.; Swager, T.M. Wireless gas detection with a smartphone via RF communication. PNAS 2014, 111, 18162–18166. [Google Scholar] [CrossRef]
- Steinberg, M.D.; Kassal, P.; Kereković, I.; Steinberg, I.M. A wireless potentiostat for mobile chemical sensing and biosensing. Talanta 2015, 143, 178–183. [Google Scholar] [CrossRef]
- Kassal, P.; Zubak, M.; Scheipl, G.; Mohr, G.J.; Steinberg, M.D.; Steinberg, I.M. Smart bandage with wireless connectivity for optical monitoring of pH. Sens. Actuators B Chem. 2017, 246, 455–460. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2011; ISBN 0-471-04372-9. [Google Scholar]
- Wang, J. Analytical Electrochemistry, 3rd ed.; Wiley: New York, NY, USA, 2006; ISBN 0-471-67879-1. [Google Scholar]
- Scholz, F. (Ed.) Electroanalytical Methods; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-642-02914-1. [Google Scholar]
- Koryta, J. Ion-Selective Electrodes. Ann. Rev. Mater. Sci. 1986, 16, 13–27. [Google Scholar] [CrossRef]
- Wolfrum, B.; Zevenbergen, M.; Lemay, S. Nanofluidic Redox Cycling Amplification for the Selective Detection of Catechol. Anal. Chem. 2008, 80, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Goluch, E.D.; Wolfrum, B.; Singh, P.S.; Zevenbergen, M.A.G.; Lemay, S.G. Redox cycling in nanofluidic channels using interdigitated electrodes. Anal. Bioanal. Chem. 2010, 394, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Straver, M.G.; Odijk, M.; Olthuisa, W.; van den Berg, A. A simple method to fabricate electrochemical sensor systems with predictable high-redox cycling amplification. Lab Chip 2012, 12, 1548–1553. [Google Scholar] [CrossRef]
- Barnes, E.O.; Lewis, G.E.M.; Dale, S.E.C.; Marken, F.; Compton, R.G. Generator-collector double electrode systems: A review. Analyst 2012, 137, 1068. [Google Scholar] [CrossRef] [PubMed]
- del Campo, F.J.; Abad Muñoz, Ll.; Illa, X.; Tsai, Y.C. Determination of heterogeneous electron transfer rate constants at interdigitated nanoband electrodes fabricated by an optical mix-and-match process. Sens. Actuators B Chem. 2014, 194, 86–95. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; Wiley: New York, NY, USA, 2008; ISBN 978-0-470-04140-6. [Google Scholar]
- Gabrielli, C. Identification of Electrochemical Processes by Frequency Response Analysis; Solartron Instrumentation Group: Farnborough, UK, 1980. [Google Scholar]
- Tsividis, Y.; Milios, J. A detailed look at electrical equivalents of uniform electrochemical diffusion using nonuniform resistance–capacitance ladders. J. Electroanal. Chem. 2013, 707, 156–165. [Google Scholar] [CrossRef]
- Enz, C.C.; Temes, G.C. Circuit techniques for reducing the effects of op-amp imperfections: autozeroing, correlated double sampling, and chopper stabilization. Proc. IEEE 1996, 84, 1584–1614. [Google Scholar] [CrossRef]
- Dei, M.; Bruschi, P.; Piotto, M. Design of CMOS chopper amplifiers for thermal sensor interfacing. In Proceedings of the IEEE PhD Research in Microelectronics and Electronics, Istanbul, Turkey, 22 June–25 April 2008. [Google Scholar] [CrossRef]
- Dei, M.; Bruschi, P.; Piotto, M. A compact CMOS Gm-C biquadratic cell for chopper amplifier band limiting. In Proceedings of the IEEE PhD Research in Microelectronics and Electronics, Cork, Ireland, 12–17 July 2009. [Google Scholar] [CrossRef]
- Chandrakumar, H. A 0.6uW/Channel, Frequency Division Multiplexed Amplifier for Neural Recording Systems. Ph.D. Thesis, University of California, Los Angeles, CA, USA, 2012. [Google Scholar]
- Schreier, R.; Temes, G.C. Understanding Delta-Sigma Data Converters, 1st ed.; Wiley-IEEE Press: Piscataway, NJ, USA, 2005; ISBN 0471465852. [Google Scholar]
- Menolfi, C.; Huang, Q. A Fully Integrated, Untrimmed CMOS Instrumentation Amplifier with Submicrovolt Offset. IEEE J. Solid-State Circuits 1999, 34, 415–450. [Google Scholar] [CrossRef]
- Wu, R.; Makinwa, K.A.A.; Huijsing, J.H. A chopper current-feedback instrumentation amplifier with a 1 mHz 1/f noise corner and an AC-coupled ripple reduction loop. IEEE J. Solid-State Circuits 2009, 44, 3232–3243. [Google Scholar] [CrossRef]
- Fan, Q.; Huijsing, J.H.; Makinwa, K.A.A. A 21 nV/ chopper-stabilized multi-path current-feedback instrumentation amplifier with 2 μV offset. IEEE J. Solid-State Circuits 2012, 47, 464–475. [Google Scholar] [CrossRef]
- Kusuda, Y. Auto correction feedback for ripple suppression in a chopper amplifier. IEEE J. Solid-State Circuits 2010, 45, 1436–1445. [Google Scholar] [CrossRef]
- Chandrakumar, H.; Marcović, D. A Simple Area-Efficient Ripple-Rejection Technique for Chopped Biosignal Amplifiers. IEEE Trans. Circuits Syst. II 2015, 62, 189–193. [Google Scholar] [CrossRef]
- Bilotti, A.; Monreal, G. Chopper-stabilized amplifiers with a track-and-hold signal demodulator. IEEE Trans. Circuits Syst. I 1999, 46, 490–495. [Google Scholar] [CrossRef]
- Belloni, M.; Bonizzoni, E.; Fornasari, A.; Maloberti, F. A micropower chopper—CDS operational amplifier. IEEE J. Solid-State Circuits 2010, 45, 2521–2529. [Google Scholar] [CrossRef]
- Pertijs, M.A.P.; Kindt, W.J. A 140 dB-CMRR current-feedback instrumentation amplifier employing ping-pong auto-zeroing and chopping. IEEE J. Solid-State Circuits 2010, 45, 2044–2056. [Google Scholar] [CrossRef]
- Butti, F.; Bruschi, P.; Dei, M.; Piotto, M. A compact instrumentation amplifier for MEMS thermal sensor interfacing. Analog Integr. Circuits Signal Process. 2012, 72, 585–594. [Google Scholar] [CrossRef]
- Butti, F.; Piotto, M.; Bruschi, P. A chopper instrumentation amplifier with input resistance boosting by means of Synchronous Dynamic Element Matching. IEEE Trans. Circuits Syst. I Regul. Pap. 2016, 64, 753–764. [Google Scholar] [CrossRef]
- Muller, R.; Gambini, S.; Rabaey, J.M. A 0.013 mm2, 5μW, DC-coupled neural signal acquisition IC with 0.5 V supply. IEEE J. Solid-State Circuits 2012, 47, 232–243. [Google Scholar] [CrossRef]
- Akita, I.; Ishida, M. A chopper-stabilized instrumentation amplifier using area-efficient self-trimming technique. Analog Integr. Circuits Process. 2014, 81, 571–582. [Google Scholar] [CrossRef]
- Sherry, A. Chopping on Σ−Δ ADCs; Analog Devices–Application Note, AN-609. Norwood, MA, USA, 2003. Available online: https://www.analog.com/media/en/technical-documentation/application-notes/AN-609.pdf (accessed on 30 January 2019).
- Pertijs, M.A.P.; Makinwa, K.A.A.; Huijsing, J.H. A CMOS Smart Temperature Sensor With a 3σ Inaccuracy of ±0.1 °C From −55 °C to 125 °C. IEEE J. Solid-State Circuits 2005, 40, 2805–2815. [Google Scholar] [CrossRef]
- Catania, A.; Ria, A.; Del Cesta, S.; Piotto, M.; Bruschi, P. Analysis and Simulation of Chopper Stabilization Techniques Applied to Delta-Sigma Converters. In Proceedings of the International Conference on Synthesis, Modeling, Analysis and Simulation Methods and Applications to Circuit Design, Prague, Czech Republic, 2–5 July 2018. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Tang, S.K.; Ko, C.T.; Pun, K.P. A chopper-stabilized high-pass Delta–Sigma Modulator with reduced chopper charge injection. Microelectron. J. 2011, 42, 733–739. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Barfidokht, A.; Park, J.; Wang, J.; Mercier, P.P. A Battery-Powered Wireless Ion Sensing System Consuming 5.5 nW of Average Power. IEEE J. Solid-State Circuits 2018, 53, 2043–2053. [Google Scholar] [CrossRef]
- Craninckx, J.; van der Plas, G. A 65fJ/Conversion-Step 0-to-50MS/s 0-to-0.7mW 9b Charge-Sharing SAR ADC in 90nm Digital CMOS. In Proceedings of the IEEE International Solid-State Circuits Conference, San Francisco, CA, USA, 11–15 February 2007. [Google Scholar] [CrossRef]
- Wang, H.; Mercier, P.P. A Reference-Free Capacitive-Discharging Oscillator Architecture Consuming 44.4 pW/75.6 nW at 2.8 Hz/6.4 kHz. IEEE J. Solid-State Circuits 2011, 51, 1423–1435. [Google Scholar] [CrossRef]
- Harpe, P.J.A.; Zhou, C.; Bi, Y.; van der Meijs, N.P.; Wang, X.; Philips, K.; Dolmans, G.; de Groot, H. A 26 μW 8 bit 10 MS/s Asynchronous SAR ADC for Low Energy Radios. IEEE J. Solid-State Circuits 2011, 46, 1585–1595. [Google Scholar] [CrossRef]
- Ghoreishizadeh, S.S.; Baj-Rossi, C.; Cavallini, A.; Carrara, S.; De Micheli, G. An Integrated Control and Readout Circuit for Implantable Multi-Target Electrochemical Biosensing. IEEE Trans. Biomed. Circuits Syst. 2014, 8, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Tan, X.; Chen, X.; Chen, S.; Zhang, Z.; Zhang, H.; Wang, J.; Huang, Y.; Zhang, P.; Zheng, L.; Min, H. An Implantable RFID Sensor Tag toward Continuous Glucose Monitoring. IEEE J. Biomed. Health Inform. 2015, 19, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Islam, S.K.; Mahbub, I.; Quaiyum, F. A Low-Power 1-V Potentiostat for Glucose Sensors. IEEE Trans. Circuits Syst. II Exp. Briefs 2015, 62, 204–208. [Google Scholar] [CrossRef]
- Ghodsevali, E.; Morneau-Gamache, S.; Mathault, J.; Landari, H.; Boisselier, É.; Boukadoum, M.; Gosselin, B.; Miled, A. Miniaturized FDDA and CMOS Based Potentiostat for Bio-Applications. Sensors 2017, 17, 810. [Google Scholar] [CrossRef]
- Martin, S.M.; Gebara, F.H.; Strong, T.D.; Brown, R.B. A Fully Differential Potentiostat. IEEE Sens. J. 2009, 6, 135–142. [Google Scholar] [CrossRef]
- Giagkoulovits, C.; Cheah, B.C.; Al-Rawhani, M.A.; Accarino, C.; Busche, C.; Grant, J.P.; Cumming, D.R.S. A 16 × 16 CMOS amperometric microelectrode array for simultaneous electrochemical measurements. IEEE Trans. Circuits Syst. I Regul. Pap. 2018, 65, 2821–2831. [Google Scholar] [CrossRef]
- Li, H.; Parsnejad, S.; Ashoori, E.; Thompson, C.; Purcell, E.K.; Mason, A.J. Ultracompact Microwatt CMOS Current Readout With Picoampere Noise and Kilohertz Bandwidth for Biosensor Arrays. IEEE Trans. Biomed. Circuits Syst. 2017, 12, 35–46. [Google Scholar] [CrossRef]
- Crescentini, M.; Bennati, M.; Carminati, M.; Tartagni, M. Noise Limits of CMOS Current Interfaces for Biosensors: A Review. IEEE Trans. Biomed. Circuits Syst. 2014, 8, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.M.; Jullien, G.A. Current-Mirror-Based Potentiostats for Three-Electrode Amperometric Electrochemical Sensors. IEEE Trans. Circuits Syst. I Regul. Pap. 2009, 56, 1339–1348. [Google Scholar] [CrossRef]
- Al Mamun, K.A.; Islam, S.K.; Hensley, D.K.; McFarlane, N. A Glucose Biosensor Using CMOS Potentiostat and Vertically Aligned Carbon Nanofibers. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Lu, S.; Liao, Y. A 2.2μW, -12 dBm RF-Powered Wireless Current Sensing Readout Interface IC With Injection-Locking Clock Generation. IEEE Trans. Circuits Syst. I Regul. Pap. 2016, 63, 950–959. [Google Scholar] [CrossRef]
- Tsai, J.; Kuo, C.; Lin, S.; Lin, F.; Liao, Y. A Wirelessly Powered CMOS Electrochemical Sensing Interface With Power-Aware RF-DC Power Management. IEEE Trans. Circuits Syst. I Regul. Pap. 2018, 65, 2810–2820. [Google Scholar] [CrossRef]
- Razavi, B. Design of Analog CMOS Integrated Circuits; McGraw-Hill: New York, NY, USA, 2001; ISBN 0072380322. [Google Scholar]
- Levine, P.M.; Gong, P.; Levicky, R.; Shepard, K.L. Active CMOS Sensor Array for Electrochemical Biomolecular Detection. IEEE J. Solid-State Circuits 2008, 43, 1859–1871. [Google Scholar] [CrossRef]
- Yang, C.; Huang, Y.; Hassler, B.L.; Worden, R.M.; Mason, A.J. Amperometric Electrochemical Microsystem for a Miniaturized Protein Biosensor Array. IEEE Trans. Biomed. Circuits Syst. 2009, 3, 160–168. [Google Scholar] [CrossRef]
- Nazari, M.H.; Mazhab-Jafari, H.; Leng, L.; Guenther, A.; Genov, R. CMOS Neurotransmitter Microarray: 96-Channel Integrated Potentiostat With On-Die Microsensors. IEEE Trans. Biomed. Circuits Syst. 2013, 7, 338–348. [Google Scholar] [CrossRef]
- Jafari, H.M.; Abdelhalim, K.; Soleymani, L.; Sargent, E.H.; Kelley, S.O.; Genov, R. Nanostructured CMOS Wireless Ultra-Wideband Label-Free PCR-Free DNA Analysis SoC. IEEE J. Solid-State Circuits 2014, 49, 1223–1241. [Google Scholar] [CrossRef]
- Liu, Y.T.; Chen, M.; Li, Z.C.; Wang, Y.; Chen, J. A High Dynamic Range Analog-front-end IC for Electrochemical Amperometric and Voltammetric Sensor. Microelectron. J. 2015, 46, 716–722. [Google Scholar] [CrossRef]
- Ghoreishizadeh, S.S.; Taurino, I.; De Micheli, G.; Carrara, S.; Georgiou, P. A Differential Electrochemical Readout ASIC With Heterogeneous Integration of Bio-Nano Sensors for Amperometric Sensing. IEEE Trans. Biomed. Circuits Syst. 2016, 11, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Mu, X.; Li, H.; Liu, X.; Mason, A.J. CMOS Monolithic Electrochemical Gas Sensor Microsystem Using Room Temperature Ionic Liquid. IEEE Sens. J. 2018, 18, 7899–7906. [Google Scholar] [CrossRef]
- Murmann, B. Thermal Noise in Track-and-Hold Circuits: Analysis and Simulation Techniques. IEEE Solid State Circuits Mag. 2012, 4, 46–54. [Google Scholar] [CrossRef]
- Hasting, A. The Art of Analog Layout; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2006; ISBN 0131464108. [Google Scholar]
- Tan, X.; Chen, X.; Zhang, Y.; Wang, J.; Min, H.; Huang, Y.; Mason, A.J. 4.3 μW 10 fA-sensitivity dual-mode current converter for implantable glucose monitoring. Electron. Lett. 2015, 51, 1484–1486. [Google Scholar] [CrossRef]
- Dei, M.; Sacristán, J.; Marigó, E.; Soundara, M.; Terés, L.; Serra-Graells, F. A 10-bit Linearity Current-Controlled Ring Oscillator with Rolling Regulation for Smart Sensing. In Proceedings of the IEEE International Symposium on Circuits and Systems, Baltimore, MD, USA, 28–31 May 2017. [Google Scholar] [CrossRef]
- Massicotte, G.; Carrara, S.; Di Micheli, G.; Sawan, M. A CMOS Amperometric System for Multi-Neurotransmitter Detection. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 731–741. [Google Scholar] [CrossRef]
- Dai, S.; Perera, R.T.; Yang, Z.; Rosenstein, J.K. A 155-dB Dynamic Range Current Measurement Front End for Electrochemical Biosensing. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 935–944. [Google Scholar] [CrossRef]
- Dei, M.; Figueras, R.; Margarit, J.M.; Terés, L.; Serra-Graells, F. Highly Linear Integrate-and-Fire Modulators with Soft Reset for Low-Power High-Speed Imagers. In Proceedings of the IEEE International Symposium on Circuits and Systems, Baltimore, MD, USA, 28–31 May 2017. [Google Scholar] [CrossRef]
- Stanacevic, M.; Murari, K.; Rege, A.; Cauwenberghs, G.; Thakor, N.V. VLSI Potentiostat Array With Oversampling Gain Modulation for Wide-Range Neurotransmitter Sensing. IEEE Trans. Biomed. Circuits Syst. 2007, 1, 63–72. [Google Scholar] [CrossRef]
- Sutula, S.; Pallarés Cuxart, J.; Gonzalo-Ruiz, J.; Muñoz-Pascual, F.X.; Terés, L.; Serra-Graells, F. A 25-μW All-MOS Potentionstatic Delta-Sigma ADC for Smart Electrochemical Sensors. IEEE Trans. Circuits Syst. I Regul. Pap. 2014, 61, 671–679. [Google Scholar] [CrossRef]
- Li, H.; Boling, C.S.; Mason, A.J. CMOS Amperometric ADC With High Sensitivity, Dynamic Range and Power Efficiency for Air Quality Monitoring. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 817–827. [Google Scholar] [CrossRef]
- Aymerich, J.; Dei, M.; Terés, L.; Serra-Graells, F. Design of a Low-Power Potentiostatic Second-Order CT Delta-Sigma ADC for Electrochemical Sensors. In Proceedings of the IEEE PhD Research in Microelectronics and Electronics, Taormina, Italy, 12–15 June 2017. [Google Scholar] [CrossRef]
- Aymerich, J.; Dei, M.; Terés, L.; Serra-Graells, F. A 6.5-μW 70-dB 0.18-μm CMOS Potentiostatic Delta-Sigma for Electrochemical Sensors. In Proceedings of the IEEE PhD Research in Microelectronics and Electronics, Prague, Czech Republic, 2–5 July 2018. [Google Scholar] [CrossRef]
- Aymerich, J.; Márquez, A.; Terés, L.; Muñoz-Berbel, X.; Jiménez, C.; Domínguez, C.; Serra-Graells, F.; Dei, M. Cost-effective smartphone-based reconfigurable electrochemical instrument for alcohol determination in whole blood samples. Biosens. Bioelectron. 2018, 117, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, S.; Habibi, M.; Magierowski, S. A High-Efficiency Discrete Current Mode Output Stage Potentiostat Instrumentation for Self-Powered Electrochemical Devices. IEEE Trans. Instrum. Meas. 2018, 67, 2247–2255. [Google Scholar] [CrossRef]
- Razavi, B. The StrongARM Latch. IEEE Solid State Circuits Mag. 2015, 12–17. [Google Scholar] [CrossRef]
- Bergveld, P. Development, Operation, and Application of the Ion-Sensitive Field-Effect Transistor as a Tool for Electrophysiology. IEEE Trans. Biomed. Eng. 1972, 5, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Bergveld, P. Thirty years of ISFETOLOGY: What happened in the past 30 years and what may happen in the next 30 years. Sens. Actuator B Chem. 2003, 88, 1–20. [Google Scholar] [CrossRef]
- Bausells, J.; Carrabina, J.; Errachid, A.; Merlos, A. Ion-sensitive field-effect transistors fabricated in a commercial CMOS technology. Sens. Actuator B Chem. 2003, 57, 56–62. [Google Scholar] [CrossRef]
- The International Technology Roadmap for Semiconductors (ITRS) 2.0, “More Moore”. 2015. Available online: http://www.itrs2.net (accessed on 21 January 2019).
- Nakata, S.; Arie, T.; Akita, S.; Takei, K. Wearable, Flexible, and Multifunctional Healthcare Device with an ISFET Chemical Sensor for Simultaneous Sweat pH and Skin Temperature Monitoring. ACS Sens. 2017, 2, 443–448. [Google Scholar] [CrossRef] [PubMed]
- van Hal, R.E.G.; Eijkel, J.C.T.; Bergveld, P. A novel description of ISFET sensitivity with the buffer capacity and double-layer capacitance as key parameters. Sens. Actuator B Chem. 1995, 24, 201–205. [Google Scholar] [CrossRef]
- Georgiou, P.; Toumazou, C. ISFET characteristics in CMOS and their application to weak inversion operation. Sens. Actuator B Chem. 2009, 143, 211–217. [Google Scholar] [CrossRef]
- Moser, N.; Lande, T.S.; Toumazou, C.; Georgiou, P. ISFETs in CMOS and Emergent Trends in Instrumentation: A Review. IEEE Sens. J. 2016, 16, 6496–6514. [Google Scholar] [CrossRef]
- Hanazato, Y.; Nakako, M.; Shiono, S.; Maeda, M. Integrated multi-biosensors based on an ion-sensitive field-effect transistor using photolithographic techniques. IEEE Trans. Electron Devices 1989, 36, 1303–1310. [Google Scholar] [CrossRef]
- Enz, C.C.; Krummenacher, F.; Vittoz, E.A. An Analytical MOS Transistor Model Valid in All Regions of Operation and Dedicated to Low-Voltage and Low-Current Applications. Analog Integr. Circuits Signal Process. 1995, 8, 83–114. [Google Scholar] [CrossRef]
- Jimenez, C.; Orozco, J.; Baldi, A. ISFET based sensors: Fundamentals and applications. Encycl. Sens. 2006, 5, 151–196. [Google Scholar]
- Miscourides, N.; Yu, L.; Rodriguez-Manzano, J.; Georgiou, P. A 12.8 k Current-Mode Velocity-Saturation ISFET Array for On-Chip Real-Time DNA Detection. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Moser, N.; Rodriguez-Manzano, J.; Lande, T.S.; Georgiou, P. A Scalable ISFET Sensing and Memory Array With Sensor Auto-Calibration for On-Chip Real-Time DNA Detection. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Alhoshany, A.; Sivashankar, S.; Mashraei, Y.; Omran, H.; Salama, K.N. A Biosensor-CMOS Platform and Integrated Readout Circuit in 0.18-μm CMOS Technology for Cancer Biomarker Detection. Sensors 2017, 17, 1942. [Google Scholar] [CrossRef] [PubMed]
- Laborde, C.; Pittino, F.; Verhoeven, H.A.; Lemay, S.G.; Selmi, L.; Jongsma, M.A.; Widdershoven, F.P. Real-time imaging of microparticles and living cells with CMOS nanocapacitor arrays. Nat. Nanotechnol. 2015, 10, 791. [Google Scholar] [CrossRef]
- Segura-Quintano, F.; Sacristán-Riquelme, J.; García-Cantón, J.; Osés, M.T.; Baldi, A. Towards Fully Integrated Wireless Impedimetric Sensors. Sensors 2010, 10, 4071–4082. [Google Scholar] [CrossRef]
- Rodriguez, S.; Ollmar, S.; Waqar, M.; Rusu, A. A Batteryless Sensor ASIC for Implantable Bio-Impedance Applications. IEEE Trans. Biomed. Circuits Syst. 2015, 10, 533–544. [Google Scholar] [CrossRef]
- Xu, J.; Harpe, P.; Pettine, J.; Van Hoof, C.; Yazicioglu, R.F. A low power configurable bio-impedance spectroscopy (BIS) ASIC with simultaneous ECG and respiration recording functionality. In Proceedings of the European Solid-State Circuits Conference, Graz, Austria, 14–18 September 2015. [Google Scholar] [CrossRef]
- Jafari, H.; Soleymani, L.; Genov, R. 16-Channel CMOS Impedance Spectroscopy DNA Analyzer With Dual-Slope Multiplying ADCs. IEEE Trans. Biomed. Circuits Syst. 2012, 6, 468–478. [Google Scholar] [CrossRef]
- Manickam, A.; Chevalier, A.; McDermott, M.; Ellington, A.D.; Hassibi, A. A CMOS Electrochemical Impedance Spectroscopy (EIS) Biosensor Array. IEEE Trans. Biomed. Circuits Syst. 2010, 4, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Min, M.; Parve, T. Improvement of lock-in electrical bio-impedance analyzer for implantable medical devices. IEEE Trans. Instrum. Meas. 2007, 56, 968–974. [Google Scholar] [CrossRef]
- Wei, C.; Wang, Y.; Liu, B. Wide-range filter-based sinusoidal wave synthesizer for electrochemical impedance spectroscopy measurements. IEEE Trans. Biomed. Circuits Syst. 2014, 8, 442–450. [Google Scholar] [CrossRef]
- Crescentini, M.; Bennati, M.; Tartagni, M. Recent trends for (bio) chemical impedance sensor electronic interfaces. Electroanal 2012, 24, 563–572. [Google Scholar] [CrossRef]
- Qu, G.; Wang, H.; Zhao, Y.; O’Donnell, J.; Lyden, C.; Liu, Y.; Ding, J.; Dempsey, D.; Chen, L.; Bourke, D.; Gu, S.; Gao, J.; Lu, L.; Wang, L.; Li, X.; Li, H.; Chu, C.; Yang, L. A 0.28 mΩ-sensitivity 105 dB-dynamic-range electrochemical impedance spectroscopy soc for electrochemical gas detection. In Proceedings of the International Solid-State Circuits Conference, San Francisco, CA, USA, 11–15 February 2018. [Google Scholar] [CrossRef]
- Chen, T.; Wu, W.; Wei, C.; Darling, R.B.; Liu, B. Novel 10-Bit Impedance-to-Digital Converter for Electrochemical Impedance Spectroscopy Measurements. IEEE Trans. Biomed. Circuits Syst. 2016, 11, 370–379. [Google Scholar] [CrossRef]
- Shleev, S.; Bergel, A.; Gorton, L. Biological fuel cells: Divergence of opinion. Bioelectrochemistry 2015, 106, 1–2. [Google Scholar] [CrossRef]
- Katz, E.; Bückmann, A.F.; Willner, I. Self-Powered Enzyme-Based Biosensors. J. Am. Chem. Soc. 2001, 123, 10752–10753. [Google Scholar] [CrossRef]
- Jeerapan, I.; Sempionatto, J.R.; Pavinatto, A.; Youa, J.M.; Wang, J. Stretchable biofuel cells as wearable textile-based self-powered sensors. J. Mater. Chem. A 2016, 4, 18342–18353. [Google Scholar] [CrossRef]
- Yeknami, A.F.; Wang, X.; Jeerapan, I.; Imani, S.; Nikoofard, A.; Wang, J.; Mercier, P.P. A 0.3-V CMOS Biofuel-Cell-Powered Wireless Glucose/Lactate Biosensing System. IEEE J. Solid-State Circuits 2018. [Google Scholar] [CrossRef]
- Niitsu, K.; Kobayashi, A.; Nishio, Y.; Hayashi, K.; Ikeda, K.; Ando, T.; Ogawa, Y.; Kai, H.; Nishizawa, M.; Nakazato, K. A Self-Powered Supply-Sensing Biosensor Platform Using Bio Fuel Cell and Low-Voltage, Low-Cost CMOS Supply-Controlled Ring Oscillator With Inductive-Coupling Transmitter for Healthcare IoT. IEEE Trans. Circuits Syst. I Regul. Pap. 2018, 65, 2784–2796. [Google Scholar] [CrossRef]



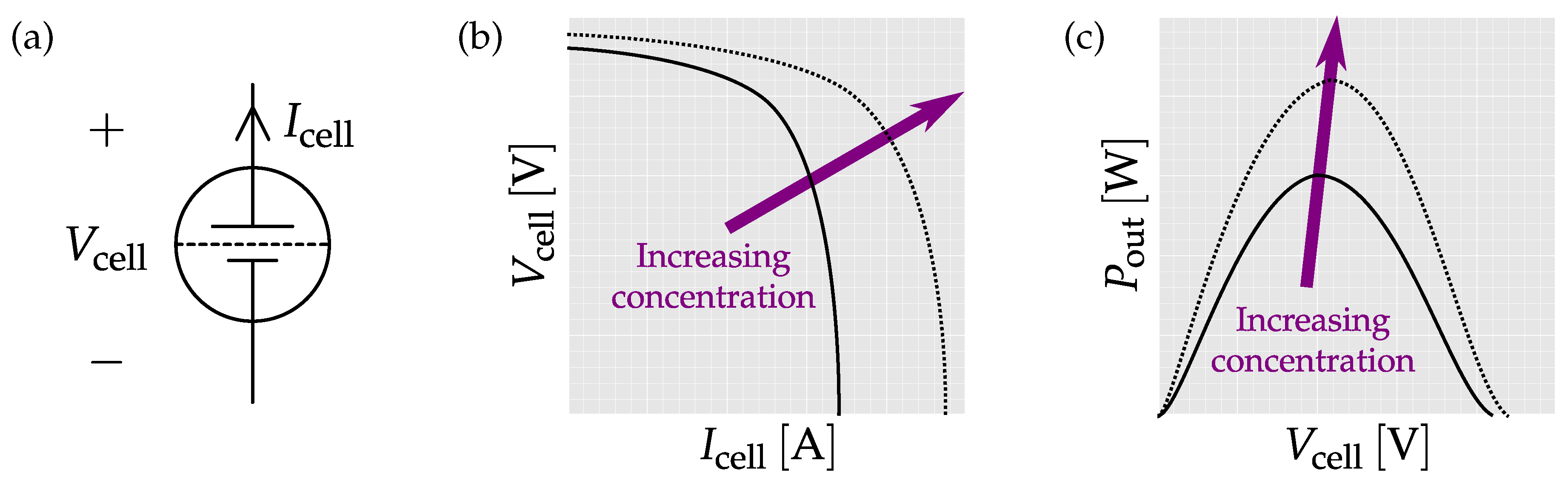
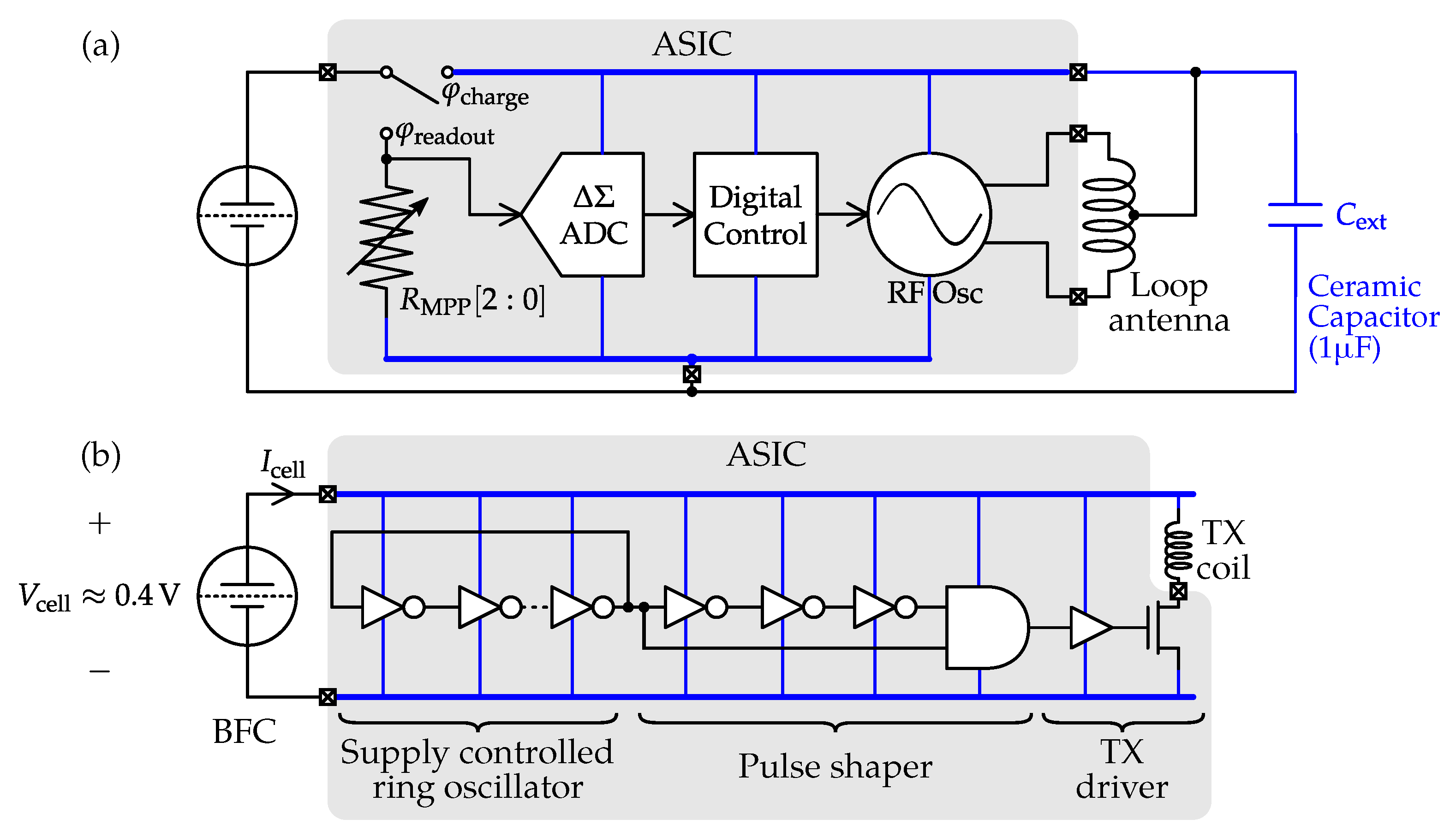
| Body Fluids | Blood | Sweat | Saliva | Tear | Urine |
|---|---|---|---|---|---|
| References | [16] | [17,18,19,20] | [8,9] | [16,21,22,23] | [24,25,26,27] |
| Glucose [mM] | 3.3–6.7 | 0.06–0.11 | 0.22–0.72 | 0.2–1 | 2.78–5.5 |
| pH | 7.36–7.44 | 4–5.5–7 | 6.2–7.6 | 6.5–7.6 7.14–7.82 | 4–8 |
| [Na+] [mM] | 136–145 | 10–40 | 20–80 | 80–161 | 40–220 |
| [K+] [mM] | 3.5–5 | 4–5 | 20 | not found | 25–125 |
| [Cl−] [mM] | 98–107 | <40 | 30–100 | 106–130 | 110–250 |
| Lactate[mM] | 0.3–1.3 0.36–0.75 | 20–60 5–110 | 0–0.4 | unknown | 0.5–2.2 |
| IL-6[pG/mL] | 0–4.3 | 7–16 | 2.5 | 100–200 | 20–30 |
| Radio | Off-The-Shelf Component(s): Radio + MCU | Reference(s) |
|---|---|---|
| Zigbee (IEEE 802.15.4) | Xbee-PRO RF + PIC24HJ12GP201 | [79] |
| Xbee + Arduino Lilypad | [80] | |
| nRF2401 + ATMega128L | [81] | |
| CC2530 | [82] | |
| Bluetooth Low Energy | CC2541 | [83,84,85] |
| (IEEE 802.15.1) | HC-06 + ATMega328p | [43,64,86] |
| Bluetooth 2.0 | CC2560 + MSP430BT5190 | [87] |
| NFC/RFID (ISO 15693) | Commercial tag, custom modified | [88] |
| SL13A | [89] | |
| MLX90129 | [62,90] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dei, M.; Aymerich, J.; Piotto, M.; Bruschi, P.; del Campo, F.J.; Serra-Graells, F. CMOS Interfaces for Internet-of-Wearables Electrochemical Sensors: Trends and Challenges. Electronics 2019, 8, 150. https://doi.org/10.3390/electronics8020150
Dei M, Aymerich J, Piotto M, Bruschi P, del Campo FJ, Serra-Graells F. CMOS Interfaces for Internet-of-Wearables Electrochemical Sensors: Trends and Challenges. Electronics. 2019; 8(2):150. https://doi.org/10.3390/electronics8020150
Chicago/Turabian StyleDei, Michele, Joan Aymerich, Massimo Piotto, Paolo Bruschi, Francisco Javier del Campo, and Francesc Serra-Graells. 2019. "CMOS Interfaces for Internet-of-Wearables Electrochemical Sensors: Trends and Challenges" Electronics 8, no. 2: 150. https://doi.org/10.3390/electronics8020150
APA StyleDei, M., Aymerich, J., Piotto, M., Bruschi, P., del Campo, F. J., & Serra-Graells, F. (2019). CMOS Interfaces for Internet-of-Wearables Electrochemical Sensors: Trends and Challenges. Electronics, 8(2), 150. https://doi.org/10.3390/electronics8020150







