Development and Validation of an Unobtrusive Automatic Sleep Quality Assessment Index (ASQI) for Elderly Individuals
Abstract
1. Introduction
2. Materials and Methods
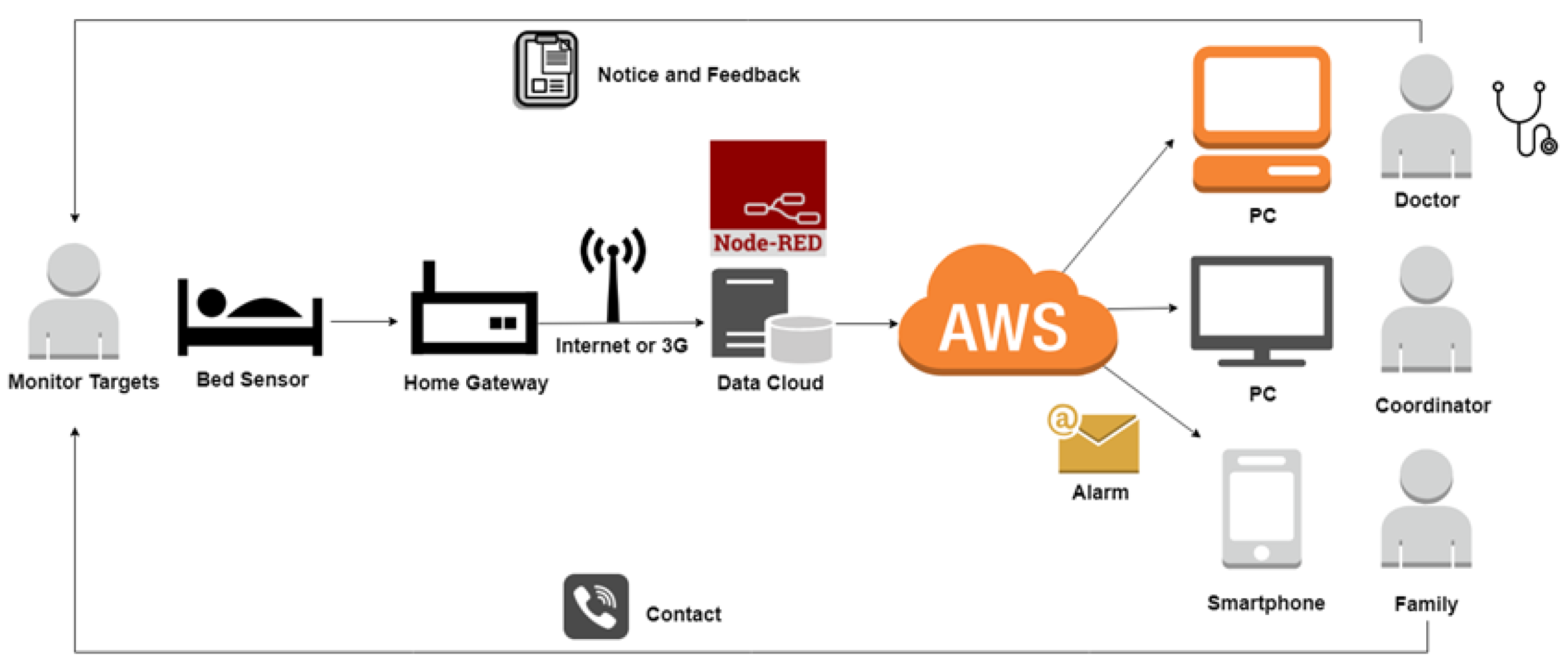
2.1. System Design
2.2. Scale Design of Sleep Quality Index
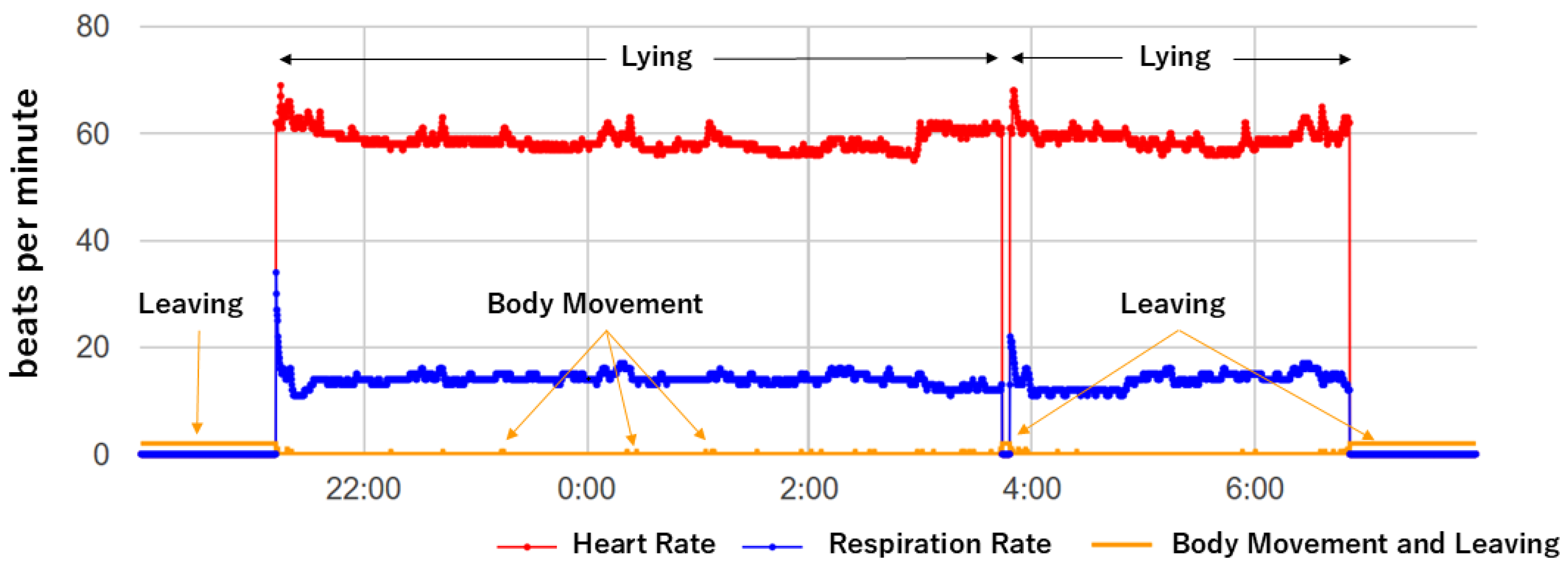
2.3. Signal Processing and Data Analysis
2.4. Participants
2.5. Study Protocol
2.6. Statistics
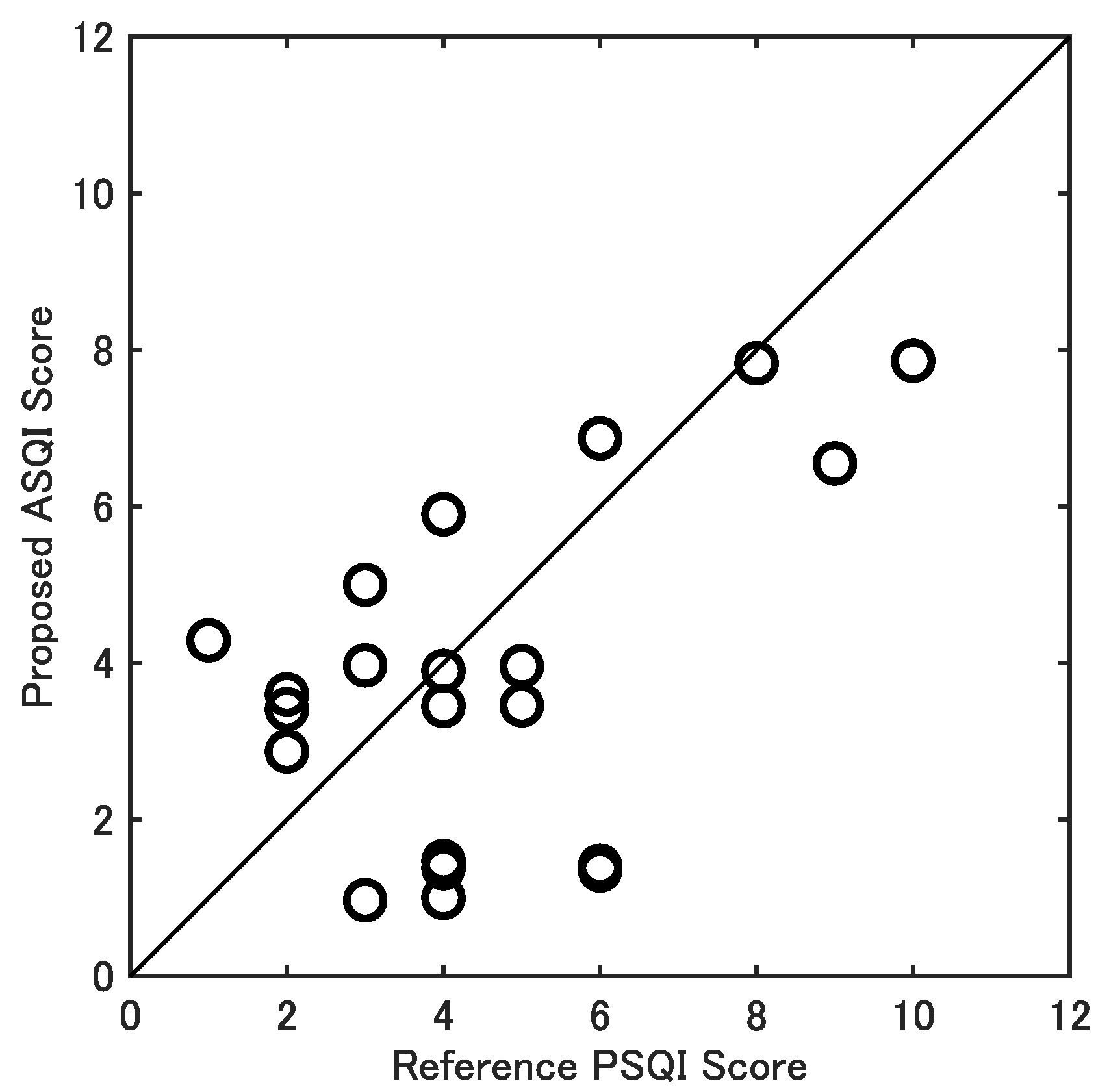
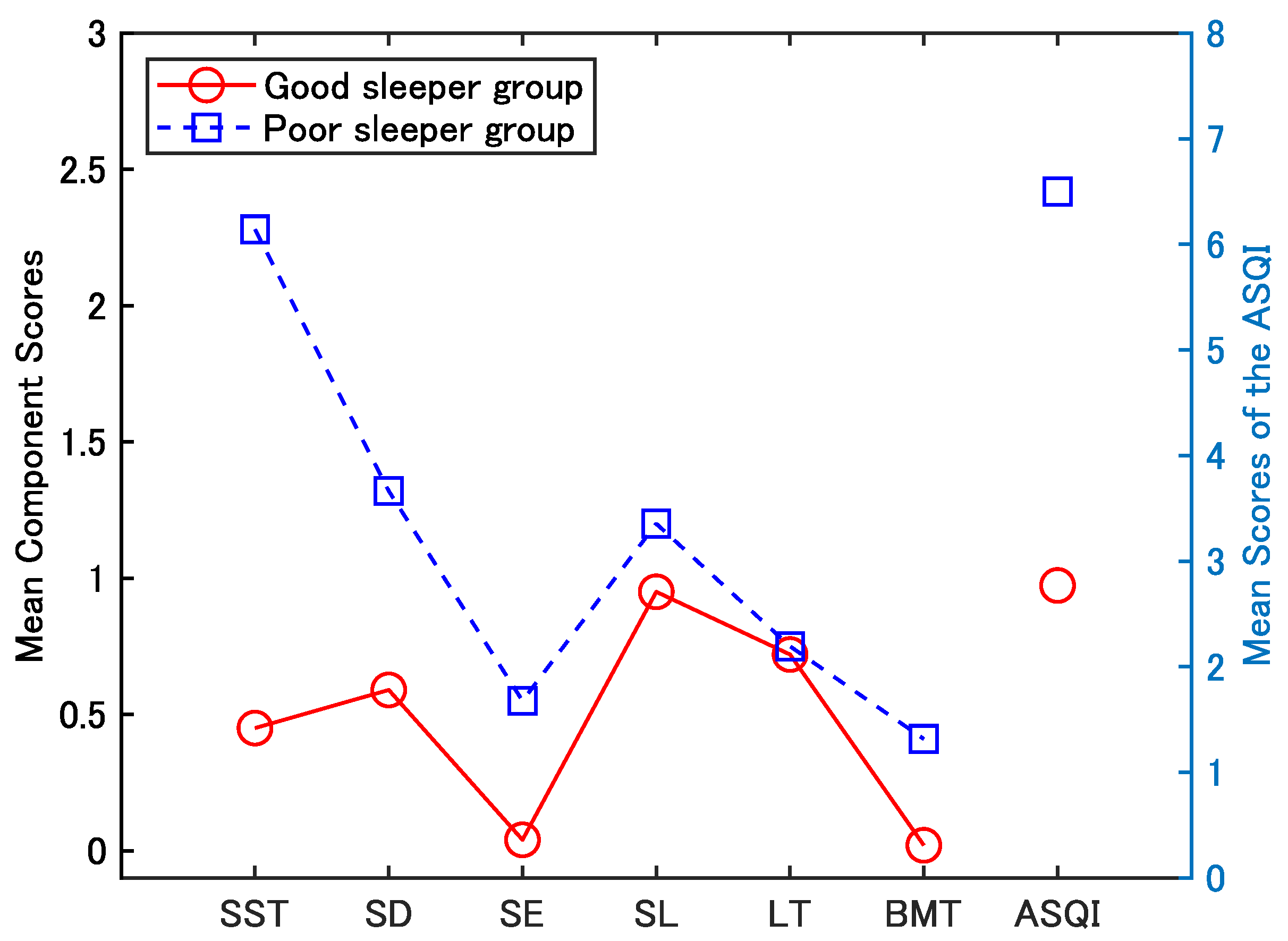
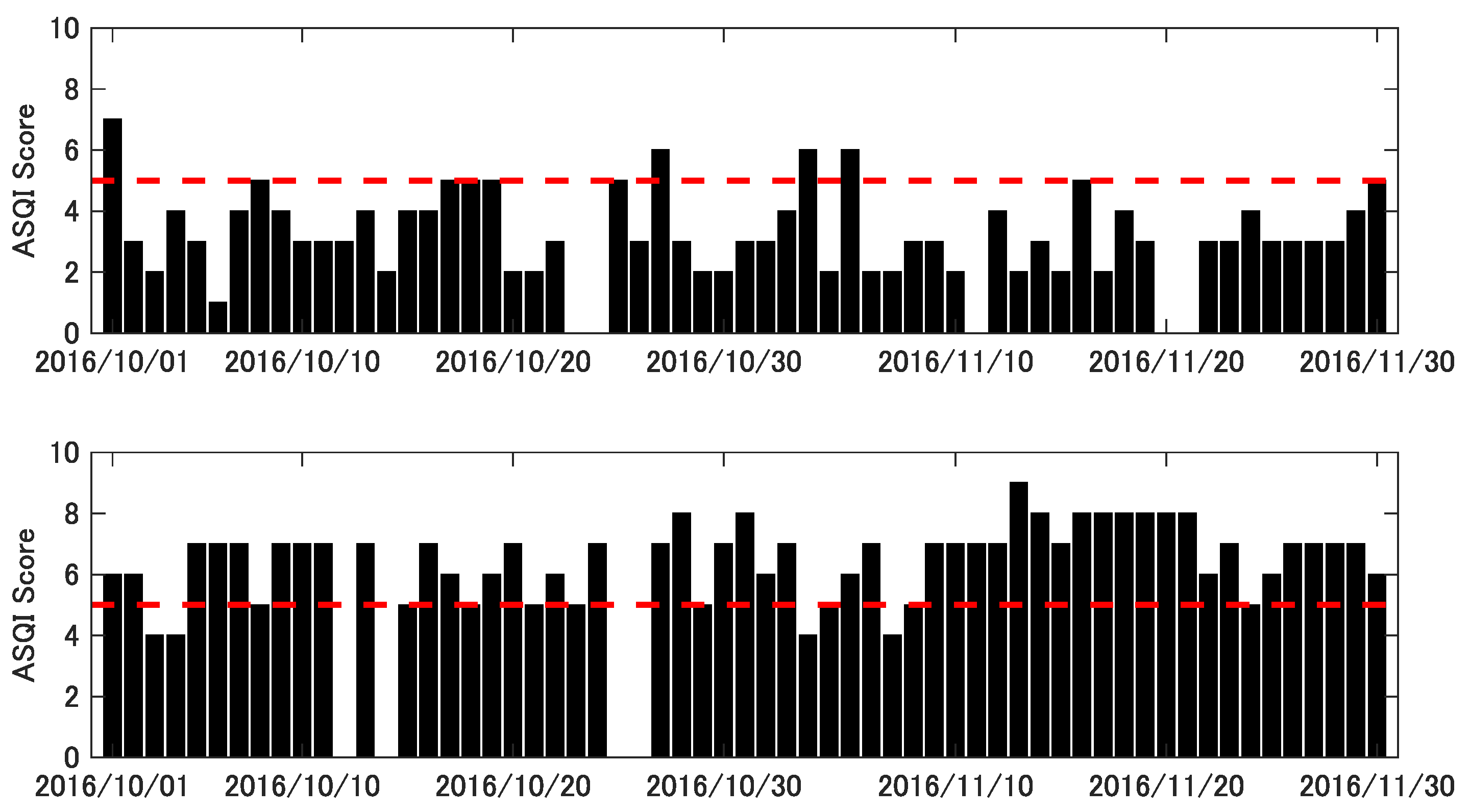
3. Results
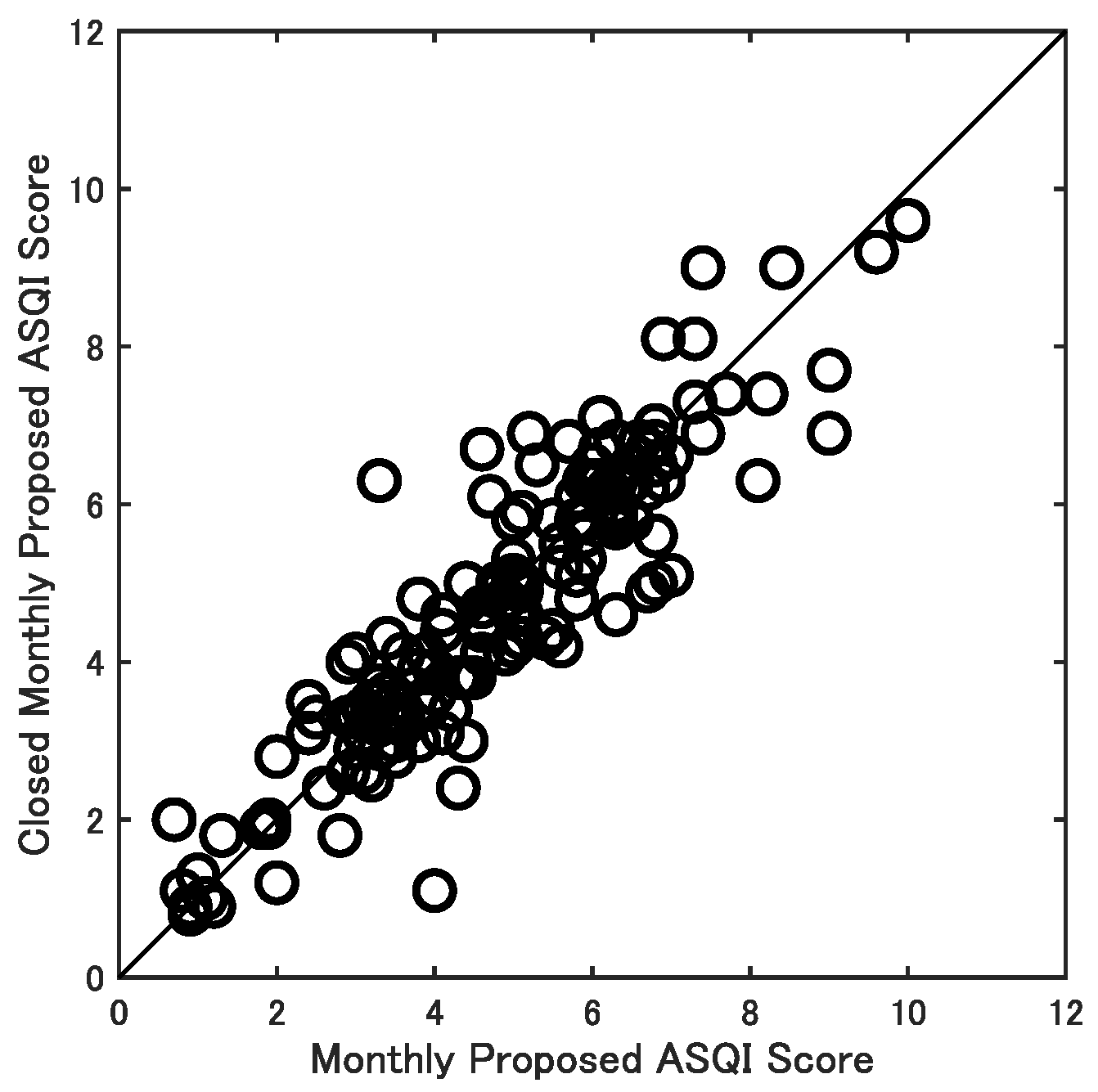
3.1. Performance Consistency (Test–Retest Reliability)
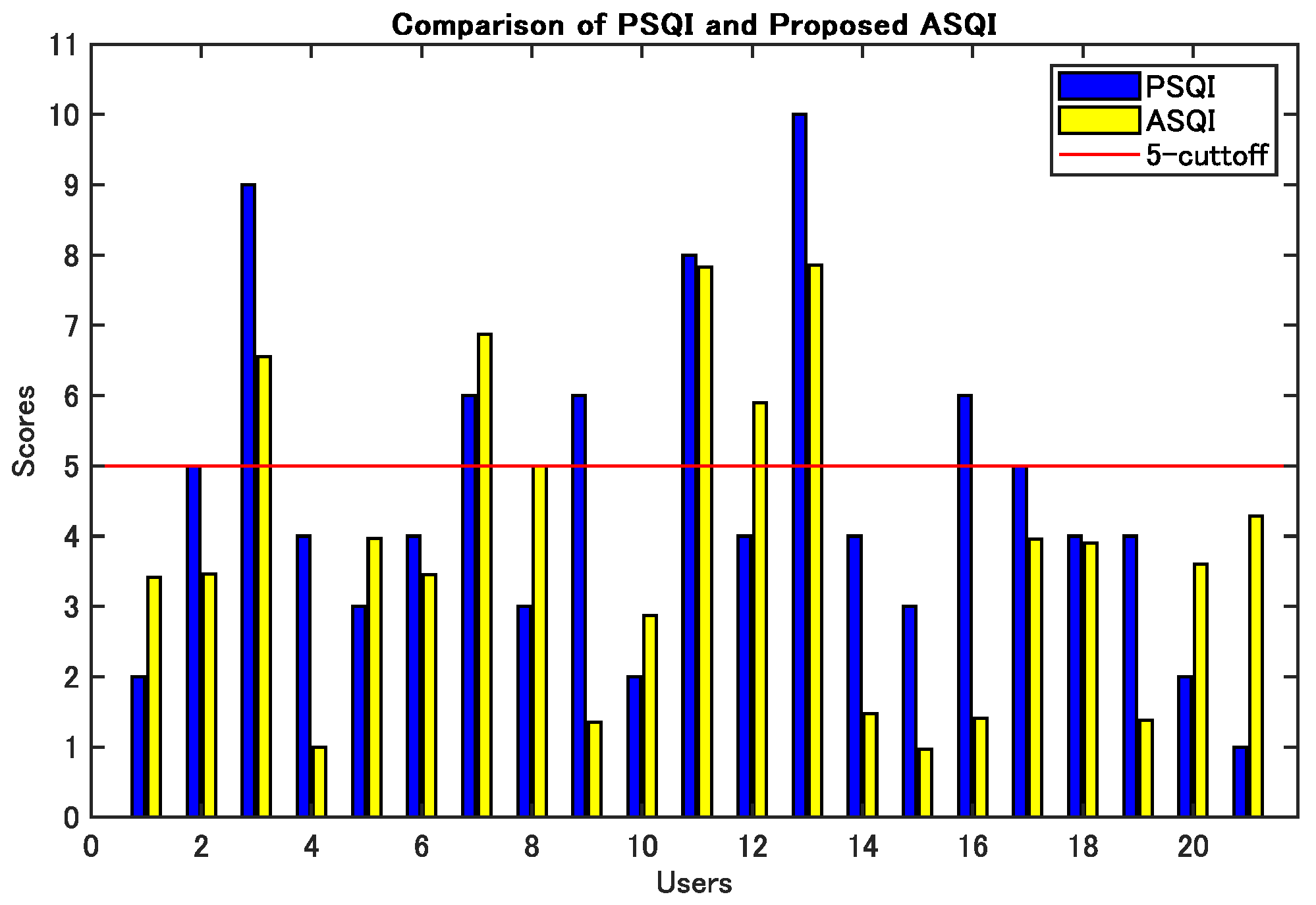
3.2. Validity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASQI | Automatic Sleep Quality Index |
| PSQI | Pittsburgh Sleep Quality Index |
| PSG | Polysomnography |
| PPG | Photoplethysmography |
| BCG | Ballistocardiography |
| HR | Heart Rate |
| RR | Respiratory Rate |
| BW | Body Movement |
| SST | Sleep Start Time |
| SD | Sleep Duration |
| SE | Sleep Efficiency |
| SL | Sleep Latency |
| LT | Leaving Times |
| BMT | Body Movement Times |
References
- Kim, K.; Uchiyama, M.; Okawa, M.; Liu, X.; Ogihara, R. An epidemiological study of insomnia among the Japanese general population. Sleep 2000, 23, 41–47. [Google Scholar] [CrossRef]
- Itani, O.; Kaneita, Y.; Munezawa, T.; Mishima, K.; Jike, M.; Nakagome, S.; Tokiya, M.; Ohida, T. Nationwide epidemiological study of insomnia in Japan. Sleep Med. 2016, 25, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Penzel, T. Sleep Quality Assessment: Challenges and Opportunities. IEEE Pulse 2016, 7, 3. [Google Scholar] [CrossRef]
- Fabbri, M.; Beracci, A.; Martoni, M.; Meneo, D.; Tonetti, L.; Natale, V. Measuring Subjective Sleep Quality: A Review. Int. J. Environ. Res. Public Health 2021, 18, 1082. [Google Scholar] [CrossRef]
- Chen, W.H.; Chen, C.T.; Cheng, K.H.; Tsao, Y.C.; Lin, Y.H.; Chen, J.Y. Associations Between Sleep Quality and Self-Reported Health Status in Middle-Aged and Older Adults: A Community-Based, Cross-Sectional Study in Northern Taiwan. Healthcare 2025, 13, 1272. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Hinz, A.; Glaesmer, H.; Brähler, E.; Löffler, M.; Engel, C.; Enzenbach, C.; Hegerl, U.; Sander, C. Sleep quality in the general population: Psychometric properties of the Pittsburgh Sleep Quality Index, derived from a German community sample of 9284 people. Sleep Med. 2017, 30, 57–63. [Google Scholar] [CrossRef]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M.; Marcus, C.; Vaughn, B.V. The AASM Manual for the Scoring of Sleep and Associated Events. Rules, Terminology and Technical Specifications; American Academy of Sleep Medicine: Darien, IL, USA, 2012; Volume 176, p. 7. [Google Scholar]
- Kaplan, K.A.; Hirshman, J.; Hernandez, B.; Stefanick, M.L.; Hoffman, A.R.; Redline, S.; Ancoli-Israel, S.; Stone, K.; Friedman, L.; Zeitzer, J.M.; et al. When a gold standard isn’t so golden: Lack of prediction of subjective sleep quality from sleep polysomnography. Biol. Psychol. 2017, 123, 37–46. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, E.; Wang, Y.; Wu, Y.; Xu, G.; Chen, L. The past, present, and future of sleep quality assessment and monitoring. Brain Res. 2023, 1810, 148333. [Google Scholar] [CrossRef] [PubMed]
- Girschik, J.; Fritschi, L.; Heyworth, J.; Waters, F. Validation of self-reported sleep against actigraphy. J. Epidemiol. 2012, 22, 462–468. [Google Scholar] [CrossRef]
- Masaki, M.; Tsumoto, S.; Tani, A.; Tominaga, M.; Seol, J.; Chiba, S.; Miyanishi, K.; Nishida, K.; Kawana, F.; Amemiya, T.; et al. Discrepancies between subjective and objective sleep assessments revealed by in-home electroencephalography during real-world sleep. Proc. Natl. Acad. Sci. USA 2025, 122, e2412895121. [Google Scholar] [CrossRef] [PubMed]
- Lechat, B.; Hirotsu, C.; Appleton, S.; Younes, M.; Adams, R.J.; Vakulin, A.; Hansen, K.; Zajamsek, B.; Wittert, G.; Catcheside, P.; et al. A novel EEG marker predicts perceived sleepiness and poor sleep quality. Sleep 2022, 45, zsac051. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Yan, C.; Dong, K.; Kang, Z.; Zhang, H.; Liu, C. Sleep Quality Evaluation Based on Single-Lead Wearable Cardiac Cycle Acquisition Device. Sensors 2023, 23, 328. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, V.; Silva, J.; Cauli, O. A survey on sleep questionnaires and diaries. Sleep Med. 2018, 42, 90–96. [Google Scholar] [CrossRef]
- Tamura, T.; Huang, M. Unobtrusive Bed Monitor State of the Art. Sensors 2025, 25, 1879. [Google Scholar] [CrossRef]
- Yamakawa, M.; Kang, H.S.; Wang, H.; Konno, R. Sleep quality assessment of adults in care settings using non-wearable sleep trackers: Scoping review. Int. J. Nurs. Pract. 2024, 30, e13240. [Google Scholar] [CrossRef] [PubMed]
- Baniassadi, A.; Manor, B.; Yu, W.; Travison, T.; Lipsitz, L. Nighttime ambient temperature and sleep in community-dwelling older adults. Sci. Total Environ. 2023, 899, 165623. [Google Scholar] [CrossRef]
- Dashkevych, O.; Portnov, B.A. Using Low-Cost Technology Devices for Monitoring Sleep and Environmental Factors Affecting It: A Systematic Review of the Literature. Appl. Sci. 2025, 15, 1188. [Google Scholar] [CrossRef]
- Jiang, L.; Tang, Z.; Liu, Z.; Chen, W.; Kitamura, K.-i.; Nemoto, T. Automatic sleep monitoring system for home healthcare. In Proceedings of the 2012 IEEE-EMBS International Conference on Biomedical and Health Informatics, Hong Kong, China, 5–7 January 2012; pp. 894–897. [Google Scholar]
- Cay, G.; Ravichandran, V.; Sadhu, S.; Zisk, A.; Salisbury, A.; Solanki, D.; Mankodiya, K. Recent Advancement in Sleep Technologies: A Literature Review on Clinical Standards, Sensors, Apps, and AI Methods. IEEE Access 2022, 10, 104737–104756. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, W.; Nemoto, T.; Kanemitsu, Y.; Kitamura, K.i.; Yamakoshi, K.i.; Wei, D. Real-time monitoring of respiration rhythm and pulse rate during sleep. IEEE Trans. Biomed. Eng. 2006, 53, 2553–2563. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, W.; Tang, Z.; Nemoto, T.; Wei, D. Automatic home care system for monitoring HR/RR during sleep. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 21–24 August 2008; pp. 522–525. [Google Scholar]
- Widasari, E.R.; Tanno, K.; Tamura, H. Automatic sleep quality assessment for obstructive sleep apnea patients based on HRV spectrum analysis. In Proceedings of the 2019 IEEE International Conference on Systems, Man and Cybernetics (SMC), Bari, Italy, 6–9 October 2019; pp. 1187–1192. [Google Scholar]
- Kalintha, W.; Kato, T.; Fukui, K. SleepAge: Sleep Quality Assessment from Nocturnal Sounds in Home Environment. Procedia Comput. Sci. 2020, 176, 898–907. [Google Scholar] [CrossRef]
- Nakajima, K.; Matsumoto, Y.; Tamura, T. Development of real-time image sequence analysis for evaluating posture change and respiratory rate of a subject in bed. Physiol. Meas. 2001, 22, N21. [Google Scholar] [CrossRef]
- Liao, W.H.; Kuo, J.H. Sleep monitoring system in real bedroom environment using texture-based background modeling approaches. J. Ambient. Intell. Humaniz. Comput. 2013, 4, 57–66. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, M.; Chen, F.; Lane, N.D.; Cardone, G.; Wang, R.; Li, T.; Chen, Y.; Choudhury, T.; Campbell, A.T. Unobtrusive sleep monitoring using smartphones. In Proceedings of the 2013 7th International Conference on Pervasive Computing Technologies for Healthcare (PervasiveHealth), Venice, Italy, 5–8 May 2013; pp. 145–152. [Google Scholar]
- Ghazi, S.N.; Behrens, A.; Berner, J.; Sanmartin Berglund, J.; Anderberg, P. Objective sleep monitoring at home in older adults: A scoping review. J. Sleep Res. 2025, 34, e14436. [Google Scholar] [CrossRef]
- Lam, A.; Simonetti, S.; D’Rozario, A.; Ireland, D.; Bradford, D.; Fripp, J.; Naismith, S.L. Perceptions of the Use of Mobile Apps to Assess Sleep-Dependent Memory in Older Adults With Subjective and Objective Cognitive Impairment: Focus Group Approach. JMIR Aging 2025, 8, e68147. [Google Scholar] [CrossRef]
- Tuominen, J.; Peltola, K.; Saaresranta, T.; Valli, K. Sleep parameter assessment accuracy of a consumer home sleep monitoring ballistocardiograph beddit sleep tracker: A validation study. J. Clin. Sleep Med. 2019, 15, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, K.; Masuyama, T.; Iizuka, Y.; Makino, J.; Shiotsuka, J.; Sanui, M. Validity of an under-mattress sensor for objective sleep measurement in critically ill patients: A prospective observational study. J. Intensive Care 2020, 8, 16. [Google Scholar] [CrossRef]
- Ikuta, K.; Aishima, M.; Noguchi-Watanabe, M.; Fukui, S. Feasibility of Monitoring Heart and Respiratory Rates Using Nonwearable Devices and Consistency of the Measured Parameters: Pilot Feasibility Study. JMIR Hum. Factors 2024, 11, e56547. [Google Scholar] [CrossRef]
- Tang, Z.; Jiang, L.; Sakatani, K. Development of Sleep Quality Assessment Method for Elderly People Based on Unobtrusive Sleep Monitoring System. In Proceedings of the Life Engineering Symposium 2018 (LE 2018), Aizu, Japan, 10–12 September 2018; pp. 1–4. [Google Scholar]
- Chaput, J.P.; Dutil, C.; Featherstone, R.; Ross, R.; Giangregorio, L.; Saunders, T.J.; Janssen, I.; Poitras, V.J.; Kho, M.E.; Ross-White, A.; et al. Sleep timing, sleep consistency, and health in adults: A systematic review. Appl. Physiol. Nutr. Metab. 2020, 45, S232–S247. [Google Scholar] [CrossRef]
- Sletten, T.L.; Weaver, M.D.; Foster, R.G.; Gozal, D.; Klerman, E.B.; Rajaratnam, S.M.; Roenneberg, T.; Takahashi, J.S.; Turek, F.W.; Vitiello, M.V.; et al. The importance of sleep regularity: A consensus statement of the National Sleep Foundation sleep timing and variability panel. Sleep Health 2023, 9, 801–820. [Google Scholar] [CrossRef] [PubMed]
- Higami, Y.; Yamakawa, M.; Shigenobu, K.; Kamide, K.; Makimoto, K. High frequency of getting out of bed in patients with Alzheimer’s disease monitored by non-wearable actigraphy. Geriatr. Gerontol. Int. 2019, 19, 130–134. [Google Scholar] [CrossRef]
- Walsh, L.; Moloney, E.; McLoone, S. Identification of nocturnal movements during sleep using the non-contact under mattress bed sensor. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 1660–1663. [Google Scholar]
- Huysmans, D.; Borzée, P.; Testelmans, D.; Buyse, B.; Willemen, T.; Van Huffel, S.; Varon, C. Evaluation of a commercial ballistocardiography sensor for sleep apnea screening and sleep monitoring. Sensors 2019, 19, 2133. [Google Scholar] [CrossRef]
- Saleh, D.; Bertisch, S.M.; Reid, M.; Lim, A.; Purcell, S.; Redline, S. Actigraphy-derived sleep fragmentation index: Convergent validity and associations with clinical outcomes. J. Clin. Sleep Med. 2025, 21, 11754. [Google Scholar] [CrossRef]
- Peng, Y.T.; Lin, C.Y.; Sun, M.T.; Landis, C.A. Multimodality sensor system for long-term sleep quality monitoring. IEEE Trans. Biomed. Circuits Syst. 2008, 1, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Minowa, M.; Uchiyama, M.; Okawa, M.; Kim, K.; Shibui, K.; Kamei, Y. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res. 2000, 97, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]

| Assessment Criteria | ||||
|---|---|---|---|---|
| Levels | Good | Fair | Poor | Very Poor |
| Sleep start time | 19:00–21:00 | 21:00–22:30 | 22:30–24:00 | >24:00 |
| Sleep latency (minutes) | ≤15 | 16–30 | 31–60 | >60 |
| Sleep duration (hours) | >7 | 6–7 | 5–6 | <5 |
| Sleep efficiency (%) | >85% | 75–84% | 65–74% | <65% |
| Body movement times | <1000 | 1000–3000 | 3001–6000 | >6000 |
| Leaving times | ≤1 | 2 | 3–4 | ≥5 |
| Select Factor | Number | Sex | Age (Year) |
|---|---|---|---|
| Good sleeper group | 8 | M: 5; F: 3 | 80.6 ± 4.3 |
| Poor sleeper group | 3 | M: 2; F: 1 | 67.3 ± 0.6 |
| Total | 11 | M: 7; F: 4 | 77.0 ± 7.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Murayama, Y.; Jiang, L.; Chen, W. Development and Validation of an Unobtrusive Automatic Sleep Quality Assessment Index (ASQI) for Elderly Individuals. Electronics 2025, 14, 4531. https://doi.org/10.3390/electronics14224531
Tang Z, Murayama Y, Jiang L, Chen W. Development and Validation of an Unobtrusive Automatic Sleep Quality Assessment Index (ASQI) for Elderly Individuals. Electronics. 2025; 14(22):4531. https://doi.org/10.3390/electronics14224531
Chicago/Turabian StyleTang, Zunyi, Yoshinobu Murayama, Linlin Jiang, and Wenxi Chen. 2025. "Development and Validation of an Unobtrusive Automatic Sleep Quality Assessment Index (ASQI) for Elderly Individuals" Electronics 14, no. 22: 4531. https://doi.org/10.3390/electronics14224531
APA StyleTang, Z., Murayama, Y., Jiang, L., & Chen, W. (2025). Development and Validation of an Unobtrusive Automatic Sleep Quality Assessment Index (ASQI) for Elderly Individuals. Electronics, 14(22), 4531. https://doi.org/10.3390/electronics14224531









