Vapor-Deposited Inorganic Perovskite Solar Cells from Fundamentals to Scalable Commercial Pathways
Abstract
1. Introduction
2. Deposition Techniques and Growth Pathways
2.1. Mechanism of Perovskite Thin-Film Formation
2.2. Significance of Vapor-Phase Deposition
2.3. Co-Evaporation and Sequential Deposition
2.4. Electron Beam and CVD Approaches
2.5. High-Throughput Methods: CSS and CFS
2.6. Pulsed Laser Deposition (PLD)
| Deposition Method | Key Advantages | Limitations | Ref. |
|---|---|---|---|
| Co-evaporation | - Enables high-quality perovskite films. - Precise composition control. - Produces uniform, pinhole-free layers. - Excellent film reproducibility. - Multiple evaporation sources can be co-utilized. - All-dry fabrication process. | - Low deposition rates (typically 1–10 nm/min). - Impractical for high-volume manufacturing. - Requires high-vacuum systems and careful calibration of multiple sources. - Organic salts can thermally decompose during heating. | [24,28,29] |
| Sequential Deposition | - Deposits components in separate steps. - Avoids the flux-balancing difficulties of co-evaporation. - Optimizes processing for each component. - Allows for better control of crystallization. - Different cations or halides can be introduced in successive evaporation steps. | - Incomplete conversion can create sub-optimal interfaces and trap states. - Requires a post-deposition anneal to form the perovskite. - Increases the process time and complexity relative to one-step co-evaporation. - Multiple sequential vacuum steps in a large-scale manufacturing line can be challenging. | [28] |
| Chemical Vapor Deposition (CVD) | - Excellent uniformity and coverage. - Feasible to calibrate the input of each precursor. - Widely used in industry for large-area deposition. - Can be run in continuous mode. - Solvent-free approach. - Feasible to integrate CVD with other processes in a single reactor sequence. | - High temperature 150–300 °C (or higher) requirements. - Equipment complexity and safety. - Batch processing in practice. | [28] |
| Close-Spaced Sublimation (CSS) | - Low vacuum requirement (~1–100 mbar) and high throughput. - Enables rapid deposition over large areas. - High material utilization with minimal waste. - Simplified process control. - Scalability and industrial relevance in device manufacturing. | - Involves a multi-step process. - Equipment and uniformity considerations. - Slight efficiency gap in fully dry CSS. - Limits the use of temperature-sensitive substrates. | [30] |
| Continuous Flash Sublimation (CFS) | - Ultrafast deposition rates, faster than conventional co-evaporation. - Critical for industrial roll-to-roll processing. - Continuous processing capability. - Scalable manufacturing for perovskite films. - The flash process yields homogeneous nucleation and crystal growth. | - Currently limited to inorganic compositions. - Pre-synthesis of source material adds complexity and cost. - Post-annealing (e.g., >300 °C) requirement limits compatible substrates and slightly complicates a continuous process. - Equipment and scale-up challenges. - Powder handling and safety issues. | [24] |
| Pulsed Laser deposition (PLD) | - Deposits a wide range of materials. - Allows for fine control of film thickness, enabling high-quality films. - High deposition rates (~6 to 80 nm/min). - Multiple targets can be used sequentially. - An all-dry, physical vapor process. | - The deposition plume is highly directional. - PLD systems are relatively expensive due to the need for high-power pulsed lasers and high vacuum power. - The high-energy laser can risk damaging or decomposing volatile components. | [31,32] |
3. Composition and Phase Engineering
3.1. CsPbX3-Based Systems
Dopants and Defect Passivation
3.2. Lead-Free Alternatives
3.2.1. CsSnX3-Based Systems

3.2.2. Double Perovskites
4. Device Performance and Functional Integration
5. Interface Engineering and Emerging Applications
5.1. Energy-Level Control
5.2. Patterned Growth and Photonic Integration
6. Scalability and Industrial Pathways
6.1. Process Modeling and Throughput Optimization
6.2. Continuous Processes and Tandem Integration
7. Conclusions and Future Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCE | Power conversion efficiency |
| PSCs | Perovskite solar cells |
| VTE | Vacuum thermal evaporation |
| CSS | Close space sublimation |
| CFS | Continuous flash sublimation |
| CVD | Chemical vapor deposition |
| PeLEDs | Perovskite light-emitting diodes |
| PLQY | Photoluminescence quantum yield |
| DTPT | 4-(dimethylamino)-1-(2,2,2-trifluoroacetyl) pyridin-1-ium 2,2,2-trifluoroacetate |
| SEM | Scanning electron microscopy |
| HTL | Hole transport layer |
| PL | Photoluminescence |
| EQE | External quantum efficiency |
| SEAPVD | Surface energy-assisted patterning and vapor deposition |
| GIXRD | Grazing incidence X-ray diffraction |
| RT | Room temperature |
| N2 | Nitrogen |
| GB | Glove box |
| RH | Relative humidity |
References
- Wu, T.; Qin, Z.; Wang, Y.; Wu, Y.; Chen, W.; Zhang, S.; Cai, M.; Dai, S.; Zhang, J.; Liu, J. The main progress of perovskite solar cells in 2020–2021. Nano-Micro Lett. 2021, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Liu, X.; Liu, Z.; Zhu, Y.; Lu, J.; Chen, Z.; Li, C.; Wang, J.; Xue, Q.; He, F. Annual research review of perovskite solar cells in 2023. Mater. Futures 2024, 3, 022102. [Google Scholar] [CrossRef]
- Chen, P.; Xiao, Y.; Li, S.; Jia, X.; Luo, D.; Zhang, W.; Snaith, H.J.; Gong, Q.; Zhu, R. The promise and challenges of inverted perovskite solar cells. Chem. Rev. 2024, 124, 10623–10700. [Google Scholar] [CrossRef]
- Ullah, S.; Wang, J.; Yang, P.; Liu, L.; Yang, S.-E.; Xia, T.; Guo, H.; Chen, Y. All-inorganic CsPbBr3 perovskite: A promising choice for photovoltaics. Mater. Adv. 2021, 2, 646–683. [Google Scholar] [CrossRef]
- Du, Y.; Tian, Q.; Chang, X.; Fang, J.; Gu, X.; He, X.; Ren, X.; Zhao, K.; Liu, S. Ionic liquid treatment for highest-efficiency ambient printed stable all-inorganic CsPbI3 perovskite solar cells. Adv. Mater. 2022, 34, 2106750. [Google Scholar] [CrossRef]
- Ullah, S.; Khan, F.; AlZahrani, A. Comprehensive assessment of all-inorganic CsPbI3–xBrx perovskite-based solar cells: Interface engineering, stability, and economic aspects. Coord. Chem. Rev. 2024, 516, 215957. [Google Scholar] [CrossRef]
- Fu, S.; Li, X.; Wan, J.; Zhang, W.; Song, W.; Fang, J. In situ stabilized CsPbI3 for air-fabricated inverted inorganic perovskite photovoltaics with wide humidity operating window. Adv. Funct. Mater. 2022, 32, 2111116. [Google Scholar] [CrossRef]
- Hutter, E.M.; Sutton, R.J.; Chandrashekar, S.; Abdi-Jalebi, M.; Stranks, S.D.; Snaith, H.J.; Savenije, T.J. Vapour-deposited cesium lead iodide perovskites: Microsecond charge carrier lifetimes and enhanced photovoltaic performance. ACS Energy Lett. 2017, 2, 1901–1908. [Google Scholar] [CrossRef]
- Chiang, Y.-H.; Frohna, K.; Salway, H.; Abfalterer, A.; Pan, L.; Roose, B.; Anaya, M.; Stranks, S.D. Vacuum-deposited wide-bandgap perovskite for all-perovskite tandem solar cells. ACS Energy Lett. 2023, 8, 2728–2737. [Google Scholar] [CrossRef]
- Bonomi, S.; Malavasi, L. Physical and chemical vapor deposition methods applied to all-inorganic metal halide perovskites. J. Vac. Sci. Technol. A 2020, 38, 060803. [Google Scholar] [CrossRef]
- Ullah, S.; Wang, J.; Yang, P.; Liu, L.; Li, Y.; Rehman, A.-U.; Yang, S.-E.; Xia, T.; Guo, H.; Chen, Y. Evaporation Deposition Strategies for All-Inorganic CsPb(I1–xBrx)3 Perovskite Solar Cells: Recent Advances and Perspectives. Sol. Rrl 2021, 5, 2100172. [Google Scholar] [CrossRef]
- Ihrenberger, J.; Roux, F.; Ledee, F.; Emieux, F.; Anglade, C.; Lemercier, T.; Lorin, G.; Gros-Daillon, E.; Grenet, L. Solution-free growth of CsPbBr3 perovskite films using a fast and scalable close space sublimation method. Cryst. Growth Des. 2024, 24, 5542–5548. [Google Scholar] [CrossRef]
- Petry, J.; Škorjanc, V.; Diercks, A.; Feeney, T.; Morsa, A.; Kimmig, S.R.; Baumann, J.; Löffler, F.; Auschill, S.; Damm, J. Industrialization of perovskite solar cell fabrication: Strategies to achieve high-throughput vapor deposition processes. EES Sol. 2025, 1, 404–418. [Google Scholar] [CrossRef]
- Soto-Montero, T.; Soltanpoor, W.; Morales-Masis, M. Pressing challenges of halide perovskite thin film growth. APL Mater. 2020, 8, 110903. [Google Scholar] [CrossRef]
- Venables, J.; Spiller, G.; Hanbucken, M. Nucleation and growth of thin films. Rep. Prog. Phys. 1984, 47, 399. [Google Scholar] [CrossRef]
- Guesnay, Q.; Sahli, F.; Ballif, C.; Jeangros, Q. Vapor deposition of metal halide perovskite thin films: Process control strategies to shape layer properties. APL Mater. 2021, 9, 100703. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, R.; Kroll, M.; Hofstetter, Y.J.; Jia, X.; Becker-Koch, D.; Paulus, F.; Löffler, M.; Nehm, F.; Leo, K. Efficient thermally evaporated γ-CsPbI3 perovskite solar cells. Adv. Energy Mater. 2021, 11, 2100299. [Google Scholar] [CrossRef]
- Abib, M.H.; Li, J.; Yang, H.; Wang, M.; Chen, T.; Jiang, Y. Direct deposition of Sn-doped CsPbBr3 perovskite for efficient solar cell application. RSC Adv. 2021, 11, 3380–3389. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, D.W.; Noh, Y.W.; Kim, H.S.; Han, J.; Lee, H.; Choi, K.J.; Cho, S.; Song, M.H. Controlled Crystal Growth of All-Inorganic CsPbI2Br via Sequential Vacuum Deposition for Efficient Perovskite Solar Cells. ACS Nano 2024, 18, 17764–17773. [Google Scholar] [CrossRef]
- Gupta, Y.; Heydarian, M.; Heydarian, M.; Er-raji, O.; Günthel, M.; Fischer, O.; Baretzky, C.; Schulze, P.S.; Bivour, M.; De Wolf, S. Photostable Inorganic Perovskite Absorber via Thermal Evaporation for Monolithic Perovskite/Perovskite/Silicon Triple-Junction Solar Cells. Prog. Photovolt. Res. Appl. 2025, 33, 782–794. [Google Scholar] [CrossRef]
- Musálek, T.; Liška, P.; Morsa, A.; Arregi, J.A.; Klok, P.; Kratochvíl, M.; Sergeev, D.; Müller, M.; Šikola, T.; Kolíbal, M. Single-vs dual-source vapor deposition of inorganic halide perovskites: A case study of CsPbBr3. APL Mater. 2025, 13, 031118. [Google Scholar] [CrossRef]
- Liu, L.; Yang, S.-E.; Liu, P.; Chen, Y. High-quality and full-coverage CsPbBr3 thin films via electron beam evaporation with post-annealing treatment for all-inorganic perovskite solar cells. Sol. Energy 2022, 232, 320–327. [Google Scholar] [CrossRef]
- Su, Z.; Cao, Z.; Cao, F.; He, Y.; Zhang, J.; Weng, G.; Hu, X.; Chu, J.; Akiyama, H.; Chen, S. Crystallization mechanism and lasing properties of CsPbBr3 perovskites by chemical vapor deposition. Chem. Eng. J. 2023, 472, 144906. [Google Scholar] [CrossRef]
- Abzieher, T.; Muzzillo, C.P.; Mirzokarimov, M.; Lahti, G.; Kau, W.F.; Kroupa, D.M.; Cira, S.G.; Hillhouse, H.W.; Kirmani, A.R.; Schall, J. Continuous flash sublimation of inorganic halide perovskites: Overcoming rate and continuity limitations of vapor deposition. J. Mater. Chem. A 2024, 12, 8405–8419. [Google Scholar] [CrossRef]
- Zhou, Y.; Yuan, B.; Wei, H.; Xu, F.; Li, Y.; Chen, X.; Cao, B. Stable CsPbX3 mixed halide alloyed epitaxial films prepared by pulsed laser deposition. Appl. Phys. Lett. 2022, 120, 112109. [Google Scholar] [CrossRef]
- Soto-Montero, T.; Kralj, S.; Azmi, R.; Reus, M.A.; Solomon, J.S.; Cunha, D.M.; Soltanpoor, W.; Utomo, D.S.; Ugur, E.; Vishal, B. Single-source pulsed laser-deposited perovskite solar cells with enhanced performance via bulk and 2D passivation. Joule 2024, 8, 3412–3425. [Google Scholar] [CrossRef]
- Kralj, S.; Artuk, K.; Wieczorek, A.; Orlov, N.; Eftekhari, Z.; Saive, R.; Garnett, E.; Siol, S.; Wolff, C.M.; Morales-Masis, M. Template-assisted growth of CsxFA1-xPbI3 with pulsed laser deposition for single junction perovskite solar cells. Adv. Energy Mater. 2025, 15, 2406033. [Google Scholar] [CrossRef]
- Hwang, J.-K.; Jeong, S.-H.; Kim, D.; Lee, H.-S.; Kang, Y. A review on dry deposition techniques: Pathways to enhanced perovskite solar cells. Energies 2023, 16, 5977. [Google Scholar] [CrossRef]
- Abzieher, T.; Moore, D.T.; Roß, M.; Albrecht, S.; Silvia, J.; Tan, H.; Jeangros, Q.; Ballif, C.; Hoerantner, M.T.; Kim, B.-S. Vapor phase deposition of perovskite photovoltaics: Short track to commercialization? Energy Environ. Sci. 2024, 17, 1645–1663. [Google Scholar] [CrossRef]
- Rodkey, N.; Gomar-Fernández, I.; Ventosinos, F.; Roldan-Carmona, C.; Koster, L.J.A.; Bolink, H.J. Close-space sublimation as a scalable method for perovskite solar cells. ACS Energy Lett. 2024, 9, 927–933. [Google Scholar] [CrossRef]
- Lu, X.; Fan, X.; Zhang, H.; Xu, Q.; Ijaz, M. Review on preparation of perovskite solar cells by pulsed laser deposition. Inorganics 2024, 12, 128. [Google Scholar] [CrossRef]
- Kliner, V.; Soto-Montero, T.; Nespoli, J.; Savenije, T.J.; Ledinskyý, M.; Morales-Masis, M. Pulsed Laser Deposition of Halide Perovskites with over 10-Fold Enhanced Deposition Rates. J. Phys. Chem. Lett. 2025, 16, 1453–1460. [Google Scholar] [CrossRef]
- Huang, Q.; Li, F.; Wang, M.; Xiang, Y.; Ding, L.; Liu, M. Vapor-deposited CsPbI3 solar cells demonstrate an efficiency of 16. Sci. Bull. 2021, 66, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhao, G.; Liu, X.; Ma, J.; Chen, S.; Song, Y.; Pi, X.; Yu, X.; Yang, D.; Zhang, Y. Highly efficient and stable inorganic CsPbBr3 perovskite solar cells via vacuum co-evaporation. Appl. Surf. Sci. 2021, 562, 150153. [Google Scholar] [CrossRef]
- Liao, Y.; Tian, N.; Wang, J.; Yao, D.; Zheng, G.; Zhou, B.; Yang, Y.; Long, F. Performance enhancement of evaporated CsPbI2Br perovskite solar cells with a CuSCN hole transport layer via a cesium bromide buffer layer. ACS Appl. Energy Mater. 2022, 5, 9542–9548. [Google Scholar] [CrossRef]
- Guo, R.; Xia, J.; Gu, H.; Chu, X.; Zhao, Y.; Meng, X.; Wu, Z.; Li, J.; Duan, Y.; Li, Z. Effective defect passivation with a designer ionic molecule for high-efficiency vapour-deposited inorganic phase-pure CsPbBr 3 perovskite solar cells. J. Mater. Chem. A 2023, 11, 408–418. [Google Scholar] [CrossRef]
- Sosa Acosta, A.; Angel, F.A. Thermally Evaporated CsPbBr3 for Green Perovskite Light-Emitting Diodes: Challenges and Perspectives. ACS Appl. Electron. Mater. 2025, 7, 1361–1376. [Google Scholar] [CrossRef]
- Sebastia-Luna, P.; Pokharel, U.; Huisman, B.A.; Koster, L.J.A.; Palazon, F.; Bolink, H.J. Vacuum-deposited cesium tin iodide thin films with tunable thermoelectric properties. ACS Appl. Energy Mater. 2022, 5, 10216–10223. [Google Scholar] [CrossRef]
- Reo, Y.; Zou, T.; Choi, T.; Kim, S.; Go, J.-Y.; Roh, T.; Ryu, H.; Kim, Y.-S.; Liu, A.; Zhu, H. Vapour-deposited high-performance tin perovskite transistors. Nat. Electron. 2025, 8, 403–410. [Google Scholar] [CrossRef]
- Bonomi, S.; Patrini, M.; Bongiovanni, G.; Malavasi, L. Versatile vapor phase deposition approach to cesium tin bromide materials CsSnBr3, CsSn2Br5 and Cs2SnBr6. RSC Adv. 2020, 10, 28478–28482. [Google Scholar] [CrossRef]
- Jung, H.; Liu, Z.; Sotome, M.; Kondo, T. Vapor phase deposition of lead-free halide perovskite alloy CsSn1−xZnxBr3. Jpn. J. Appl. Phys. 2023, 63, 01SP24. [Google Scholar] [CrossRef]
- Yin, Y.; Shi, X.; Wang, C.; Zhang, X.; Zhou, D.; Li, S.; Liao, K.; Yuan, J.; Zhan, G.; He, J. Space-Confined Chemical Vapor Deposition Synthesis of All-Inorganic CsSnI3 Perovskite Nanosheets. J. Phys. Chem. C 2024, 128, 8324–8330. [Google Scholar] [CrossRef]
- Li, H.; Ye, C.; Jin, Y.; Wu, L.; Ma, Y.; Dong, H.; Ono, L.K.; Qi, Y.; Donaev, S.B.; Jiang, L. Suppressed Defects and Improved Stability of All-Inorganic CsSnI3 Films by Solid Additive-Assisted Chemical Vapor Deposition Process. Small 2025, 21, 2412824. [Google Scholar] [CrossRef] [PubMed]
- Horani, F.; Gamelin, D.R. Cs2AgSbI6 Nanocrystals: A New Air-Stable Iodide Double-Perovskite (Elpasolite) Semiconductor. J. Am. Chem. Soc. 2025, 147, 16552–16559. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Tran, M.N.; Cleveland, I.J.; Liu, Y.; Sarp, S.; Aydil, E.S. Vapor Deposition and Optical Properties of Cs2AgBiCl6 Thin Films. J. Phys. Chem. C 2025, 129, 5301–5311. [Google Scholar] [CrossRef]
- Pintor Monroy, M.I.; Goldberg, I.; Elkhouly, K.; Georgitzikis, E.; Clinckemalie, L.; Croes, G.; Annavarapu, N.; Qiu, W.; Debroye, E.; Kuang, Y. All-evaporated, all-inorganic CsPbI3 Perovskite-based devices for broad-band photodetector and solar cell applications. ACS Appl. Electron. Mater. 2021, 3, 3023–3033. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Shen, C.; Chen, M.; He, B.; Su, Z.; Cao, L.; Gao, X. Distinct rubrene/CsPbI2Br interfacial energetics on spin-coated and vacuum evaporated perovskite films. Appl. Surf. Sci. 2025, 163663. [Google Scholar] [CrossRef]
- Xu, Z.; Pan, X.; Lu, H.; Lu, Q.; Liang, Y.; He, Z.; Zhu, Y.; Yu, Y.; Wu, W.; Han, X. Surface Energy-Assisted Patterning of Vapor Deposited All-Inorganic Perovskite Arrays for Wearable Optoelectronics. Adv. Sci. 2024, 11, 2402635. [Google Scholar] [CrossRef]
- Zhong, Y.; Liao, K.; Du, W.; Zhu, J.; Shang, Q.; Zhou, F.; Wu, X.; Sui, X.; Shi, J.; Yue, S. Large-scale thin CsPbBr3 single-crystal film grown on sapphire via chemical vapor deposition: Toward laser array application. ACS Nano 2020, 14, 15605–15615. [Google Scholar] [CrossRef]
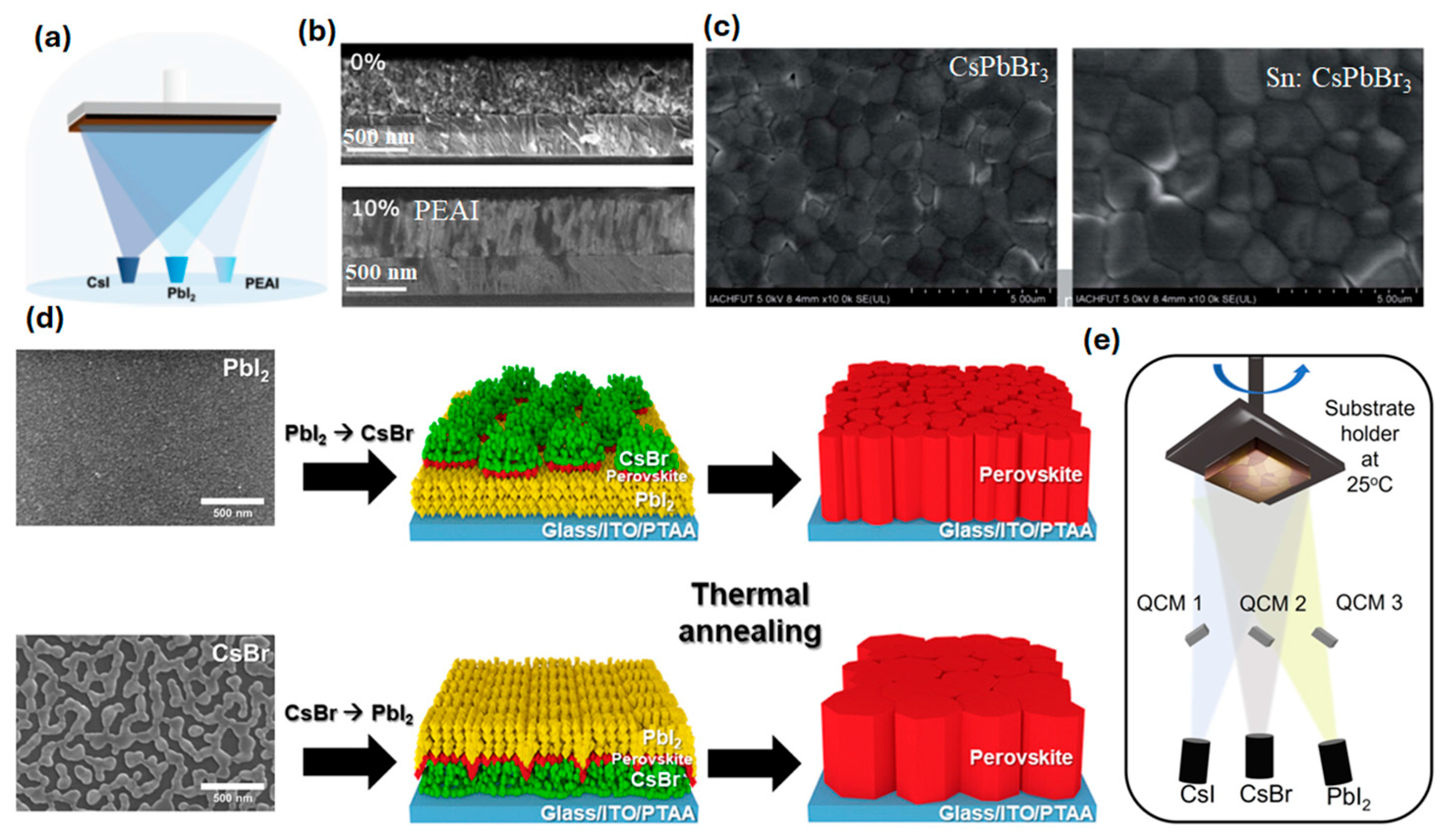

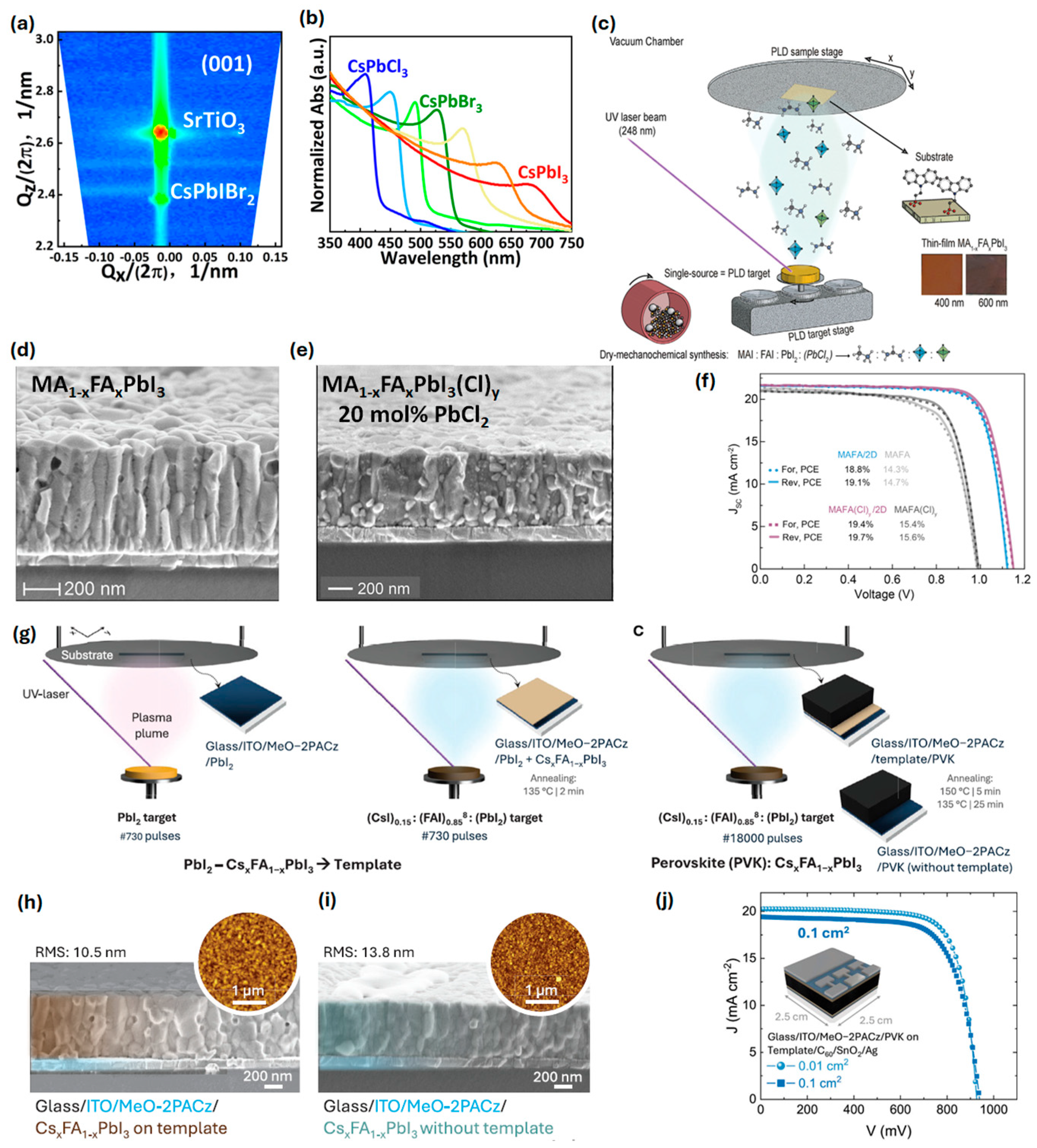


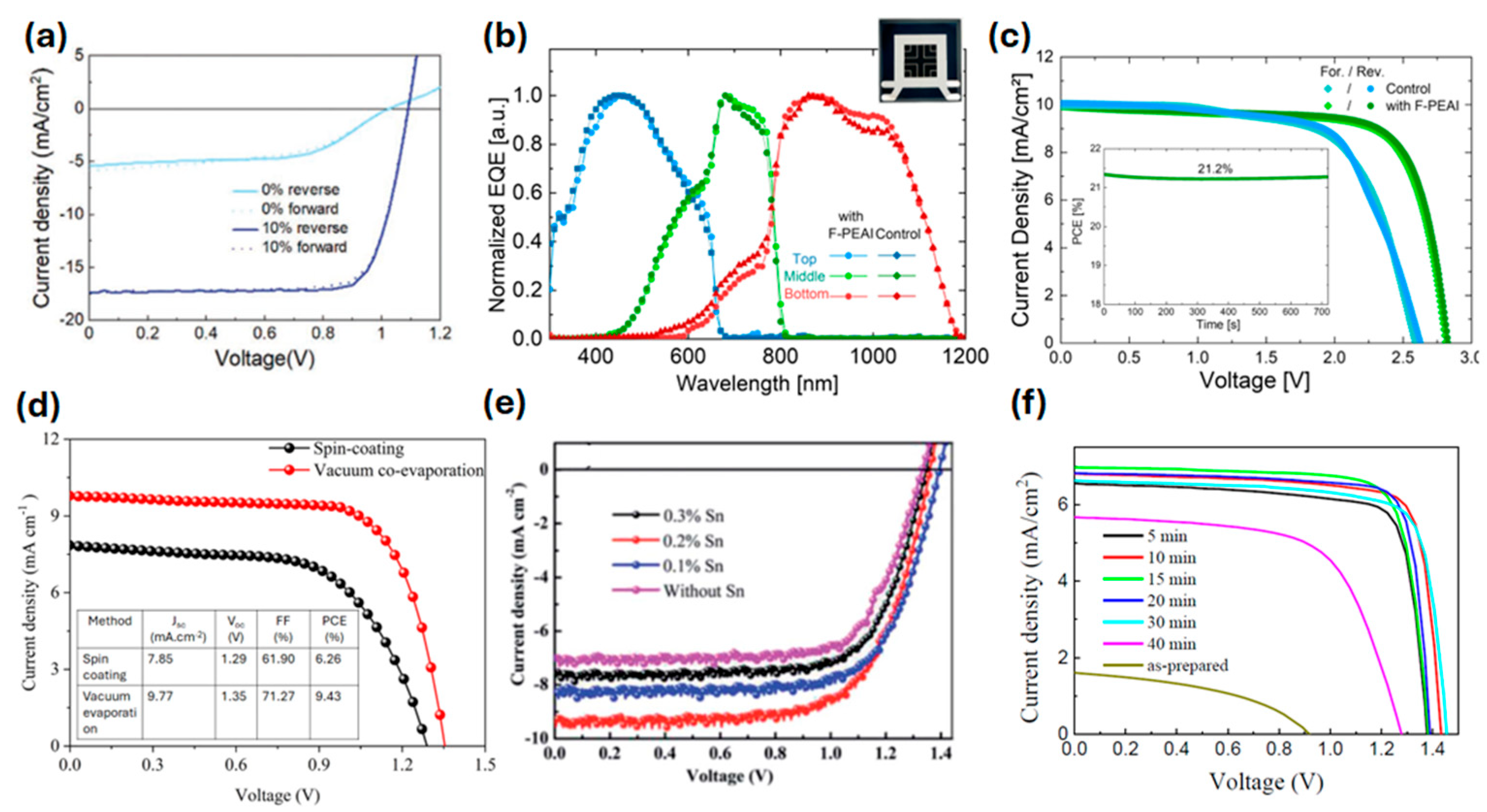

| Composition | Deposition Method | PCE (%) | Area | Jsc mA cm−2 | Voc (V) | FF | Stability | Ref. |
|---|---|---|---|---|---|---|---|---|
| γ-CsPbI3 + PEAI | Co-evaporation | 15.0 | 4.5 mm2 | 17.3 | 1.09 | 79.4 | 215 days Encapsulated (RT) | [17] |
| CsPbI2Br | Sequential evaporation | 13.41 | ~0.1 cm2 | 14.1 | 1.20 | 79.1 | 600 h Without encapsulation (under N2 GB, 85 °C) | [19] |
| CsPbI2Br single-junction solar cell | Sequential evaporation | 7.7 | 1 cm2 | 10.6 | 1.09 | 66.3 | 100 h Without encapsulation (RT), 35–40% RH | [20] |
| perovskite/perovskite/silicon triple-junction tandem solar cell | 21 | 10 | 2.83 | 74 | ||||
| CsPbBr3 | Co-evaporation | 9.43 | 0.04 cm2 | 9.77 | 1.35 | 71.2 | 480 h Without encapsulation (RT), 40% RH | [34] |
| CsPbBr3 + DTPT | Co-evaporation | 11.21 | 0.04 cm2 | 8.52 | 1.57 | 83.6 | >100 days | [36] |
| 9.18 | 1 cm2 | 7.81 | 1.50 | 77.8 | Without encapsulation (RT), 55% RH | |||
| Sn-CsPbBr3 | Direct evaporation | 8.95 | 0.09 cm2 | 9.27 | 1.36 | 71.0 | 720 h Open-air environment | [18] |
| CsPbBr3 | E-beam | 7.81 | 0.06 cm2 | 6.81 | 1.43 | 79.9 | Ambient stable | [22] |
| α-CsxFA1-xPbI3 | Pulsed laser deposition | 14.0 | 0.01 cm2 | 20.27 | 0.92 | 74.88 | Unencapsulated (stored under N2) Measured/30–40% RH, 22 °C RT | [27] |
| 12.91 | 0.1 cm2 | 19.43 | 0.93 | 70.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, P.; Kang, D.-W. Vapor-Deposited Inorganic Perovskite Solar Cells from Fundamentals to Scalable Commercial Pathways. Electronics 2025, 14, 3171. https://doi.org/10.3390/electronics14163171
Pandey P, Kang D-W. Vapor-Deposited Inorganic Perovskite Solar Cells from Fundamentals to Scalable Commercial Pathways. Electronics. 2025; 14(16):3171. https://doi.org/10.3390/electronics14163171
Chicago/Turabian StylePandey, Padmini, and Dong-Won Kang. 2025. "Vapor-Deposited Inorganic Perovskite Solar Cells from Fundamentals to Scalable Commercial Pathways" Electronics 14, no. 16: 3171. https://doi.org/10.3390/electronics14163171
APA StylePandey, P., & Kang, D.-W. (2025). Vapor-Deposited Inorganic Perovskite Solar Cells from Fundamentals to Scalable Commercial Pathways. Electronics, 14(16), 3171. https://doi.org/10.3390/electronics14163171






