Biochips on the Move: Emerging Trends in Wearable and Implantable Lab-on-Chip Health Monitors
Abstract
1. Introduction
2. Overview of Lab-on-Chip (LoC) Technology
3. Wearable LoC Biosensors
3.1. Types of Wearable Biosensors
3.1.1. Skin-Based Patches
3.1.2. Smart Textiles
3.1.3. Wearable Wristbands and Watches
3.2. Applications
3.2.1. Glucose Monitoring
3.2.2. Sweat Analysis
3.2.3. Cardiovascular Health (ECG, Blood Pressure)
3.2.4. Respiratory and Metabolic Monitoring
3.3. Design Considerations
| Category | Sub-Category | Description |
|---|---|---|
| Types of Wearable Biosensors | Skin-based patches [101] | Conformal, adhesive devices applied to the epidermis enabling continuous, real-time biochemical and physiological monitoring through non-invasive or minimally invasive means. |
| Smart textiles [65] | Biosensors integrated into textile fibers or fabrics, allowing seamless, unobtrusive data acquisition of vital signs during everyday activities or physical exertion. | |
| Wearable wristbands and watches [69] | Commercially viable platforms incorporating multi-modal sensors (e.g., photoplethysmography, ECG) for cardiovascular and metabolic monitoring with integrated data processing. | |
| Applications | Glucose monitoring [102] | Continuous and non-invasive glucose tracking through interstitial fluid or sweat analysis; crucial for diabetes management and glycemic control. |
| Sweat analysis [78] | Quantitative analysis of sweat constituents such as electrolytes, lactate, and cortisol; indicative of hydration status, physical exertion, or stress levels. | |
| Cardiovascular health [103] | Real-time monitoring of electrocardiography (ECG), heart rate, and blood pressure; instrumental in diagnosing arrhythmias and managing hypertension. | |
| Respiratory and metabolic monitoring [104] | Assessment of respiratory rate, oxygen saturation, and metabolic markers; applicable in chronic disease management and fitness optimization. | |
| Design Considerations | Biocompatibility [105] | Use of materials and interfaces that are non-toxic, non-irritating, and compatible with prolonged dermal contact to prevent adverse biological responses. |
| Power management and energy harvesting [106] | Implementation of low-power electronics, energy-efficient protocols, and self-sustaining power sources (e.g., thermoelectric, triboelectric) for extended operational lifespan. | |
| Data transmission and connectivity [107] | Integration of wireless communication protocols (e.g., Bluetooth, NFC, Wi-Fi) enabling real-time data transfer to external devices for storage, analysis, and feedback. |
| Sensing Modality/Sensor Type | Target Analyte | Sensitivity | Specificity | LOD | Response Time | Operational Lifetime | Ref. |
|---|---|---|---|---|---|---|---|
| Electrochemical Glucose Sensor (Wearable) | Glucose | ~10 µA/mM·cm2 | High (enzyme-based) | ~1 µM | ~5–10 s | ~7–14 days (enzyme-limited) | [29,79,80,108] |
| Optical Sweat Sensor (Colorimetric) | Lactate, pH, Na+ | Qualitative | Moderate (cross-talk) | ~0.1 mM (lactate) | ~2–5 min | ~1 day (disposable patches) | [78,80,109] |
| ISF Patch-Based Sensor (Microneedle) | Glucose, Ketone | ~0.1 µA/mM·cm2 | High | ~10 µM | ~15–30 s | ~7 days | [108,110] |
| FET-Based DNA Sensor (Implantable) | DNA/RNA (biomarkers) | fM–pM range | Very high (probe-specific) | ~10 fM | ~60–120 s | Weeks–months (depends on stability) | [111,112,113] |
| Capacitive Sweat Sensor | Electrolytes (Na+, K+) | ~0.1 pF/mM | Moderate | ~1 mM | ~30–60 s | ~3–5 days | [78,114,115] |
| Piezoelectric Sensor (Implantable) | Cardiac Troponin | High (~ng/mL) | High | ~0.05 ng/mL | ~1–2 min | Weeks | [116,117] |
| Wearable Immunosensor (Lateral Flow) | CRP, IL-6, SARS-CoV-2 | Qualitative/Quantitative | High (antibody-based) | ~1 ng/mL | ~5–15 min | Single-use (disposable) | [118,119,120] |
4. Implantable LoC Biosensors
4.1. Types and Insertion Sites
4.1.1. Subcutaneous Sensors
4.1.2. Intravascular and Intracranial Sensors
4.1.3. Gastrointestinal and Organ-Targeted Implants
4.2. Applications
4.2.1. Continuous Glucose Monitoring (CGM)
4.2.2. Neurological and Neural Interface Systems
4.2.3. Cardiac Monitoring
4.2.4. Drug Delivery Systems
4.3. Design Considerations
| Category | Sub-Category | Description |
|---|---|---|
| Types of Implantable Biosensors | Subcutaneous sensors [121,130] | Minimally invasive devices placed beneath the skin to access interstitial fluid. Commonly used for glucose, lactate, and ion monitoring. Known for relatively simple implantation and moderate longevity. |
| Intravascular sensors [126] | Sensors implanted in blood vessels to directly access and analyze blood components. Offer high temporal resolution for analytes like oxygen, glucose, and electrolytes. Require biocompatibility and anticoagulant surfaces to reduce thrombosis risk. | |
| Intracranial/neurological implants [127] | Highly specialized biosensors inserted into brain tissue or cerebrospinal spaces. Used for monitoring intracranial pressure, EEG, or neurochemical levels. Must maintain precise function under immune-reactive conditions. | |
| Gastrointestinal and organ-targeted implants [131] | Devices tailored for specific organs (e.g., gut, liver, bladder). Enable localized sensing or modulation (e.g., pH, motility, enzymes). Must withstand complex physiological environments and mechanical stress. | |
| Applications | Continuous glucose monitoring (CGM) [124] | Long-term monitoring of glucose levels in blood or ISF using enzymatic, optical, or electromagnetic sensors. Integration with telemetry enables real-time glycemic control in diabetes care. |
| Neural interfaces and neuroprosthetics [132] | Capture neural signals or modulate brain activity for treatment of epilepsy, Parkinson’s, or for use in BCIs. Require ultra-miniaturized electronics and neural-friendly biocompatible interfaces. | |
| Cardiac monitoring and pacemakers [26] | Integrated within pacemakers or standalone to detect arrhythmias, ischemia, and heart rate variability. LoC sensors allow closed-loop control and precise event-triggered therapy. | |
| Smart drug delivery systems [147] | LoC platforms integrated with micro-reservoirs or micropumps to enable real-time, analyte-triggered drug release (e.g., insulin, anti-inflammatories). Support precision medicine with localized, on-demand therapy. | |
| Design Considerations | Biocompatibility and encapsulation [159] | Implantable sensors require materials that resist immune rejection, inflammation, and fibrotic encapsulation. Common materials include medical-grade silicones, Parylene-C, titanium, and hydrogel coatings. |
| Power supply and energy harvesting [44] | Power sources include miniaturized batteries, inductive wireless charging, biofuel cells, or energy harvesting from body motion/heat. Energy efficiency and longevity are critical. | |
| Data transmission and telemetry [106] | Communication is achieved via radio frequency (RF), Bluetooth Low Energy (BLE), or near-field communication (NFC). Requires high signal integrity, low latency, and data encryption. | |
| Long-term stability and signal fidelity [153] | Must maintain sensitivity over weeks to years. Challenges include biofouling, corrosion, material degradation, and sensor drift. Self-calibrating or regenerable sensors are preferred for extended deployment. | |
| Miniaturization and integration [160] | Requires integration of sensors, microfluidics, and processing circuits within mm-scale footprints. Must be mechanically and electrically stable without compromising sensing performance. | |
| Safety and regulatory compliance [158] | Devices must meet stringent safety, toxicity, and sterility standards (e.g., ISO 10993, FDA Class III). Preclinical validation, human trials, and post-market surveillance are essential for clinical deployment. |
5. Materials and Fabrication Technologies
5.1. Biocompatible and Flexible Materials
5.2. Nanomaterials for Sensitivity and Selectivity
5.3. Fabrication Techniques
| Category | Material/Technique | Key Characteristics | Applications/Benefits |
|---|---|---|---|
| Biocompatible and Flexible Materials | PDMS [166,206] | Biocompatible, flexible, optically transparent, gas permeable; easy to mold and bond | Standard for microfluidics; skin-conforming devices |
| Hydrogels (PEG, alginate, polyacrylamide) [169] | High water content, ECM-like, tunable mechanics | Tissue-interfacing and drug-delivery biosensors | |
| Conductive Hydrogels [169] | Electrical conductivity + hydrogel benefits | Bioelectrical interfacing | |
| PMMA, COC, PC [173] | Rigid, strong, chemically resistant, optically clear | Rigid implantable LoC systems; compatible with molding | |
| Polyimide, TPU, conductive fabrics [207] | Flexible, durable under strain | Wearables integrated in clothing or skin-contact devices | |
| Nanomaterials for Sensitivity/Selectivity | Gold (Au), Silver (Ag) NPs [208] | High conductivity, plasmonic, easy to functionalize | Optical/electrochemical sensing, enhanced detection limits |
| Carbon Nanotubes, Graphene, Graphene Oxide [185] | Excellent electrical/mechanical properties | High-sensitivity transducers in FETs | |
| Quantum Dots (QDs) [209] | Size-tunable fluorescence, stable | Multiplexed biomarker detection | |
| Metal–Organic Frameworks (MOFs) [189] | High porosity, large surface area | Selective analyte capture | |
| Polyaniline (PANI), Polypyrrole (PPy) [210] | Conductive polymers, redox-active, functionalizable | Electrochemical biosensing | |
| Fabrication Techniques | Photolithography [194] | High-resolution, silicon/glass-based, cleanroom required | Precision microstructures for sensor arrays |
| Soft Lithography [197] | Uses PDMS stamps; flexible, cost-effective | Microfluidic channel fabrication on soft substrates | |
| Additive Manufacturing (3D Printing) [198] | Inkjet, SLA, FDM; fast prototyping | Multi-material biosensors, bioprinting | |
| Laser-based Fabrication [202] | Maskless, precise, customizable | Rapid prototyping across diverse substrates | |
| Screen and Inkjet Printing [199] | Low-cost, scalable, nanomaterial-compatible | Conductive inks, wearable sensor patches | |
| MEMS Fabrication [205] | Microelectronics integration; bulk/surface micromachining | Compact, implantable, multifunctional LoC systems |
6. Challenges and Limitations
6.1. Regulatory and Ethical Concerns
6.2. Data Accuracy and Reliability over Time
6.3. Cost, Mass Production, and User Adoption
6.4. Technical Hurdles: Miniaturization, Integration, and Power Management
7. Future Trends and Research Directions
7.1. Smart and Autonomous Systems
7.2. Multiparameter Sensing
7.3. Personalized and Precision Medicine
7.4. Advances in Wireless Power and Communication
7.5. Wearable–Implantable Hybrid Systems
8. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shiwani, M.A.; Chico, T.J.A.; Ciravegna, F.; Mihaylova, L. Continuous Monitoring of Health and Mobility Indicators in Patients with Cardiovascular Disease: A Review of Recent Technologies. Sensors 2023, 23, 5752. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Rajagopal, S.; Prieto-Simón, B.; Pogue, B.W. Recent Advances in Smart Wearable Sensors for Continuous Human Health Monitoring. Talanta 2024, 272, 125817. [Google Scholar] [CrossRef]
- Kazanskiy, N.L.; Khonina, S.N.; Butt, M.A. A Review on Flexible Wearables—Recent Developments in Non-Invasive Continuous Health Monitoring. Sens. Actuators A Phys. 2024, 366, 114993. [Google Scholar] [CrossRef]
- Gutruf, P. Towards a Digitally Connected Body for Holistic and Continuous Health Insight. Commun. Mater. 2024, 5, 2. [Google Scholar] [CrossRef]
- Rajasekaran, K.; Chakaravarthi, M.S.; Lokaswar, P. Continuous Health Monitoring System for Patients Using IoT. In Proceedings of the 2023 9th International Conference on Advanced Computing and Communication Systems (ICACCS), Coimbatore, India, 17–18 March 2023; Volume 1, pp. 987–990. [Google Scholar]
- Kazanskiy, N.L.; Khonina, S.N.; Butt, M.A. Smart Contact Lenses—A Step towards Non-Invasive Continuous Eye Health Monitoring. Biosensors 2023, 13, 933. [Google Scholar] [CrossRef]
- Butt, M.A.; Imran Akca, B.; Mateos, X. Integrated Photonic Biosensors: Enabling Next-Generation Lab-on-a-Chip Platforms. Nanomaterials 2025, 15, 731. [Google Scholar] [CrossRef]
- Zhang, Z.; Abdalwareth, A.; Flachenecker, G.; Angelmahr, M.; Schade, W. Polymer Waveguide Sensor Based on Evanescent Bragg Grating for Lab-on-a-Chip Applications. Sensors 2024, 24, 1234. [Google Scholar] [CrossRef] [PubMed]
- Parolo, C.; Idili, A.; Heikenfeld, J.; Plaxco, K.W. Conformational-Switch Biosensors as Novel Tools to Support Continuous, Real-Time Molecular Monitoring in Lab-on-a-Chip Devices. Lab Chip 2023, 23, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Singh, S.; Covone, G.; Tonietti, L.; Ricciardelli, A.; Cordone, A.; Iacono, R.; Mazzoli, A.; Moracci, M.; Rotundi, A.; et al. Reviewing the State of Biosensors and Lab-on-a- Chip Technologies: Opportunities for Extreme Environments and Space Exploration. Front. Microbiol. 2023, 14, 1215529. [Google Scholar] [CrossRef] [PubMed]
- Nikoleli, G.-P.; Siontorou, C.G.; Nikolelis, D.P.; Bratakou, S.; Karapetis, S.; Tzamtzis, N. Chapter 13—Biosensors Based on Microfluidic Devices Lab-on-a-Chip and Microfluidic Technology. In Nanotechnology and Biosensors; Nikolelis, D.P., Nikoleli, G.-P., Eds.; Advanced Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 375–394. ISBN 978-0-12-813855-7. [Google Scholar]
- Beykou, M.; Bousgouni, V.; Moser, N.; Georgiou, P.; Bakal, C. Biocompatibility Characterisation of CMOS-Based Lab-on-Chip Electrochemical Sensors for in Vitro Cancer Cell Culture Applications. Biosens. Bioelectron. 2024, 262, 116513. [Google Scholar] [CrossRef]
- Ghorbanizamani, F.; Moulahoum, H.; Guler Celik, E.; Timur, S. Material Design in Implantable Biosensors toward Future Personalized Diagnostics and Treatments. Appl. Sci. 2023, 13, 4630. [Google Scholar] [CrossRef]
- Dkhar, D.S.; Kumari, R.; Malode, S.J.; Shetti, N.P.; Chandra, P. Integrated Lab-on-a-Chip Devices: Fabrication Methodologies, Transduction System for Sensing Purposes. J. Pharm. Biomed. Anal. 2023, 223, 115120. [Google Scholar] [CrossRef]
- Morris, R.; Mancin, M. Chapter 3—Lab-on-a-Chip: Wearables as a One Stop Shop for Free-Living Assessments. In Digital Health; Godfrey, A., Stuart, S., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 43–60. ISBN 978-0-12-818914-6. [Google Scholar]
- Subudhi, S.K.; Das, S. Reliability of Lab-on-a-Chip Technologies for Wearable Electronics: A Perspective. Front. Sens. 2023, 4, 1283402. [Google Scholar] [CrossRef]
- Apoorva, S.; Nguyen, N.-T.; Sreejith, K.R. Recent Developments and Future Perspectives of Microfluidics and Smart Technologies in Wearable Devices. Lab Chip 2024, 24, 1833–1866. [Google Scholar] [CrossRef]
- Economou, A.; Kokkinos, C.; Prodromidis, M. Flexible Plastic, Paper and Textile Lab-on-a Chip Platforms for Electrochemical Biosensing. Lab Chip 2018, 18, 1812–1830. [Google Scholar] [CrossRef]
- Ma, X.; Guo, G.; Wu, X.; Wu, Q.; Liu, F.; Zhang, H.; Shi, N.; Guan, Y. Advances in Integration, Wearable Applications, and Artificial Intelligence of Biomedical Microfluidics Systems. Micromachines 2023, 14, 972. [Google Scholar] [CrossRef]
- Liao, Y.; Yu, N.; Zhou, G.; Wu, Y.; Wang, C. A Wireless Multi-Channel Low-Cost Lab-on-Chip Algae Culture Monitor AIoT System for Algae Farm. Comput. Electron. Agric. 2022, 193, 106647. [Google Scholar] [CrossRef]
- Dornhof, J.; Kieninger, J.; Muralidharan, H.; Maurer, J.; Urban, G.A.; Weltin, A. Microfluidic Organ-on-Chip System for Multi-Analyte Monitoring of Metabolites in 3D Cell Cultures. Lab Chip 2022, 22, 225–239. [Google Scholar] [CrossRef]
- Butt, M.A. A Perspective on Smart Contact Lenses: Pioneering Non-Intrusive Eye Health Monitoring. Sens. Actuators A Phys. 2025, 387, 116399. [Google Scholar] [CrossRef]
- Cooper, J.M. Challenges in Lab-on-a-Chip Technology. Front. Lab Chip Technol. 2022, 1, 979398. [Google Scholar] [CrossRef]
- Battat, S.; Weitz, D.A.; Whitesides, G.M. An Outlook on Microfluidics: The Promise and the Challenge. Lab Chip 2022, 22, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Mai, A.; Mai, C.; Steglich, P. From Lab-on-Chip to Lab-in-App: Challenges towards Silicon Photonic Biosensors Product Developments. Results Opt. 2022, 9, 100317. [Google Scholar] [CrossRef]
- Choi, Y.S.; Yin, R.T.; Pfenniger, A.; Koo, J.; Avila, R.; Benjamin Lee, K.; Chen, S.W.; Lee, G.; Li, G.; Qiao, Y.; et al. Fully Implantable and Bioresorbable Cardiac Pacemakers without Leads or Batteries. Nat. Biotechnol. 2021, 39, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, A.; Raveendran, J.; Babu, T.G.S. Chapter Five—Design, Fabrication and Assembly of Lab-on-a-Chip and Its Uses. In Progress in Molecular Biology and Translational Science; Pandya, A., Singh, V., Eds.; Micro/Nanofluidics and Lab-on-Chip Based Emerging Technologies for Biomedical and Translational Research Applications—Part B; Academic Press: Cambridge, MA, USA, 2022; Volume 187, pp. 121–162. [Google Scholar]
- Cunha, M.L.; da Silva, S.S.; Stracke, M.C.; Zanette, D.L.; Aoki, M.N.; Blanes, L. Sample Preparation for Lab-on-a-Chip Systems in Molecular Diagnosis: A Review. Anal. Chem. 2022, 94, 41–58. [Google Scholar] [CrossRef]
- Marimuthu, M.; Krishnan, V.; Sudhakaran, S.D.; Vigneswari, S.; Senthilkumar, S.; Veerapandian, M. Electrochemical-Based Biosensor Platforms in Lab-Chip Models for Point-of-Need Toxicant Analysis. Electrochem 2023, 4, 537–552. [Google Scholar] [CrossRef]
- Yoo, K.M.; Fan, K.-C.; Hlaing, M.; Jain, S.; Ning, S.; An, Y.; Chen, R.T. Lab-on-a-Chip Optical Biosensor Platform: A Micro-Ring Resonator Integrated with a near-Infrared Fourier Transform Spectrometer. Opt. Lett. 2023, 48, 5371–5374. [Google Scholar] [CrossRef]
- Wang, J.; Sanchez, M.M.; Yin, Y.; Herzer, R.; Ma, L.; Schmidt, O.G. Silicon-Based Integrated Label-Free Optofluidic Biosensors: Latest Advances and Roadmap. Adv. Mater. Technol. 2020, 5, 1901138. [Google Scholar] [CrossRef]
- Mazaher, A.; Seyed Sepehr, U.; Ali, J. Chapter 20—Miniaturized Analytical Lab-on-a-Chip Devices and Their Potential Application in Environmental Monitoring. In Recent Trends and Perspectives on Electrochemical Sensors for Environmental Monitoring; Ozkan, S.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 637–669. ISBN 978-0-443-13388-6. [Google Scholar]
- Weigel, N.; Männel, M.J.; Thiele, J. Flexible Materials for High-Resolution 3D Printing of Microfluidic Devices with Integrated Droplet Size Regulation. ACS Appl. Mater. Interfaces 2021, 13, 31086–31101. [Google Scholar] [CrossRef]
- Antonelli, G.; Mencattini, A.; Massimiani, M.; Lacconi, V.; Filippi, J.; Losardo, M.; D’Orazio, M.; Casti, P.; Bragaglia, M.; Curci, G.; et al. Development of Integrated Optical Biosensors Based on Low-Cost Stereolithography Fabrication and Multispectral Signature for Lab-On-Chip Applications. Sens. Actuators B Chem. 2024, 401, 135009. [Google Scholar] [CrossRef]
- Fu, Y.Q.; Luo, J.K.; Nguyen, N.T.; Walton, A.J.; Flewitt, A.J.; Zu, X.T.; Li, Y.; McHale, G.; Matthews, A.; Iborra, E.; et al. Advances in Piezoelectric Thin Films for Acoustic Biosensors, Acoustofluidics and Lab-on-Chip Applications. Prog. Mater. Sci. 2017, 89, 31–91. [Google Scholar] [CrossRef]
- Nestorova, G.G.; Adapa, B.S.; Kopparthy, V.L.; Guilbeau, E.J. Lab-on-a-Chip Thermoelectric DNA Biosensor for Label-Free Detection of Nucleic Acid Sequences. Sens. Actuators B Chem. 2016, 225, 174–180. [Google Scholar] [CrossRef]
- Kazanskiy, N.L.; Butt, M.A.; Khonina, S.N. Recent Advances in Wearable Optical Sensor Automation Powered by Battery versus Skin-like Battery-Free Devices for Personal Healthcare—A Review. Nanomaterials 2022, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Shinar, R.; Shinar, J. Organic Electronics—Microfluidics/Lab on a Chip Integration in Analytical Applications. Sensors 2023, 23, 8488. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Xu, J.; Ouyang, C.; Mu, X.; Schober, R. Near-Field Communications: A Tutorial Review. IEEE Open J. Commun. Soc. 2023, 4, 1999–2049. [Google Scholar] [CrossRef]
- Koulouras, G.; Katsoulis, S.; Zantalis, F. Evolution of Bluetooth Technology: BLE in the IoT Ecosystem. Sensors 2025, 25, 996. [Google Scholar] [CrossRef]
- Akkaş, T.; Reshadsedghi, M.; Şen, M.; Kılıç, V.; Horzum, N. The Role of Artificial Intelligence in Advancing Biosensor Technology: Past, Present, and Future Perspectives. Adv. Mater. 2025, 2504796. [Google Scholar] [CrossRef]
- Li, X.; Huang, X.; Yang, L.; Jung, S.; Wang, J.; Zhao, H. Implantable Physical Sensors for in Vivo Organ Monitoring. Med-X 2025, 3, 1. [Google Scholar] [CrossRef]
- Yogev, D.; Goldberg, T.; Arami, A.; Tejman-Yarden, S.; Winkler, T.E.; Maoz, B.M. Current State of the Art and Future Directions for Implantable Sensors in Medical Technology: Clinical Needs and Engineering Challenges. APL Bioeng. 2023, 7, 031506. [Google Scholar] [CrossRef]
- Roy, S.; Azad, A.N.M.W.; Baidya, S.; Alam, M.K.; Khan, F. Powering Solutions for Biomedical Sensors and Implants Inside the Human Body: A Comprehensive Review on Energy Harvesting Units, Energy Storage, and Wireless Power Transfer Techniques. IEEE Trans. Power Electron. 2022, 37, 12237–12263. [Google Scholar] [CrossRef]
- Dolson, C.M.; Harlow, E.R.; Phelan, D.M.; Gabbett, T.J.; Gaal, B.; McMellen, C.; Geletka, B.J.; Calcei, J.G.; Voos, J.E.; Seshadri, D.R. Wearable Sensor Technology to Predict Core Body Temperature: A Systematic Review. Sensors 2022, 22, 7639. [Google Scholar] [CrossRef]
- Min, S.; An, J.; Lee, J.H.; Kim, J.H.; Joe, D.J.; Eom, S.H.; Yoo, C.D.; Ahn, H.-S.; Hwang, J.-Y.; Xu, S.; et al. Wearable Blood Pressure Sensors for Cardiovascular Monitoring and Machine Learning Algorithms for Blood Pressure Estimation. Nat. Rev. Cardiol. 2025, 22, 629–648. [Google Scholar] [CrossRef]
- Budić, D.; Šimunić, D.; Sayrafian, K. Kinetic-Based Micro Energy-Harvesting for Wearable Sensors. In Proceedings of the 2015 6th IEEE International Conference on Cognitive Infocommunications (CogInfoCom), Gyor, Hungary, 19–21 October 2015; pp. 505–509. [Google Scholar]
- Morresi, N.; Cipollone, V.; Casaccia, S.; Revel, G.M. Measuring Thermal Comfort Using Wearable Technology in Transient Conditions during Office Activities. Measurement 2024, 224, 113897. [Google Scholar] [CrossRef]
- Papani, R.; Li, Y.; Wang, S. Soft Mechanical Sensors for Wearable and Implantable Applications. WIREs Nanomed. Nanobiotechnol. 2024, 16, e1961. [Google Scholar] [CrossRef] [PubMed]
- Khatsenko, K.; Khin, Y.; Maibach, H. Allergic Contact Dermatitis to Components of Wearable Adhesive Health Devices. Dermatitis 2020, 31, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, P.; Kamat, V.; Abrol, I.; Kaushik, A.; Bhansali, S. Bio-Acceptability of Wearable Sensors: A Mechanistic Study towards Evaluating Ionic Leaching Induced Cellular Inflammation. Sci. Rep. 2022, 12, 10782. [Google Scholar] [CrossRef] [PubMed]
- Hrabovska, N.; Kajati, E.; Zolotova, I. A Validation Study to Confirm the Accuracy of Wearable Devices Based on Health Data Analysis. Electronics 2023, 12, 2536. [Google Scholar] [CrossRef]
- Jorsch, C.; Guenther, M.; Gerlach, G. Biocompatible Hermetic Encapsulation for Implantable Miniaturized Biomedical Sensor System. Procedia Technol. 2017, 27, 42–43. [Google Scholar] [CrossRef]
- Oh, J.; Loeb, G.E.; Smith, B.A. The Utility of Calibrating Wearable Sensors before Quantifying Infant Leg Movements. Sensors 2024, 24, 5736. [Google Scholar] [CrossRef]
- Leung, D.P.; McCormick, D.J.; Malpas, S.C.; Budgett, D.M. Reducing Drift in Implantable Pressure Sensors. IEEE Sens. J. 2019, 19, 2458–2465. [Google Scholar] [CrossRef]
- Kargarandehkordi, A.; Li, S.; Lin, K.; Phillips, K.T.; Benzo, R.M.; Washington, P. Fusing Wearable Biosensors with Artificial Intelligence for Mental Health Monitoring: A Systematic Review. Biosensors 2025, 15, 202. [Google Scholar] [CrossRef]
- De Fazio, R.; Mastronardi, V.M.; De Vittorio, M.; Visconti, P. Wearable Sensors and Smart Devices to Monitor Rehabilitation Parameters and Sports Performance: An Overview. Sensors 2023, 23, 1856. [Google Scholar] [CrossRef]
- Ghazizadeh, E.; Naseri, Z.; Deigner, H.-P.; Rahimi, H.; Altintas, Z. Approaches of Wearable and Implantable Biosensor towards of Developing in Precision Medicine. Front. Med. 2024, 11, 1390634. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, J.; Ray, W.Z.; MacEwan, M.R. Implantable and Semi-Implantable Biosensors for Minimally Invasive Disease Diagnosis. Processes 2024, 12, 1535. [Google Scholar] [CrossRef]
- Vulpe, G.; Liu, G.; Oakley, S.; Yang, G.; Mohan, A.A.; Waldron, M.; Sharma, S. Lab on Skin: Real-Time Metabolite Monitoring with Polyphenol Film Based Subdermal Wearable Patches. Lab Chip 2024, 24, 2039–2048. [Google Scholar] [CrossRef]
- Kunnel, B.P.; Demuru, S. An Epidermal Wearable Microfluidic Patch for Simultaneous Sampling, Storage, and Analysis of Biofluids with Counterion Monitoring. Lab Chip 2022, 22, 1793–1804. [Google Scholar] [CrossRef]
- Shinde, S.; Kim, K.H.; Park, S.Y.; Kim, J.H.; Kim, J.; Joe, D.J.; Lee, H.E. Wearable Sweat-Sensing Patches for Non-Invasive and Continuous Health Tracking. Sens. Actuators Rep. 2025, 9, 100265. [Google Scholar] [CrossRef]
- Lee, H.; Song, S.; Yea, J.; Ha, J.; Oh, S.; Jekal, J.; Hong, M.S.; Won, C.; Jung, H.H.; Keum, H.; et al. Vialess Heterogeneous Skin Patch for Multimodal Monitoring and Stimulation. Nat. Commun. 2025, 16, 650. [Google Scholar] [CrossRef] [PubMed]
- Phan, D.T.; Nguyen, C.H.; Nguyen, T.D.P.; Tran, L.H.; Park, S.; Choi, J.; Lee, B.; Oh, J. A Flexible, Wearable, and Wireless Biosensor Patch with Internet of Medical Things Applications. Biosensors 2022, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Akter, A.; Apu, M.M.H.; Veeranki, Y.R.; Baroud, T.N.; Posada-Quintero, H.F. Recent Studies on Smart Textile-Based Wearable Sweat Sensors for Medical Monitoring: A Systematic Review. J. Sens. Actuator Netw. 2024, 13, 40. [Google Scholar] [CrossRef]
- Subashini, J.M.; Padmaja, P.P.; Mishra, M.K.; Palanisamy, R.; Veluswamy, P. Smart Textile Materials for Monitoring ECG Signals. In Proceedings of the 2022 IEEE International Conference on Emerging Electronics (ICEE), Bangalore, India, 11–14 December 2022; pp. 1–6. [Google Scholar]
- Nigusse, A.B.; Mengistie, D.A.; Malengier, B.; Tseghai, G.B.; Langenhove, L.V. Wearable Smart Textiles for Long-Term Electrocardiography Monitoring—A Review. Sensors 2021, 21, 4174. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, C.; Ahmed, D. A Smart Acoustic Textile for Health Monitoring. Nat. Electron. 2025, 8, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, M.; Vanhooydonck, A.; Watts, R.; De Wael, K. Wearable Wristband-Based Electrochemical Sensor for the Detection of Phenylalanine in Biofluids. Biosens. Bioelectron. 2022, 197, 113764. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.A.; Li, R.; Tse, Z.T.H. Reshaping Healthcare with Wearable Biosensors. Sci. Rep. 2023, 13, 4998. [Google Scholar] [CrossRef] [PubMed]
- Vo, D.-K.; Trinh, K.T.L. Advances in Wearable Biosensors for Healthcare: Current Trends, Applications, and Future Perspectives. Biosensors 2024, 14, 560. [Google Scholar] [CrossRef]
- Alzahrani, S.; Nadershah, M.; Alghamdi, M.; Baabdullah, R.; Bayoumi, M.; Bawajeeh, O.; Alghamdi, A.; Bayoumi, A. The Use of Apple Smartwatches to Obtain Vital Signs Readings in Surgical Patients. Sci. Rep. 2025, 15, 10920. [Google Scholar] [CrossRef]
- Volkova, E.; Perchik, A.; Pavlov, K.; Nikolaev, E.; Ayuev, A.; Park, J.; Chang, N.; Lee, W.; Kim, J.Y.; Doronin, A.; et al. Multispectral Sensor Fusion in SmartWatch for in Situ Continuous Monitoring of Human Skin Hydration and Body Sweat Loss. Sci. Rep. 2023, 13, 13371. [Google Scholar] [CrossRef]
- Martins, A.J.L.; Velásquez, R.J.; Gaillac, D.B.; Santos, V.N.; Tami, D.C.; Souza, R.N.P.; Osorio, F.C.; Fogli, G.A.; Soares, B.S.; do Rego, C.G.; et al. A Comprehensive Review of Non-Invasive Optical and Microwave Biosensors for Glucose Monitoring. Biosens. Bioelectron. 2025, 271, 117081. [Google Scholar] [CrossRef]
- Heller, A.; Feldman, B. Electrochemical Glucose Sensors and Their Applications in Diabetes Management. Chem. Rev. 2008, 108, 2482–2505. [Google Scholar] [CrossRef]
- Psoma, S.D.; Kanthou, C. Wearable Insulin Biosensors for Diabetes Management: Advances and Challenges. Biosensors 2023, 13, 719. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Qiao, C.; Liu, Y.; Wang, C.; Zeng, X.; Hou, J.; Huo, D.; Hou, C. Synergistic Enhancement of Wearable Biosensor through Pt Single-Atom Catalyst for Sweat Analysis. Biosens. Bioelectron. 2024, 258, 116354. [Google Scholar] [CrossRef]
- Gao, F.; Liu, C.; Zhang, L.; Liu, T.; Wang, Z.; Song, Z.; Cai, H.; Fang, Z.; Chen, J.; Wang, J.; et al. Wearable and Flexible Electrochemical Sensors for Sweat Analysis: A Review. Microsyst. Nanoeng. 2023, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Yang, Y.; Huang, S.; Huang, Z. Wearable Devices for Monitoring Sweat Glucose: An Integrated Strategy for Efficient Electrochemical Sensors. Sens. Actuators Rep. 2025, 9, 100339. [Google Scholar] [CrossRef]
- Xu, J.; Fang, Y.; Chen, J. Wearable Biosensors for Non-Invasive Sweat Diagnostics. Biosensors 2021, 11, 245. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.; Pérez-Ràfols, C.; Chen, C.; Cuartero, M.; Crespo, G.A. Lactate Biosensing for Reliable On-Body Sweat Analysis. ACS Sens. 2021, 6, 2763–2771. [Google Scholar] [CrossRef]
- Childs, A.; Mayol, B.; Lasalde-Ramírez, J.A.; Song, Y.; Sempionatto, J.R.; Gao, W. Diving into Sweat: Advances, Challenges, and Future Directions in Wearable Sweat Sensing. ACS Nano 2024, 18, 24605–24616. [Google Scholar] [CrossRef]
- Nan, M.; Darmawan, B.A.; Go, G.; Zheng, S.; Lee, J.; Kim, S.; Lee, T.; Choi, E.; Park, J.-O.; Bang, D. Wearable Localized Surface Plasmon Resonance-Based Biosensor with Highly Sensitive and Direct Detection of Cortisol in Human Sweat. Biosensors 2023, 13, 184. [Google Scholar] [CrossRef]
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Yin Yin Nyein, H.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z.; et al. Autonomous Sweat Extraction and Analysis Applied to Cystic Fibrosis and Glucose Monitoring Using a Fully Integrated Wearable Platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630. [Google Scholar] [CrossRef]
- Homayounfar, S.Z.; Andrew, T.L. Wearable Sensors for Monitoring Human Motion: A Review on Mechanisms, Materials, and Challenges. SLAS Technol. 2020, 25, 9–24. [Google Scholar] [CrossRef]
- Pinheiro, G.P.M.; Miranda, R.K.; Praciano, B.J.G.; Santos, G.A.; Mendonça, F.L.L.; Javidi, E.; da Costa, J.P.J.; de Sousa, R.T. Multi-Sensor Wearable Health Device Framework for Real-Time Monitoring of Elderly Patients Using a Mobile Application and High-Resolution Parameter Estimation. Front. Hum. Neurosci. 2022, 15, 750591. [Google Scholar] [CrossRef]
- Wan, J.; AAH Al-awlaqi, M.; Li, M.; O’Grady, M.; Gu, X.; Wang, J.; Cao, N. Wearable IoT Enabled Real-Time Health Monitoring System. EURASIP J. Wirel. Commun. Netw. 2018, 2018, 298. [Google Scholar] [CrossRef]
- Kaisti, M.; Leppänen, J.; Lahdenoja, O.; Kostiainen, P.; Pankaaia, M.; Meriheina, U.; Koivisto, T. Wearable Pressure Sensor Array for Health Monitoring. In Proceedings of the 2017 Computing in Cardiology (CinC), Rennes, France, 24–27 September 2017; pp. 1–4. [Google Scholar]
- Dai, B.; Gao, C.; Xie, Y. Flexible Wearable Devices for Intelligent Health Monitoring. VIEW 2022, 3, 20220027. [Google Scholar] [CrossRef]
- Buendia, R.; Karpefors, M.; Folkvaljon, F.; Hunter, R.; Sillen, H.; Luu, L.; Docherty, K.; Cowie, M.R. Wearable Sensors to Monitor Physical Activity in Heart Failure Clinical Trials: State-of-the-Art Review. J. Card. Fail. 2024, 30, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P.; Ha, G.; Wright, D.E.; Ma, Y.; Sen-Gupta, E.; Haubrich, N.R.; Branche, P.C.; Li, W.; Huppert, G.L.; Johnson, M.; et al. Highly Flexible, Wearable, and Disposable Cardiac Biosensors for Remote and Ambulatory Monitoring. NPJ Digit. Med. 2018, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Ullah, S.; Fernández-García, R.; Gil, I. Wearable Sensors for Respiration Monitoring: A Review. Sensors 2023, 23, 7518. [Google Scholar] [CrossRef]
- Abdul Kader, L.; Al-Shargie, F.; Tariq, U.; Al-Nashash, H. One-Channel Wearable Mental Stress State Monitoring System. Sensors 2024, 24, 5373. [Google Scholar] [CrossRef]
- Guo, H.; Lu, L.; Hatton, F.L.; Xu, L.; Yu, E.; Peijs, T.; Bilotti, E.; Zhang, H.; Liu, Y. Wearable Body Temperature Sensing with Autonomous Self-Regulated Joule Heating and Passive Cooling for Healthcare Applications. Adv. Funct. Mater. 2025, 35, 2417961. [Google Scholar] [CrossRef]
- Lee, O.; Lee, H. Development and Printing of Three-Dimensional Electrodes for the High Body Adhesion of Smart Wear. Fash. Text. 2024, 11, 29. [Google Scholar] [CrossRef]
- Park, H.; Park, W.; Lee, C.H. Electrochemically Active Materials and Wearable Biosensors for the in Situ Analysis of Body Fluids for Human Healthcare. NPG Asia Mater. 2021, 13, 23. [Google Scholar] [CrossRef]
- Bardhan, N.M.; Radisic, M.; Nurunnabi, M. Bioinspired Materials for Wearable Diagnostics and Biosensors. ACS Biomater. Sci. Eng. 2023, 9, 2015–2019. [Google Scholar] [CrossRef]
- Li, F.; Huang, T.; Pasic, P.; Easton, C.D.; Voelcker, N.H.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J.; Thissen, H. One Step Antimicrobial Coatings for Medical Device Applications Based on Low Fouling Polymers Containing Selenium Nanoparticles. Chem. Eng. J. 2023, 467, 143546. [Google Scholar] [CrossRef]
- Antolini, A.; Zavalloni, F.; Lico, A.; Quqa, S.; Greco, L.; Mangia, M.; Pareschi, F.; Pasotti, M.; Franchi Scarselli, E. The Role of Phase-Change Memory in Edge Computing and Analog In-Memory Computing: An Overview of Recent Research Contributions and Future Challenges. Sensors 2025, 25, 3618. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Jiang, N.; Yetisen, A.K. Wearable Artificial Intelligence Biosensor Networks. Biosens. Bioelectron. 2023, 219, 114825. [Google Scholar] [CrossRef]
- Chugh, V.; Basu, A.; Kaushik, A.; Basu, A.K. E-Skin—Based Advanced Wearable Technology for Health Management. Curr. Res. Biotechnol. 2023, 5, 100129. [Google Scholar] [CrossRef]
- Mansour, M.; Saeed Darweesh, M.; Soltan, A. Wearable Devices for Glucose Monitoring: A Review of State-of-the-Art Technologies and Emerging Trends. Alex. Eng. J. 2024, 89, 224–243. [Google Scholar] [CrossRef]
- Sana, F.; Isselbacher, E.M.; Singh, J.P.; Heist, E.K.; Pathik, B.; Armoundas, A.A. Wearable Devices for Ambulatory Cardiac Monitoring. JACC 2020, 75, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Keshet, A.; Reicher, L.; Bar, N.; Segal, E. Wearable and Digital Devices to Monitor and Treat Metabolic Diseases. Nat. Metab. 2023, 5, 563–571. [Google Scholar] [CrossRef]
- Lu, T.; Ji, S.; Jin, W.; Yang, Q.; Luo, Q.; Ren, T.-L. Biocompatible and Long-Term Monitoring Strategies of Wearable, Ingestible and Implantable Biosensors: Reform the Next Generation Healthcare. Sensors 2023, 23, 2991. [Google Scholar] [CrossRef]
- Gao, M.; Yao, Y.; Wang, Y.; Wang, B.; Wang, P.; Wang, Y.; Dai, J.; Liu, S.; Torres, J.F.; Cheng, W.; et al. Wearable Power Management System Enables Uninterrupted Battery-Free Data-Intensive Sensing and Transmission. Nano Energy 2023, 107, 108107. [Google Scholar] [CrossRef]
- Alruwaili, O.; Yousef, A.; Armghan, A. Monitoring the Transmission of Data From Wearable Sensors Using Probabilistic Transfer Learning. IEEE Access 2024, 12, 97460–97475. [Google Scholar] [CrossRef]
- Cheng, Y.; Gong, X.; Yang, J.; Zheng, G.; Zheng, Y.; Li, Y.; Xu, Y.; Nie, G.; Xie, X.; Chen, M.; et al. A Touch-Actuated Glucose Sensor Fully Integrated with Microneedle Array and Reverse Iontophoresis for Diabetes Monitoring. Biosens. Bioelectron. 2022, 203, 114026. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Gutruf, P.; Choi, J.; Lee, K.; Sekine, Y.; Reeder, J.T.; Jeang, W.J.; Aranyosi, A.J.; Lee, S.P.; Model, J.B.; et al. Battery-Free, Skin-Interfaced Microfluidic/Electronic Systems for Simultaneous Electrochemical, Colorimetric, and Volumetric Analysis of Sweat. Sci. Adv. 2019, 5, eaav3294. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gong, X.; Chen, S.; Zheng, Y.; Peng, L.; Liu, B.; Chen, Z.; Xie, X.; Yi, C.; Jiang, L. Development of Smartphone-Controlled and Microneedle-Based Wearable Continuous Glucose Monitoring System for Home-Care Diabetes Management. ACS Sens. 2023, 8, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Jeong, Y.-T.; Park, H.-J.; Shin, J.-K.; Choi, P.; Lee, J.-H.; Lim, G. An FET-Type Charge Sensor for Highly Sensitive Detection of DNA Sequence. Biosens. Bioelectron. 2004, 20, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Sreejith, S.; Ajayan, J.; Radhika, J.M.; Sivasankari, B.; Tayal, S.; Saravanan, M. A Comprehensive Review on Graphene FET Bio-Sensors and Their Emerging Application in DNA/RNA Sensing & Rapid COVID-19 Detection. Measurement 2023, 206, 112202. [Google Scholar] [CrossRef]
- Janićijević, Ž.; Baraban, L. Integration Strategies and Formats in Field-Effect Transistor Chemo- and Biosensors: A Critical Review. ACS Sens. 2025, 10, 2431–2452. [Google Scholar] [CrossRef]
- Islam, M.S.; Cha, S.; Cai, W.; Ferdoushi, M.; Khan, Y. A Wearable Sweat Rate Sensor With Adaptive Sweat Ion Concentration Calibration. IEEE Sens. Lett. 2025, 9, 5502404. [Google Scholar] [CrossRef]
- Aguilar-Torán, J.; Rabost-Garcia, G.; Toinga-Villafuerte, S.; Álvarez-Carulla, A.; Colmena-Rubil, V.; Fajardo-Garcia, A.; Cardona-Bonet, A.; Casals-Terré, J.; Muñoz-Pascual, X.; Miribel-Català, P.; et al. Novel Sweat-Based Wearable Device for Advanced Monitoring of Athletic Physiological Biometrics. Sensors 2023, 23, 9473. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Hong, M.; Venezuela, J.; Shi, W.; Dargusch, M. Advances in Biodegradable Piezoelectrics for Medical Implants. Nano Today 2023, 52, 101945. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Dai, J.; Qu, Z.; Li, X.; Wu, Y.; Hu, S.; Fang, J.; Shen, Z.; Xiao, M.; et al. Biodegradable Piezoelectric Implant for Wirelessly Delivering Electrical Stimulation to the Heart under Ultrasound Stress. Adv. Funct. Mater. 2025, 35, 2418708. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, B.-C.; Oh, B.-K.; Choi, J.-W. Highly Sensitive Localized Surface Plasmon Resonance Immunosensor for Label-Free Detection of HIV-1. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1018–1026. [Google Scholar] [CrossRef]
- Nah, J.S.; Barman, S.C.; Zahed, M.A.; Sharifuzzaman, M.; Yoon, H.; Park, C.; Yoon, S.; Zhang, S.; Park, J.Y. A Wearable Microfluidics-Integrated Impedimetric Immunosensor Based on Ti3C2Tx MXene Incorporated Laser-Burned Graphene for Noninvasive Sweat Cortisol Detection. Sens. Actuators B Chem. 2021, 329, 129206. [Google Scholar] [CrossRef]
- Manisha Inukonda, S.; Panda, S. Bio-Inspired Wettability Patterning for Flexible and Wearable Electrochemical Immunosensors Utilizing PDMS-TiO2 Coating. Microchem. J. 2024, 200, 110425. [Google Scholar] [CrossRef]
- Kim, S.; Malik, J.; Seo, J.M.; Cho, Y.M.; Bien, F. Subcutaneously Implantable Electromagnetic Biosensor System for Continuous Glucose Monitoring. Sci. Rep. 2022, 12, 17395. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Redouté, J.-M.; Yuce, M.R. A Wireless Implantable Sensor Design With Subcutaneous Energy Harvesting for Long-Term IoT Healthcare Applications. IEEE Access 2018, 6, 35801–35808. [Google Scholar] [CrossRef]
- Moussy, F.; Harrison, D.J.; O’Brien, D.W.; Rajotte, R.V. Performance of Subcutaneously Implanted Needle-Type Glucose Sensors Employing a Novel Trilayer Coating. Anal. Chem. 1993, 65, 2072–2077. [Google Scholar] [CrossRef]
- Gerritsen, M.; Jansen, J.A.; Lutterman, J.A. Performance of Subcutaneously Implanted Glucose Sensors for Continuous Monitoring. Neth. J. Med. 1999, 54, 167–179. [Google Scholar] [CrossRef]
- Brancato, L.; Keulemans, G.; Verbelen, T.; Meyns, B.; Puers, R. An Implantable Intravascular Pressure Sensor for a Ventricular Assist Device. Micromachines 2016, 7, 135. [Google Scholar] [CrossRef]
- Starr, P.; Bartels, K.; Agrawal, C.M.; Bailey, S. A Thin-Film Pressure Transducer for Implantable and Intravascular Blood Pressure Sensing. Sens. Actuators A Phys. 2016, 248, 38–45. [Google Scholar] [CrossRef]
- Li, J.; Zhang, F.; Xia, X.; Zhang, K.; Wu, J.; Liu, Y.; Zhang, C.; Cai, X.; Lu, J.; Xu, L.; et al. An Ultrasensitive Multimodal Intracranial Pressure Biotelemetric System Enabled by Exceptional Point and Iontronics. Nat. Commun. 2024, 15, 9557. [Google Scholar] [CrossRef]
- Lo, Y.-K.; Wang, P.-M.; Dubrovsky, G.; Wu, M.-D.; Chan, M.; Dunn, J.C.Y.; Liu, W. A Wireless Implant for Gastrointestinal Motility Disorders. Micromachines 2018, 9, 17. [Google Scholar] [CrossRef]
- Pons-Faudoa, F.P.; Ballerini, A.; Sakamoto, J.; Grattoni, A. Advanced Implantable Drug Delivery Technologies: Transforming the Clinical Landscape of Therapeutics for Chronic Diseases. Biomed. Microdevices 2019, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Leduc, C.; Ravussin, Y.; Li, S.; Davis, E.; Song, B.; Wang, Q.; Accili, D.; Leibel, R.; Lin, Q. Continuous Monitoring of Glucose in Subcutaneous Tissue Using Microfabricated Differential Affinity Sensors. J. Diabetes Sci. Technol. 2012, 6, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Payne, S.C.; Furness, J.B.; Stebbing, M.J. Bioelectric Neuromodulation for Gastrointestinal Disorders: Effectiveness and Mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 89–105. [Google Scholar] [CrossRef]
- Hatsopoulos, N.G.; Donoghue, J.P. The Science of Neural Interface Systems. Annu. Rev. Neurosci. 2009, 32, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Valeriani, D.; Santoro, F.; Ienca, M. The Present and Future of Neural Interfaces. Front. Neurorobot. 2022, 16, 953968. [Google Scholar] [CrossRef]
- Chen, B.; Lu, J.; Chen, Z.; Han, X.; Sun, Y.; Lin, X.; Tang, Z.; Jia, S.; Xie, G.; Huo, F.; et al. Long-Term Implantable Flexible Neural Interfaces for Electrophysiological Monitoring. J. Mater. Chem. C 2025, 13, 5951–5980. [Google Scholar] [CrossRef]
- Donoghue, J.P. Bridging the Brain to the World: A Perspective on Neural Interface Systems. Neuron 2008, 60, 511–521. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Wang, H.; Zhao, Y.; Zhang, X.-D. Neuron Devices: Emerging Prospects in Neural Interfaces and Recognition. Microsyst. Nanoeng. 2022, 8, 128. [Google Scholar] [CrossRef]
- Cho, Y.U.; Lim, S.L.; Hong, J.-H.; Yu, K.J. Transparent Neural Implantable Devices: A Comprehensive Review of Challenges and Progress. NPJ Flex. Electron. 2022, 6, 53. [Google Scholar] [CrossRef]
- Sifringer, L.; Fratzl, A.; Clément, B.F.; Chansoria, P.; Mönkemöller, L.S.; Duru, J.; Ihle, S.J.; Steffens, S.; Beltraminelli, A.; Ceylan, E.; et al. An Implantable Biohybrid Neural Interface Toward Synaptic Deep Brain Stimulation. Adv. Funct. Mater. 2025, 35, 2416557. [Google Scholar] [CrossRef]
- Madhavan, M.; Mulpuru, S.K.; McLeod, C.J.; Cha, Y.-M.; Friedman, P.A. Advances and Future Directions in Cardiac Pacemakers: Part 2 of a 2-Part Series. J. Am. Coll. Cardiol. 2017, 69, 211–235. [Google Scholar] [CrossRef] [PubMed]
- Harfoush, A. Implantable Loop Recorder Migration: Case-Based Review and Implications for Clinical Practice. Am. Heart J. Plus Cardiol. Res. Pract. 2025, 51, 100505. [Google Scholar] [CrossRef] [PubMed]
- Bisignani, A.; De Bonis, S.; Mancuso, L.; Ceravolo, G.; Bisignani, G. Implantable Loop Recorder in Clinical Practice. J. Arrhythmia 2019, 35, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Chaurasia, R. Secure Implantable Cardiac Pacemaker for Medical Consumer Electronics. NPJ Biomed. Innov. 2025, 2, 5. [Google Scholar] [CrossRef]
- Mulpuru, S.K.; Madhavan, M.; McLeod, C.J.; Cha, Y.-M.; Friedman, P.A. Cardiac Pacemakers: Function, Troubleshooting, and Management: Part 1 of a 2-Part Series. J. Am. Coll. Cardiol. 2017, 69, 189–210. [Google Scholar] [CrossRef]
- Das, D.; Rytkin, E.; Das, J.; Deng, J.; Trampel, K.; Efimov, I.; Kelley, S. Implantable Cardiac Patch Continuously Monitors Acute Heart Failure Biomarkers In Vivo and Ex Vivo. JACC Basic Transl. Sci. 2025, 10, 273–275. [Google Scholar] [CrossRef]
- Huanbutta, K.; Puri, V.; Sharma, A.; Singh, I.; Sriamornsak, P.; Sangnim, T. Rise of Implantable Drugs: A Chronicle of Breakthroughs in Drug Delivery Systems. Saudi Pharm. J. 2024, 32, 102193. [Google Scholar] [CrossRef]
- Meng, E.; Hoang, T. Micro- and Nano-Fabricated Implantable Drug-Delivery Systems. Ther. Deliv. 2012, 3, 1457–1467. [Google Scholar] [CrossRef]
- Kar, A.; Ahamad, N.; Dewani, M.; Awasthi, L.; Patil, R.; Banerjee, R. Wearable and Implantable Devices for Drug Delivery: Applications and Challenges. Biomaterials 2022, 283, 121435. [Google Scholar] [CrossRef]
- Fayzullin, A.; Bakulina, A.; Mikaelyan, K.; Shekhter, A.; Guller, A. Implantable Drug Delivery Systems and Foreign Body Reaction: Traversing the Current Clinical Landscape. Bioengineering 2021, 8, 205. [Google Scholar] [CrossRef]
- He, G.-Q.; Li, H.; Liu, J.; Hu, Y.-L.; Liu, Y.; Wang, Z.L.; Jiang, P. Recent Progress in Implantable Drug Delivery Systems. Adv. Mater. 2024, 36, 2312530. [Google Scholar] [CrossRef]
- Beck, R.W.; Riddlesworth, T.; Ruedy, K.; Ahmann, A.; Bergenstal, R.; Haller, S.; Kollman, C.; Kruger, D.; McGill, J.B.; Polonsky, W.; et al. Effect of Continuous Glucose Monitoring on Glycemic Control in Adults With Type 1 Diabetes Using Insulin Injections: The DIAMOND Randomized Clinical Trial. JAMA 2017, 317, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.B.; Gao, P.; Derdzinski, M.; Puhr, S.; Johnson, T.K.; Walker, T.C.; Graham, C. Accuracy, Utilization, and Effectiveness Comparisons of Different Continuous Glucose Monitoring Systems. Diabetes Technol. Ther. 2019, 21, 128–132. [Google Scholar] [CrossRef] [PubMed]
- LINQ IITM Insertable Cardiac Monitor. Available online: https://www.medtronic.com/en-us/healthcare-professionals/products/cardiac-rhythm/cardiac-monitoring/insertable-cardiac-monitors/linq-ii-icm.html (accessed on 31 July 2025).
- Xu, J.; Lee, H. Anti-Biofouling Strategies for Long-Term Continuous Use of Implantable Biosensors. Chemosensors 2020, 8, 66. [Google Scholar] [CrossRef]
- Arshad, F.; Hassan, I.U.; AlGhamadi, J.M.; Naikoo, G.A. Biofouling-Resistant Nanomaterials for Non-Enzymatic Glucose Sensors: A Critical Review. Mater. Today Bio 2025, 32, 101746. [Google Scholar] [CrossRef]
- Amar, A.B.; Kouki, A.B.; Cao, H. Power Approaches for Implantable Medical Devices. Sensors 2015, 15, 28889–28914. [Google Scholar] [CrossRef]
- Gray, M.E.; Meehan, J.; Blair, E.O.; Ward, C.; Langdon, S.P.; Morrison, L.R.; Marland, J.R.K.; Tsiamis, A.; Kunkler, I.H.; Murray, A.; et al. Biocompatibility of Common Implantable Sensor Materials in a Tumor Xenograft Model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1620–1633. [Google Scholar] [CrossRef]
- Ramsden, J.J. 7—Biocompatibility of Implantable Systems. In Implantable Sensor Systems for Medical Applications; Inmann, A., Hodgins, D., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2013; pp. 215–252. ISBN 978-1-84569-987-1. [Google Scholar]
- Smith, J.J.; Henderson, J.A. FDA Regulation of Implantable Sensors: Demonstrating Safety and Effectiveness for Marketing in the U.S. IEEE Sens. J. 2008, 8, 52–56. [Google Scholar] [CrossRef]
- Ibrahim, S.; Fahmy, H.; Elkhawas, K.; Labeeb, A. Smart Packaging Materials Based Nanoencapsulated Bromothymol as Monitoring Sensors for Spoilage of Chilled Fillet. J. Food Sci. Technol. 2025. [Google Scholar] [CrossRef]
- Madhvapathy, S.R.; Bury, M.I.; Wang, L.W.; Ciatti, J.L.; Avila, R.; Huang, Y.; Sharma, A.K.; Rogers, J.A. Miniaturized Implantable Temperature Sensors for the Long-Term Monitoring of Chronic Intestinal Inflammation. Nat. Biomed. Eng. 2024, 8, 1040–1052. [Google Scholar] [CrossRef]
- Koruprolu, A.; Hack, T.; Ghadami, O.; Jain, A.; Hall, D.A. From Wearables to Implantables: Harnessing Sensor Technologies for Continuous Health Monitoring. IEEE Trans. Biomed. Circuits Syst. 2025, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Ban, S.; Lee, H.; Chen, J.; Kim, H.-S.; Hu, Y.; Cho, S.J.; Yeo, W.-H. Recent Advances in Implantable Sensors and Electronics Using Printable Materials for Advanced Healthcare. Biosens. Bioelectron. 2024, 257, 116302. [Google Scholar] [CrossRef]
- Butt, M.A. Emerging Trends in Thermo-Optic and Electro-Optic Materials for Tunable Photonic Devices. Materials 2025, 18, 2782. [Google Scholar] [CrossRef] [PubMed]
- Khan, P.D.; Khahro, S.H. Advancement in Biomedical Implant Materials—A Mini Review. Front. Bioeng. Biotechnol. 2024, 12, 1400918. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Peng, K.; Li, Z.; Gao, Y.; Tian, Q.; Zhou, Z.; Chen, Y. Recent Progress in Flexible Materials for Wearable Devices for Body Function and Athletic Performance Monitoring. Chem. Eng. J. 2025, 505, 159659. [Google Scholar] [CrossRef]
- Ibarlucea, B.; Díez-Gil, C.; Ratera, I.; Veciana, J.; Caballero, A.; Zapata, F.; Tárraga, A.; Molina, P.; Demming, S.; Büttgenbach, S.; et al. PDMS Based Photonic Lab-on-a-Chip for the Selective Optical Detection of Heavy Metal Ions. Analyst 2013, 138, 839–844. [Google Scholar] [CrossRef]
- Velázquez-Carreón, F.; Pérez-Alonzo, A.; Sandoval-Romero, G.E.; Sánchez-Pérez, C. Enhanced PDMS-Embedded FBG Devices for Displacement Sensing. Opt. Laser Technol. 2024, 179, 111269. [Google Scholar] [CrossRef]
- Lima, R.A. The Impact of Polydimethylsiloxane (PDMS) in Engineering: Recent Advances and Applications. Fluids 2025, 10, 41. [Google Scholar] [CrossRef]
- Lee, D.; Song, J.; Kim, J.; Lee, J.; Son, D.; Shin, M. Soft and Conductive Polyethylene Glycol Hydrogel Electrodes for Electrocardiogram Monitoring. Gels 2023, 9, 957. [Google Scholar] [CrossRef]
- Cai, C.; Fu, J.; Zhang, C.; Wang, C.; Sun, R.; Guo, S.; Zhang, F.; Wang, M.; Liu, Y.; Chen, J. Highly Flexible Reduced Graphene Oxide@polypyrrole–Polyethylene Glycol Foam for Supercapacitors. RSC Adv. 2020, 10, 29090–29099. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, M.; Xiang, C.; Lin, Y.; Guan, Y.; Ren, K.; Ning, C.; Zhou, L.; Lu, L.; Fu, R.; et al. Polyethylene Glycol-Based Conductive Hydrogels with Anti-Freezing, Water Retention and Self-Adhesion for Flexible Sensors. ACS Appl. Polym. Mater. 2024, 6, 11828–11839. [Google Scholar] [CrossRef]
- Kassanos, P.; Hourdakis, E. Implantable Passive Sensors for Biomedical Applications. Sensors 2025, 25, 133. [Google Scholar] [CrossRef] [PubMed]
- Al-Othman, A.; Al-Nashash, H.; Tawalbeh, M.; Elhariri, Y.; Alami, A.H.; Salameh, T. Bio-Electrodes Based on Poly(Methy1 Methacrylate) (PMMA) for Neural Sensing. In Proceedings of the 2020 IEEE 20th International Conference on Nanotechnology (IEEE-NANO), Virtual, 28–31 July 2020; pp. 253–256. [Google Scholar]
- Cesaria, M.; Calcagnile, M.; Arima, V.; Bianco, M.; Alifano, P.; Cataldo, R. Cyclic Olefin Copolymer (COC) as a Promising Biomaterial for Affecting Bacterial Colonization: Investigation on Vibrio campbellii. Int. J. Biol. Macromol. 2024, 271, 132550. [Google Scholar] [CrossRef]
- Agha, A.; Waheed, W.; Alamoodi, N.; Mathew, B.; Alnaimat, F.; Abu-Nada, E.; Abderrahmane, A.; Alazzam, A. A Review of Cyclic Olefin Copolymer Applications in Microfluidics and Microdevices. Macromol. Mater. Eng. 2022, 307, 2200053. [Google Scholar] [CrossRef]
- Sadeghi, F.; Zamani, Y.; Bear, K.L.; Kheradvar, A. Material Characterization and Biocompatibility of Polycarbonate-Based Polyurethane for Biomedical Implant Applications. RSC Adv. 2025, 15, 8839–8850. [Google Scholar] [CrossRef]
- Khonina, S.N.; Kazanskiy, N.L. Trends and Advances in Wearable Plasmonic Sensors Utilizing Surface-Enhanced Raman Spectroscopy (SERS): A Comprehensive Review. Sensors 2025, 25, 1367. [Google Scholar] [CrossRef]
- Goldys, E.M.; Xie, F. Metallic Nanomaterials for Sensitivity Enhancement of Fluorescence Detection. Sensors 2008, 8, 886–896. [Google Scholar] [CrossRef]
- Tang, K.; Chen, X.; Ding, X.; Zhao, X.; Yu, X.; Yu, X.; Chen, X. Humidity Sensitivity Enhancement Effects of Metal Nanoparticles Loaded Fullerene. Sens. Actuators B Chem. 2021, 329, 129086. [Google Scholar] [CrossRef]
- Hemdan, M.; Abuelhaded, K.; Shaker, A.A.S.; Ashour, M.M.; Abdelaziz, M.M.; Dahab, M.I.; Nassar, Y.A.; Sarguos, A.M.M.; Zakaria, P.S.; Fahmy, H.A.; et al. Recent Advances in Nano-Enhanced Biosensors: Innovations in Design, Applications in Healthcare, Environmental Monitoring, and Food Safety, and Emerging Research Challenges. Sens. Bio-Sens. Res. 2025, 48, 100783. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, X.; Li, X.; Gong, P.; Zhang, Y.; Zhao, Y. Recent Advancements of LSPR Fiber-Optic Biosensing: Combination Methods, Structure, and Prospects. Biosensors 2023, 13, 405. [Google Scholar] [CrossRef]
- Meira, D.I.; Barbosa, A.I.; Borges, J.; Reis, R.L.; Correlo, V.M.; Vaz, F. Label-Free Localized Surface Plasmon Resonance (LSPR) Biosensor, Based on Au-Ag NPs Embedded in TiO2 Matrix, for Detection of Ochratoxin-A (OTA) in Wine. Talanta 2025, 284, 127238. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, B.; Angelomé, P.C.; Lechuga, L.M.; Liz-Marzán, L.M. LSPR-Based Nanobiosensors. Nano Today 2009, 4, 244–251. [Google Scholar] [CrossRef]
- Hao, X.; St-Pierre, J.-P.; Zou, S.; Cao, X. Localized Surface Plasmon Resonance Biosensor Chip Surface Modification and Signal Amplifications toward Rapid and Sensitive Detection of COVID-19 Infections. Biosens. Bioelectron. 2023, 236, 115421. [Google Scholar] [CrossRef] [PubMed]
- Sreekanth, S.P.; Alodhayb, A.; Assaifan, A.K.; Alzahrani, K.E.; Muthuramamoorthy, M.; Alkhammash, H.I.; Pandiaraj, S.; Alswieleh, A.M.; Van Le, Q.; Mangaiyarkarasi, R.; et al. Multi-Walled Carbon Nanotube-Based Nanobiosensor for the Detection of Cadmium in Water. Environ. Res. 2021, 197, 111148. [Google Scholar] [CrossRef]
- Lee, B.Y.; Seo, S.M.; Lee, D.J.; Lee, M.; Lee, J.; Cheon, J.-H.; Cho, E.; Lee, H.; Chung, I.-Y.; Park, Y.J.; et al. Biosensor System-on-a-Chip Including CMOS-Based Signal Processing Circuits and 64 Carbon Nanotube-Based Sensors for the Detection of a Neurotransmitter. Lab Chip 2010, 10, 894–898. [Google Scholar] [CrossRef]
- Kanjwal, M.A.; Ghaferi, A.A. Advanced Waveguide Based LOC Biosensors: A Minireview. Sensors 2022, 22, 5443. [Google Scholar] [CrossRef]
- Venkataramana, M.; Kurkuri, M.D. Simple Quantum Dot Bioprobe/Label for Sensitive Detection of Staphylococcus aureus TNase. Sens. Actuators B Chem. 2016, 222, 1201–1208. [Google Scholar] [CrossRef]
- Theyagarajan, K.; Kim, Y.-J. Metal Organic Frameworks Based Wearable and Point-of-Care Electrochemical Sensors for Healthcare Monitoring. Biosensors 2024, 14, 492. [Google Scholar] [CrossRef]
- Li, M.; Zhang, G.; Boakye, A.; Chai, H.; Qu, L.; Zhang, X. Recent Advances in Metal-Organic Framework-Based Electrochemical Biosensing Applications. Front. Bioeng. Biotechnol. 2021, 9, 797067. [Google Scholar] [CrossRef]
- Lakard, B. Electrochemical Biosensors Based on Conducting Polymers: A Review. Appl. Sci. 2020, 10, 6614. [Google Scholar] [CrossRef]
- Aydemir, N.; Malmström, J.; Travas-Sejdic, J. Conducting Polymer Based Electrochemical Biosensors. Phys. Chem. Chem. Phys. 2016, 18, 8264–8277. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Wang, S.; Li, C.; Liu, T.; Zhao, D.; Yin, Y.; Qin, G.; Wang, F.; Zhang, D. Monolithic Integration of Waveguide Amplifiers and Passive Polymer Photonic Devices Using Photolithography. Opt. Express 2024, 32, 38285–38291. [Google Scholar] [CrossRef] [PubMed]
- Urness, A.C.; McLeod, R.R. Liquid Deposition Photolithography for Photonic Device Fabrication. In CLEO: Science and Innovations; Optica Publishing Group: Washington, DC, USA, 2013; p. CTh3J.2. [Google Scholar]
- Kocabas, A.; Aydinli, A. Polymeric Waveguide Bragg Grating Filter Using Soft Lithography. Opt. Express 2006, 14, 10228–10232. [Google Scholar] [CrossRef]
- Moran, I.W.; Cheng, D.F.; Jhaveri, S.B.; Carter, K.R. High-Resolution Soft Lithography of Thin Film Resists Enabling Nanoscopic Pattern Transfer. Soft Matter 2007, 4, 168–176. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Angew. Chem. Int. Ed. 1998, 37, 550–575. [Google Scholar] [CrossRef]
- Ma, Q.; Dong, K.; Li, F.; Jia, Q.; Tian, J.; Yu, M.; Xiong, Y. Additive Manufacturing of Polymer Composite Millimeter-Wave Components: Recent Progress, Novel Applications, and Challenges. Polym. Compos. 2025, 46, 14–37. [Google Scholar] [CrossRef]
- Klestova, A.; Cheplagin, N.; Keller, K.; Slabov, V.; Zaretskaya, G.; Vinogradov, A.V. Inkjet Printing of Optical Waveguides for Single-Mode Operation. Adv. Opt. Mater. 2019, 7, 1801113. [Google Scholar] [CrossRef]
- Mirshafiei, M.; Rashedi, H.; Yazdian, F.; Rahdar, A.; Baino, F. Advancements in Tissue and Organ 3D Bioprinting: Current Techniques, Applications, and Future Perspectives. Mater. Des. 2024, 240, 112853. [Google Scholar] [CrossRef]
- Ravariu, C.; Parvulescu, C.C.; Manea, E.; Tucureanu, V. Optimized Technologies for Cointegration of MOS Transistor and Glucose Oxidase Enzyme on a Si-Wafer. Biosensors 2021, 11, 497. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, Y.; Han, X.; Hu, J.; Qiu, J. Laser-Direct-Lithography of Large-Area 3D Integrated Photonics: Technological Challenges and Advances. Chip 2025, 100157. [Google Scholar] [CrossRef]
- Li, J.; Yan, J.; Jiang, L.; Yu, J.; Guo, H.; Qu, L. Nanoscale Multi-Beam Lithography of Photonic Crystals with Ultrafast Laser. Light Sci. Appl. 2023, 12, 164. [Google Scholar] [CrossRef]
- Al-Amri, A.M. Recent Progress in Printed Photonic Devices: A Brief Review of Materials, Devices, and Applications. Polymers 2023, 15, 3234. [Google Scholar] [CrossRef]
- Kotzar, G.; Freas, M.; Abel, P.; Fleischman, A.; Roy, S.; Zorman, C.; Moran, J.M.; Melzak, J. Evaluation of MEMS Materials of Construction for Implantable Medical Devices. Biomaterials 2002, 23, 2737–2750. [Google Scholar] [CrossRef]
- Baczyński, S.; Sobotka, P.; Marchlewicz, K.; Dybko, A.; Rutkowska, K. Low-Cost, Widespread and Reproducible Mold Fabrication Technique for PDMS-Based Microfluidic Photonic Systems. Photonics Lett. Pol. 2020, 12, 22–24. [Google Scholar] [CrossRef]
- Wessa, T.; Barié, N.; Rapp, M.; Ache, H.J. Polyimide, a New Shielding Layer for Sensor Applications. Sens. Actuators B Chem. 1998, 53, 63–68. [Google Scholar] [CrossRef]
- Khdary, N.H.; Almuarqab, B.T.; El Enany, G. Nanoparticle-Embedded Polymers and Their Applications: A Review. Membranes 2023, 13, 537. [Google Scholar] [CrossRef] [PubMed]
- Ngo, G.L.; Nguyen, L.; Hermier, J.-P.; Lai, N.D. On-Chip 3D Printing of Polymer Waveguide-Coupled Single-Photon Emitter Based on Colloidal Quantum Dots. Polymers 2023, 15, 2201. [Google Scholar] [CrossRef]
- Hosseine, M.; Bakhshi, A.; Naghib, S.M.; Rabiee, N. Recent Advancements in Polyaniline-Based Biosensors for Diagnosis of Cancers: A Comprehensive Review. TrAC Trends Anal. Chem. 2024, 181, 118040. [Google Scholar] [CrossRef]
- Li, K.; Morales-Garza, M.; Cardoso, C.; Moctezuma-Ramirez, A.; Burman, A.; Titus, J.; Elgalad, A.; Perin, E. Early Changes in Acute Myocardial Infarction in Pigs: Achieving Early Detection with Wearable Devices. Diagnostics 2023, 13, 1006. [Google Scholar] [CrossRef]
- Device Approvals and Clearances|FDA. Available online: https://www.fda.gov/medical-devices/products-and-medical-procedures/device-approvals-and-clearances (accessed on 2 July 2025).
- Hassija, V.; Chamola, V.; Bajpai, B.C.; Naren; Zeadally, S. Security Issues in Implantable Medical Devices: Fact or Fiction? Sustain. Cities Soc. 2021, 66, 102552. [Google Scholar] [CrossRef]
- Camara, C.; Peris-Lopez, P.; Tapiador, J.E. Security and Privacy Issues in Implantable Medical Devices: A Comprehensive Survey. J. Biomed. Inform. 2015, 55, 272–289. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fan, S.; Qiao, Z.; Wu, Z.; Lin, B.; Li, Z.; Riegler, M.A.; Wong, M.Y.H.; Opheim, A.; Korostynska, O.; et al. Transforming Healthcare: Intelligent Wearable Sensors Empowered by Smart Materials and Artificial Intelligence. Adv. Mater. 2025, 37, 2500412. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, W.; Yin, H.; Zou, K.; Jiao, Y.; Zhang, Y. One-Dimensional Implantable Sensors for Accurately Monitoring Physiological and Biochemical Signals. Research 2024, 7, 0507. [Google Scholar] [CrossRef]
- Mou, X.; Lennartz, M.R.; Loegering, D.J.; Stenken, J.A. Long-Term Calibration Considerations during Subcutaneous Microdialysis Sampling in Mobile Rats. Biomaterials 2010, 31, 4530–4539. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Qiu, Y.; Wu, H.; Qin, W.; Liao, Y.; Yu, Q.; Cheng, H. Stretchable Piezoelectric Energy Harvesters and Self-Powered Sensors for Wearable and Implantable Devices. Biosens. Bioelectron. 2020, 168, 112569. [Google Scholar] [CrossRef]
- Fletcher, R.R.; Poh, M.-Z.; Eydgahi, H. Wearable Sensors: Opportunities and Challenges for Low-Cost Health Care. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 1763–1766. [Google Scholar]
- Chung, H.U.; Kim, B.H.; Lee, J.Y.; Lee, J.; Xie, Z.; Ibler, E.M.; Lee, K.; Banks, A.; Jeong, J.Y.; Kim, J.; et al. Binodal, Wireless Epidermal Electronic Systems with in-Sensor Analytics for Neonatal Intensive Care. Science 2019, 363, eaau0780. [Google Scholar] [CrossRef]
- Ahmad, R.; Alkhammash, E.H. Online Adaptive Kalman Filtering for Real-Time Anomaly Detection in Wireless Sensor Networks. Sensors 2024, 24, 5046. [Google Scholar] [CrossRef]
- Ma, L.; Yin, L.; Li, X.; Chen, S.; Peng, L.; Liu, G.; Ye, S.; Zhang, W.; Man, S. A Smartphone-Based Visual Biosensor for CRISPR-Cas Powered SARS-CoV-2 Diagnostics. Biosens. Bioelectron. 2022, 195, 113646. [Google Scholar] [CrossRef]
- Wu, C.-T.; Huang, H.-C.; Huang, S.; Chen, I.-M.; Liao, S.-C.; Chen, C.-K.; Lin, C.; Lee, S.-H.; Chen, M.-H.; Tsai, C.-F.; et al. Resting-State EEG Signal for Major Depressive Disorder Detection: A Systematic Validation on a Large and Diverse Dataset. Biosensors 2021, 11, 499. [Google Scholar] [CrossRef]
- Leung, D.; Malpas, S.; McCormick, D.; Budgett, D. In-Situ Re-Calibration of Implanted Pressure Sensors. In Proceedings of the 2017 IEEE SENSORS, Glasgow, UK, 29 October–1 November 2017; pp. 1–3. [Google Scholar]
- Palivela, A. The Challenges in Developing Implantable Biosensors. Scilight 2023, 2023, 391104. [Google Scholar] [CrossRef]
- Zhang, Z.; Azizi, M.; Lee, M.; Davidowsky, P.; Lawrence, P.; Abbaspourrad, A. A Versatile, Cost-Effective, and Flexible Wearable Biosensor for in Situ and Ex Situ Sweat Analysis, and Personalized Nutrition Assessment. Lab Chip 2019, 19, 3448–3460. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Kim, J.-H. Recent Advances in Materials and Manufacturing of Implantable Devices for Continuous Health Monitoring. Biosens. Bioelectron. 2024, 261, 116461. [Google Scholar] [CrossRef] [PubMed]
- Lalouani, W.; Younis, M.; White-Gittens, I.; Emokpae, R.N.; Emokpae, L.E. Energy-Efficient Collection of Wearable Sensor Data through Predictive Sampling. Smart Health 2021, 21, 100208. [Google Scholar] [CrossRef]
- Verma, N.; Pandya, A. Chapter Twelve—Challenges and Opportunities in Micro/Nanofluidic and Lab-on-a-Chip. In Progress in Molecular Biology and Translational Science; Pandya, A., Singh, V., Eds.; Micro/Nanofluidics and Lab-on-Chip Based Emerging Technologies for Biomedical and Translational Research Applications—Part A; Academic Press: Cambridge, MA, USA, 2022; Volume 186, pp. 289–302. [Google Scholar]
- Ben-Yoav, H.; Dykstra, P.H.; Bentley, W.E.; Ghodssi, R. A Microfluidic-Based Electrochemical Biochip for Label-Free Diffusion-Restricted DNA Hybridization Analysis. Biosens. Bioelectron. 2012, 38, 114–120. [Google Scholar] [CrossRef]
- Lechago, S.; García-Meca, C.; Sánchez-Losilla, N.; Griol, A.; Martí, J. High Signal-to-Noise Ratio Ultra-Compact Lab-on-a-Chip Microflow Cytometer Enabled by Silicon Optical Antennas. Opt. Express 2018, 26, 25645–25656. [Google Scholar] [CrossRef]
- Pires, N.M.M.; Dong, T.; Hanke, U.; Hoivik, N. Recent Developments in Optical Detection Technologies in Lab-on-a-Chip Devices for Biosensing Applications. Sensors 2014, 14, 15458–15479. [Google Scholar] [CrossRef]
- Bahavarnia, F.; Hasanzadeh, M.; Sadighbayan, D.; Seidi, F. Recent Progress and Challenges on the Microfluidic Assay of Pathogenic Bacteria Using Biosensor Technology. Biomimetics 2022, 7, 175. [Google Scholar] [CrossRef]
- Mukherjee, M.D.; Gupta, P.; Kumari, V.; Rana, I.; Jindal, D.; Sagar, N.; Singh, J.; Dhand, C. Wearable Biosensors in Modern Healthcare: Emerging Trends and Practical Applications. Talanta Open 2025, 12, 100486. [Google Scholar] [CrossRef]
- Min, J.; Demchyshyn, S.; Sempionatto, J.R.; Song, Y.; Hailegnaw, B.; Xu, C.; Yang, Y.; Solomon, S.; Putz, C.; Lehner, L.E.; et al. An Autonomous Wearable Biosensor Powered by a Perovskite Solar Cell. Nat. Electron. 2023, 6, 630–641. [Google Scholar] [CrossRef]
- Kadian, S.; Kumari, P.; Shukla, S.; Narayan, R. Recent Advancements in Machine Learning Enabled Portable and Wearable Biosensors. Talanta Open 2023, 8, 100267. [Google Scholar] [CrossRef]
- Un, K.-C.; Wong, C.-K.; Lau, Y.-M.; Lee, J.C.-Y.; Tam, F.C.-C.; Lai, W.-H.; Lau, Y.-M.; Chen, H.; Wibowo, S.; Zhang, X.; et al. Observational Study on Wearable Biosensors and Machine Learning-Based Remote Monitoring of COVID-19 Patients. Sci. Rep. 2021, 11, 4388. [Google Scholar] [CrossRef]
- Topsakal, E.; Asili, M.; Chen, P.; Demirci, U. Lab-on-a-Chip: Continuous Glucose Monitoring Antenna Sensors. In Proceedings of the 2014 United States National Committee of URSI National Radio Science Meeting (USNC-URSI NRSM), Boulder, CO, USA, 8–11 January 2014; p. 1. [Google Scholar]
- Jung, D.G.; Jung, D.; Kong, S.H. A Lab-on-a-Chip-Based Non-Invasive Optical Sensor for Measuring Glucose in Saliva. Sensors 2017, 17, 2607. [Google Scholar] [CrossRef]
- Xu, B.; Tian, H.; Li, X.; Hao, Q.; Ma, Y.; Liu, L.; Lei, C.; Chen, Y.; Nie, Z. On-Chip Engineered Living Materials as Field-Deployable Biosensing Laboratories for Multiplexed Detection. Adv. Funct. Mater. 2025, 35, 2416830. [Google Scholar] [CrossRef]
- van den Berg, A.; Mummery, C.L.; Passier, R.; Meer, A.D. van der Personalised Organs-on-Chips: Functional Testing for Precision Medicine. Lab Chip 2019, 19, 198–205. [Google Scholar] [CrossRef]
- Jodat, Y.A.; Kang, M.G.; Kiaee, K.; Kim, G.J.; Martinez, A.F.H.; Rosenkranz, A.; Bae, H.; Shin, S.R. Human-Derived Organ-on-a-Chip for Personalized Drug Development. Curr. Pharm. Des. 2018, 24, 5471–5486. [Google Scholar] [CrossRef]
- Crivillé-Tena, L.; Colomer-Farrarons, J.; Miribel-Català, P.L. Fully Autonomous Active Self-Powered Point-of-Care Devices: The Challenges and Opportunities. Sensors 2023, 23, 9453. [Google Scholar] [CrossRef] [PubMed]
- Özyurt, C.; Uludağ, İ.; İnce, B.; Sezgintürk, M.K. Lab-on-a-Chip Systems for Cancer Biomarker Diagnosis. J. Pharm. Biomed. Anal. 2023, 226, 115266. [Google Scholar] [CrossRef] [PubMed]
- García-Hernández, L.A.; Martínez-Martínez, E.; Pazos-Solís, D.; Aguado-Preciado, J.; Dutt, A.; Chávez-Ramírez, A.U.; Korgel, B.; Sharma, A.; Oza, G. Optical Detection of Cancer Cells Using Lab-on-a-Chip. Biosensors 2023, 13, 439. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Podder, P.S.; Russo, M.; Henry, C.; Cinti, S. Tailored Point-of-Care Biosensors for Liquid Biopsy in the Field of Oncology. Lab Chip 2022, 23, 44–61. [Google Scholar] [CrossRef]
- Campuzano, S.; Barderas, R.; Moreno-Casbas, M.T.; Almeida, Á.; Pingarrón, J.M. Pursuing Precision in Medicine and Nutrition: The Rise of Electrochemical Biosensing at the Molecular Level. Anal. Bioanal. Chem. 2024, 416, 2151–2172. [Google Scholar] [CrossRef]
- Xu, Z.; Hao, Y.; Luo, A.; Jiang, Y. Technologies and Applications in Wireless Biosensors for Real-Time Health Monitoring. Med-X 2024, 2, 24. [Google Scholar] [CrossRef]
- Puspitasari, A.A.; An, T.T.; Alsharif, M.H.; Lee, B.M. Emerging Technologies for 6G Communication Networks: Machine Learning Approaches. Sensors 2023, 23, 7709. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Nordin, A.N.; Li, F.; Voiculescu, I. A Lab-on-Chip Cell-Based Biosensor for Label-Free Sensing of Water Toxicants. Lab Chip 2014, 14, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Pei, X.; Rajendran, J.; Esfandyarpour, R. A Wireless and Battery-Free Wearable Pressure Sensing System for Human–Machine Interaction and Health Monitoring. IEEE J. Flex. Electron. 2023, 2, 439–447. [Google Scholar] [CrossRef]
- El Srouji, L.; Krishnan, A.; Ravichandran, R.; Lee, Y.; On, M.; Xiao, X.; Ben Yoo, S.J. Photonic and Optoelectronic Neuromorphic Computing. APL Photonics 2022, 7, 051101. [Google Scholar] [CrossRef]
- Abhinav, V.; Basu, P.; Verma, S.S.; Verma, J.; Das, A.; Kumari, S.; Yadav, P.R.; Kumar, V. Advancements in Wearable and Implantable BioMEMS Devices: Transforming Healthcare Through Technology. Micromachines 2025, 16, 522. [Google Scholar] [CrossRef]
- Xie, P.; Zhang, Y.; Man, Z.; Zhou, J.; Zhang, Y.; Lu, W.; Dong, H.; Wu, G. Wearable, Recoverable, and Implantable Energy Storage Devices With Heterostructure Porous COF-5/Ti3C2Tx Cathode for High-Performance Aqueous Zn-Ion Hybrid Capacitor. Adv. Funct. Mater. 2025, 35, 2421517. [Google Scholar] [CrossRef]
- Li, C.; Bian, Y.; Zhao, Z.; Liu, Y.; Guo, Y. Advances in Biointegrated Wearable and Implantable Optoelectronic Devices for Cardiac Healthcare. Cyborg Bionic Syst. 2024, 5, 0172. [Google Scholar] [CrossRef]
- Sadri, B.; Gao, W. Fibrous Wearable and Implantable Bioelectronics. Appl. Phys. Rev. 2023, 10, 031303. [Google Scholar] [CrossRef]
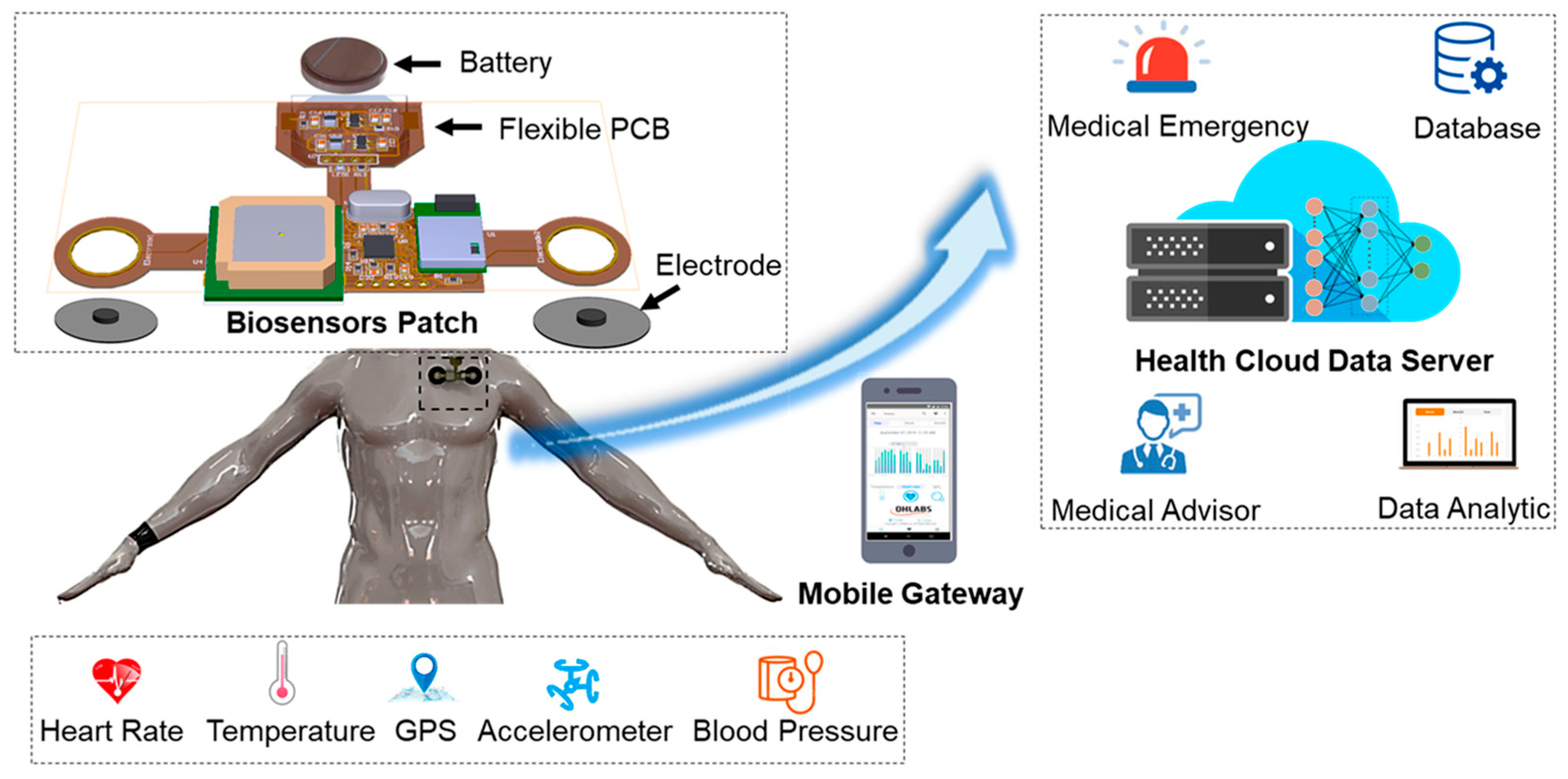





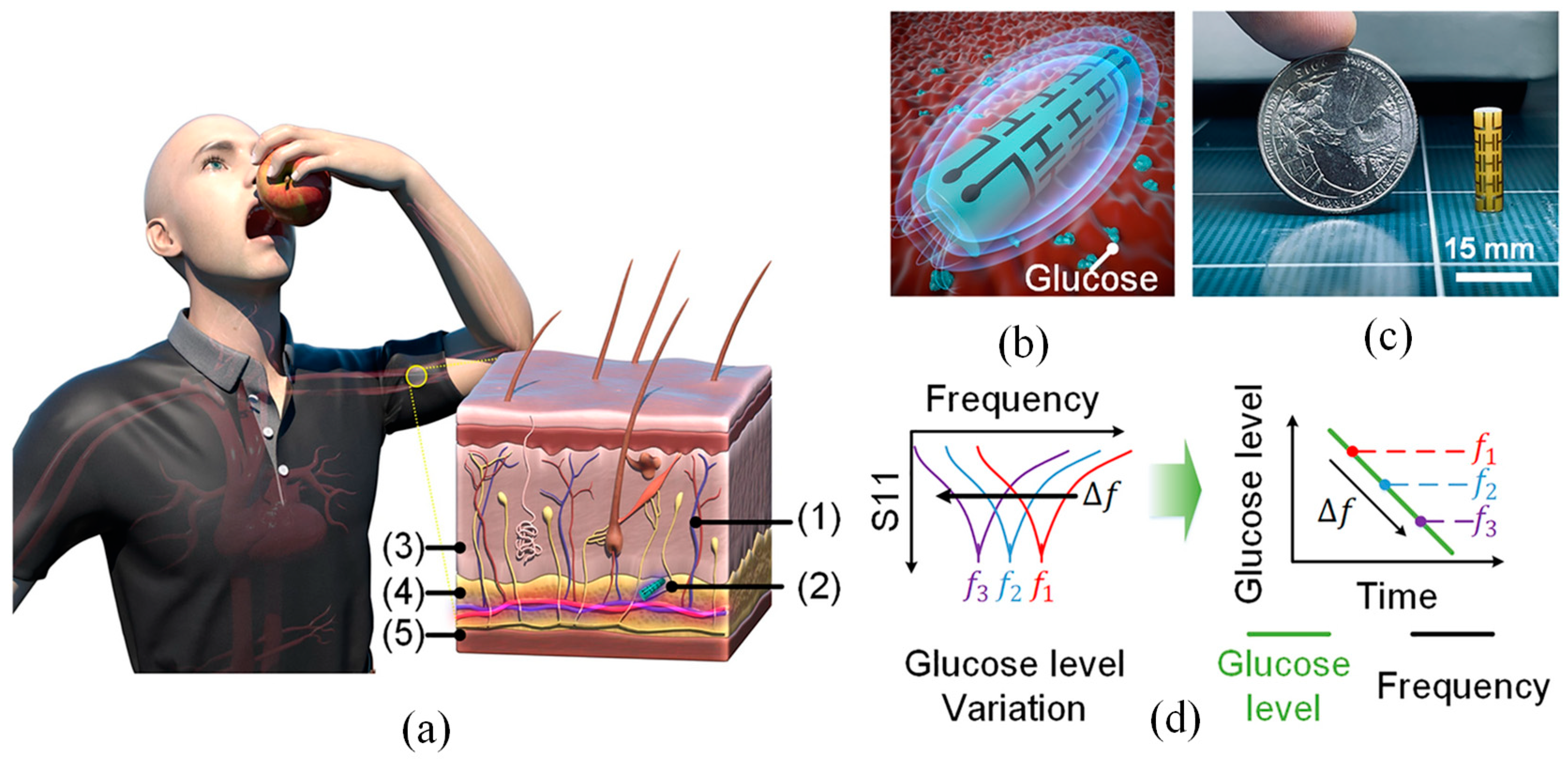

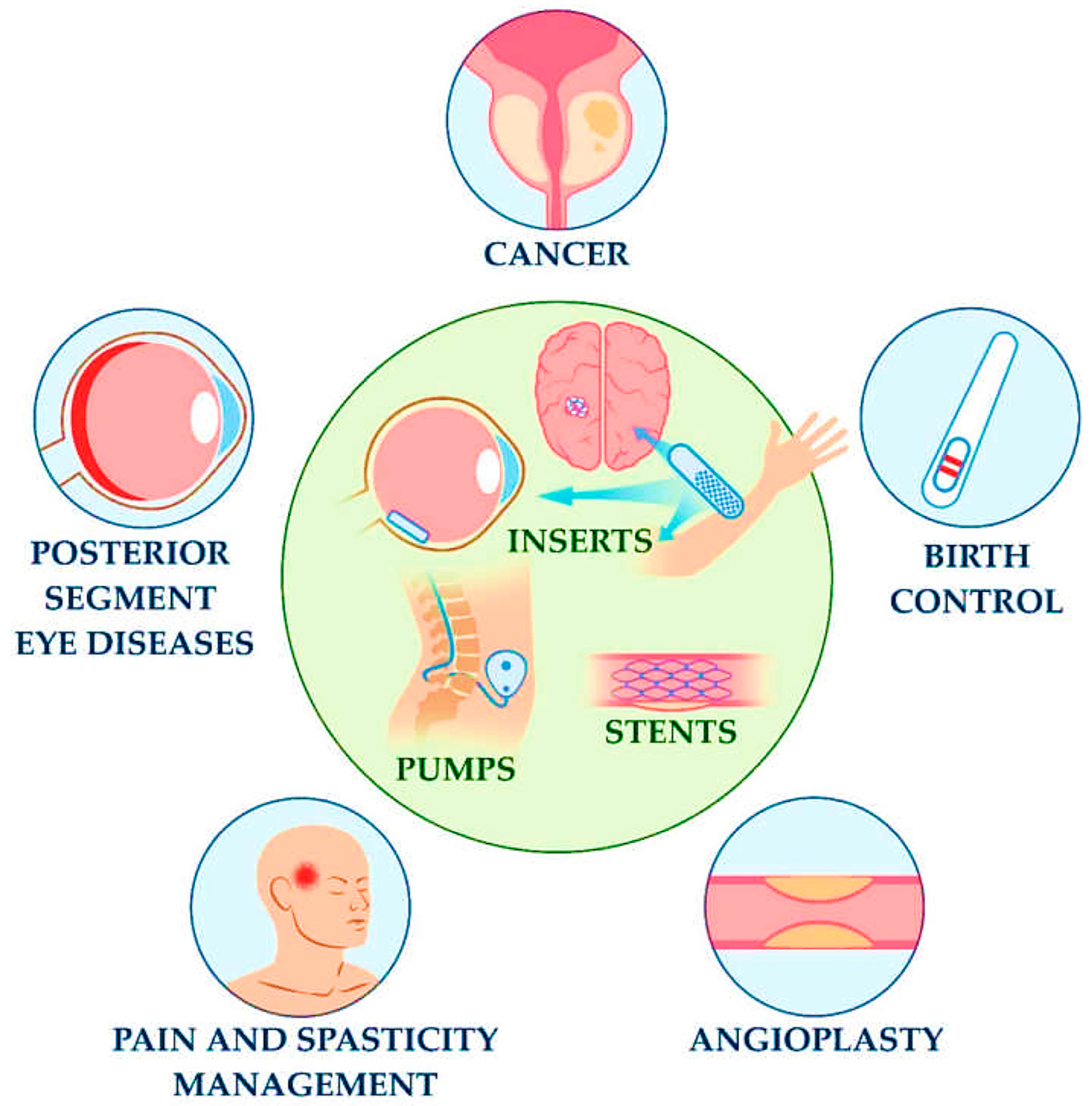
| Characteristic | Wearable Biosensors | Implantable Biosensors |
|---|---|---|
| Anatomical Placement | Non-invasive placement on external body sites such as wrist, chest, or skin surface; designed for continuous or intermittent monitoring [2] | Surgically implanted within the body’s internal environment (e.g., subcutaneous tissue, organs, vascular system) for direct physiological measurement [42] |
| Invasiveness and Biocompatibility | Non-invasive or minimally invasive; typically skin-contact-based with biocompatible adhesive or straps to minimize irritation [3] | Highly invasive; requires biocompatible materials to prevent immune rejection, fibrotic encapsulation, and long-term tissue damage [43] |
| Power Supply and Energy Harvesting | Primarily battery-powered (lithium-ion or polymer), often with recharge capability; emerging technologies include energy harvesting from body heat or motion [37] | Limited by size constraints; use of micro-batteries, wireless inductive power transfer, or biofuel cells; long-term energy sustainability remains a challenge [44] |
| Data Acquisition and Transmission | Employs optical, electrical, or mechanical transduction mechanisms; data transmitted wirelessly via Bluetooth, NFC, or Wi-Fi to external devices for processing and storage [37] | High fidelity signal acquisition close to target tissue; data transmission via wireless telemetry systems or implantable transceivers; latency and security considerations critical [44] |
| Measurement Parameters | Monitors vital signs (heart rate, respiratory rate, temperature), biochemical markers (sweat glucose, lactate), and physical activity metrics [45,46] | Measures biochemical analytes (glucose, ions, neurotransmitters), electrophysiological signals (ECG, EEG), and therapeutic feedback signals (e.g., pacemaker rhythms) [26] |
| Operational Lifetime | Limited by battery capacity, environmental exposure, and adhesive degradation; operational lifespan ranges from days to several weeks depending on use case [47] | Designed for long-term operation (months to years); reliability affected by biofouling, sensor degradation, and immune responses [42] |
| User Compliance and Comfort | High user compliance due to non-invasiveness, lightweight materials, and ergonomic design; potential for skin irritation or discomfort over prolonged use [48] | Lower user compliance due to surgical implantation, risk of pain or discomfort, and potential for complications requiring clinical intervention [49] |
| Safety and Risk Profile | Minimal safety risks primarily related to skin irritation or allergic reactions; data privacy and cybersecurity considerations are pertinent [50,51] | Significant risks include surgical complications, infection, inflammatory response, device migration, and long-term biocompatibility challenges [43] |
| Data Accuracy and Signal Quality | Susceptible to motion artifacts, environmental noise, and inconsistent skin contact leading to signal degradation; advanced signal processing required [52] | High signal-to-noise ratio due to direct tissue contact; reduced artifacts but potential signal drift over time due to biological encapsulation [53] |
| Maintenance and Calibration | User-friendly maintenance; routine calibration often performed via software updates or standardized protocols; sensors generally replaceable [54] | Requires invasive procedures for maintenance or replacement; in situ calibration challenging; efforts focus on self-calibrating and stable sensor design [55] |
| Typical Applications | Fitness and health monitoring, early disease detection, outpatient telemedicine, stress and sleep analysis [56,57] | Chronic disease management (diabetes, cardiac arrhythmias), neuroprosthetics, drug delivery control, and real-time therapeutic feedback [58,59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazanskiy, N.L.; Khorin, P.A.; Khonina, S.N. Biochips on the Move: Emerging Trends in Wearable and Implantable Lab-on-Chip Health Monitors. Electronics 2025, 14, 3224. https://doi.org/10.3390/electronics14163224
Kazanskiy NL, Khorin PA, Khonina SN. Biochips on the Move: Emerging Trends in Wearable and Implantable Lab-on-Chip Health Monitors. Electronics. 2025; 14(16):3224. https://doi.org/10.3390/electronics14163224
Chicago/Turabian StyleKazanskiy, Nikolay L., Pavel A. Khorin, and Svetlana N. Khonina. 2025. "Biochips on the Move: Emerging Trends in Wearable and Implantable Lab-on-Chip Health Monitors" Electronics 14, no. 16: 3224. https://doi.org/10.3390/electronics14163224
APA StyleKazanskiy, N. L., Khorin, P. A., & Khonina, S. N. (2025). Biochips on the Move: Emerging Trends in Wearable and Implantable Lab-on-Chip Health Monitors. Electronics, 14(16), 3224. https://doi.org/10.3390/electronics14163224









