Usability of Inexpensive Optical Pulse Sensors for Textile Integration and Heartbeat Detection Code Development
Abstract
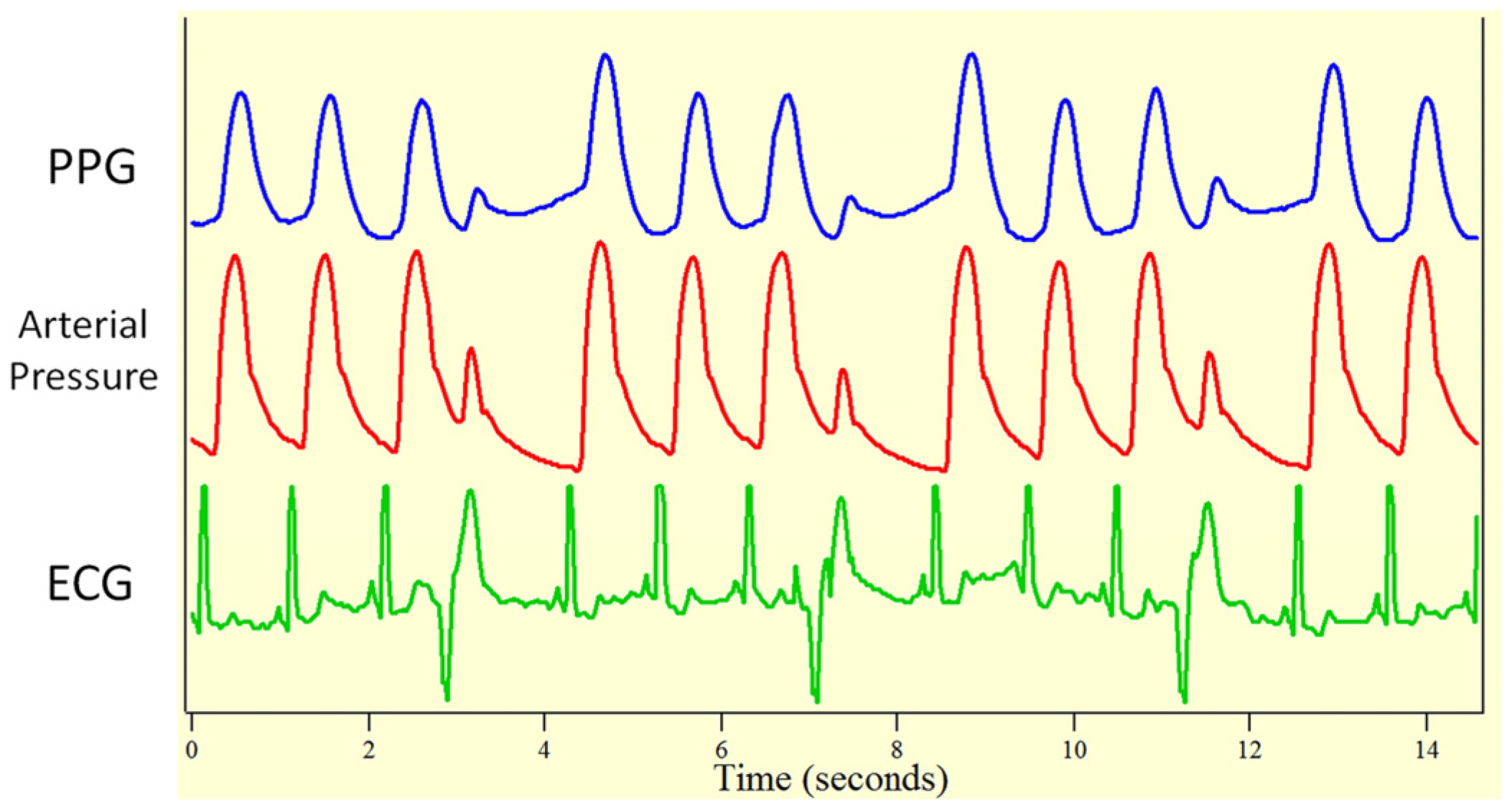
1. Introduction
2. Materials and Methods
3. Results
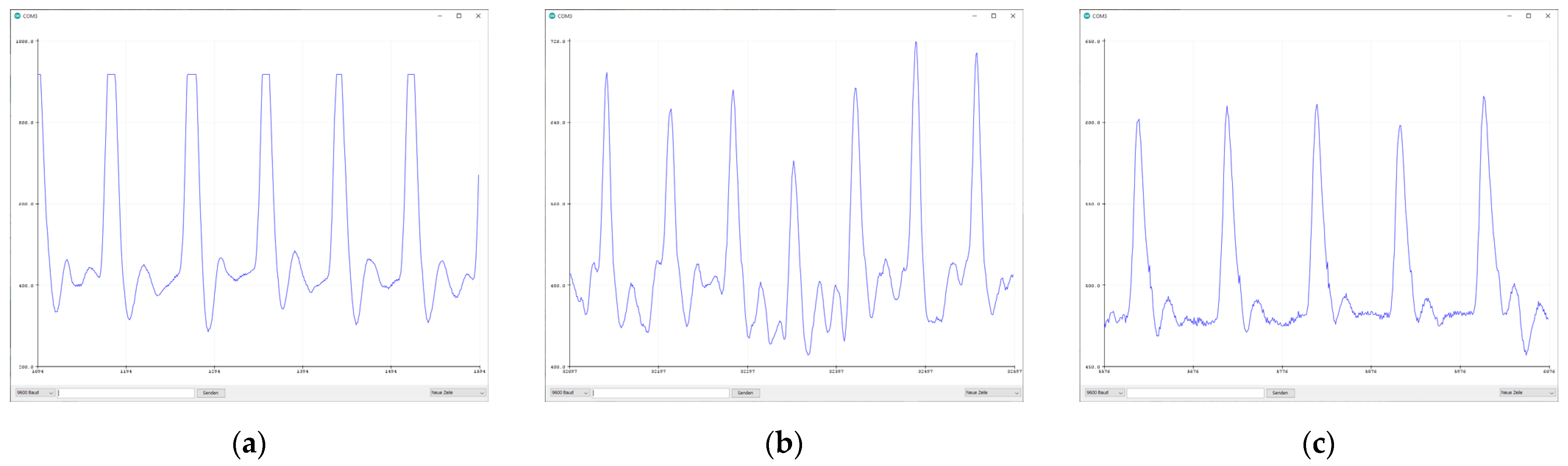
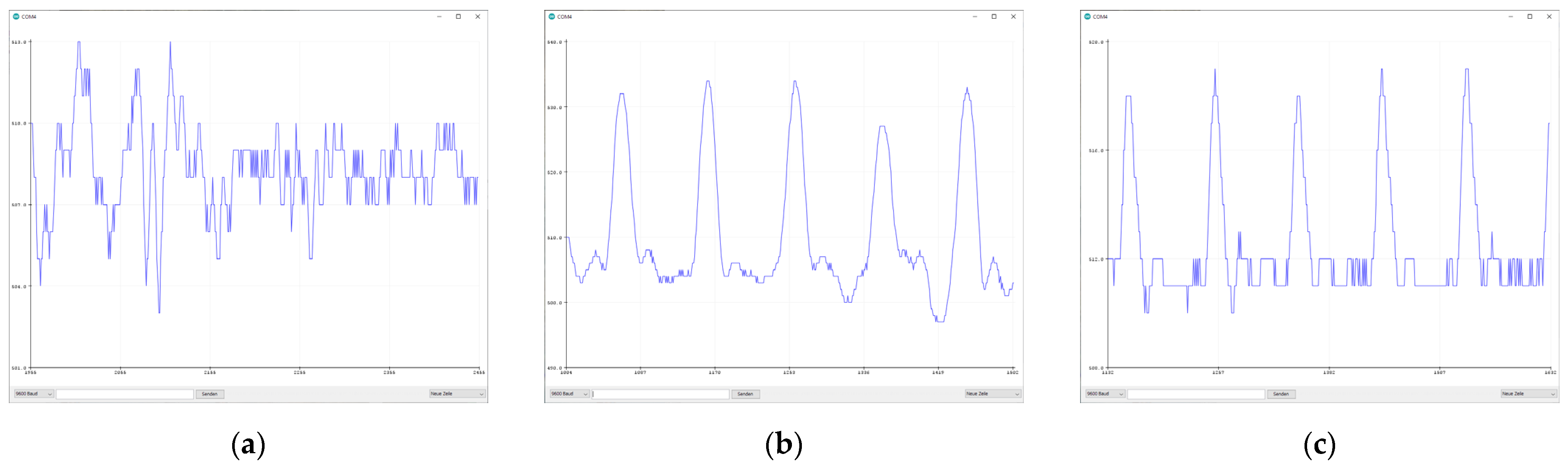
3.1. Sensor Comparison
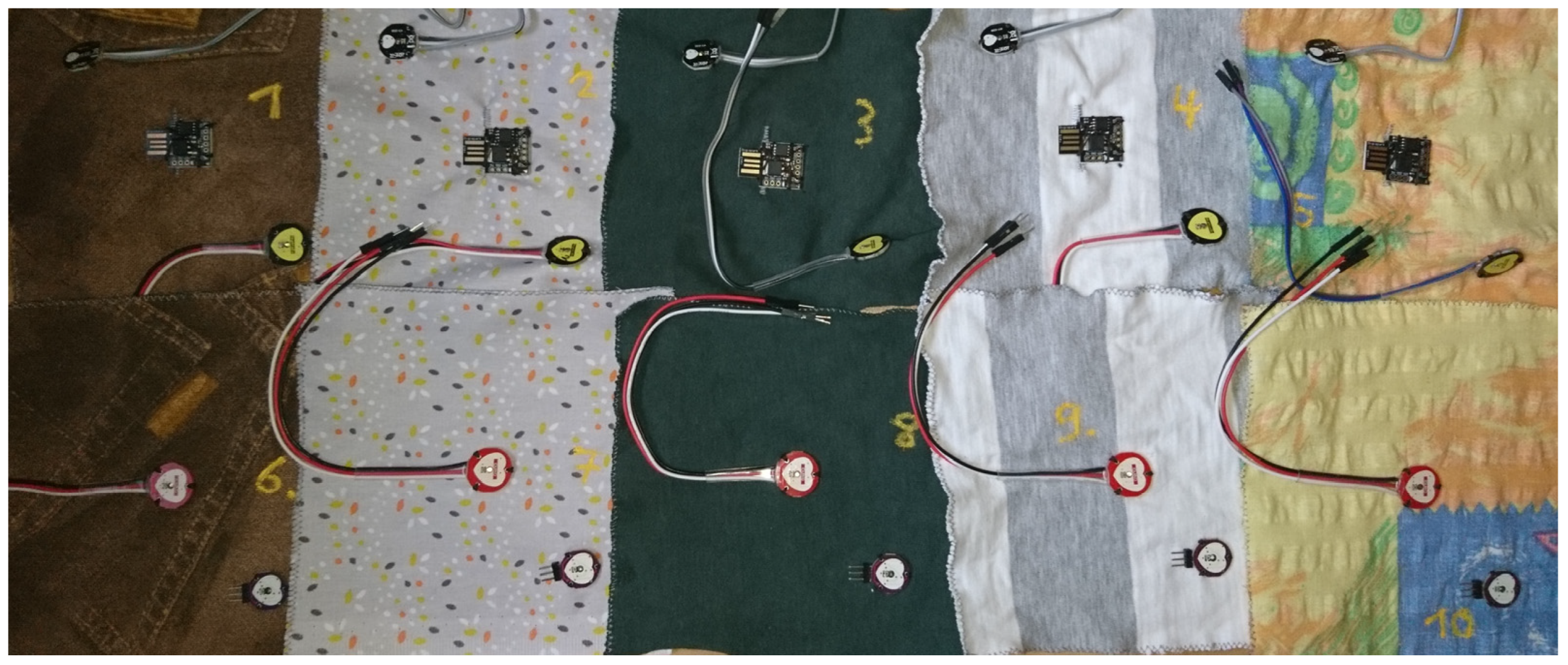
3.2. Washing Tests
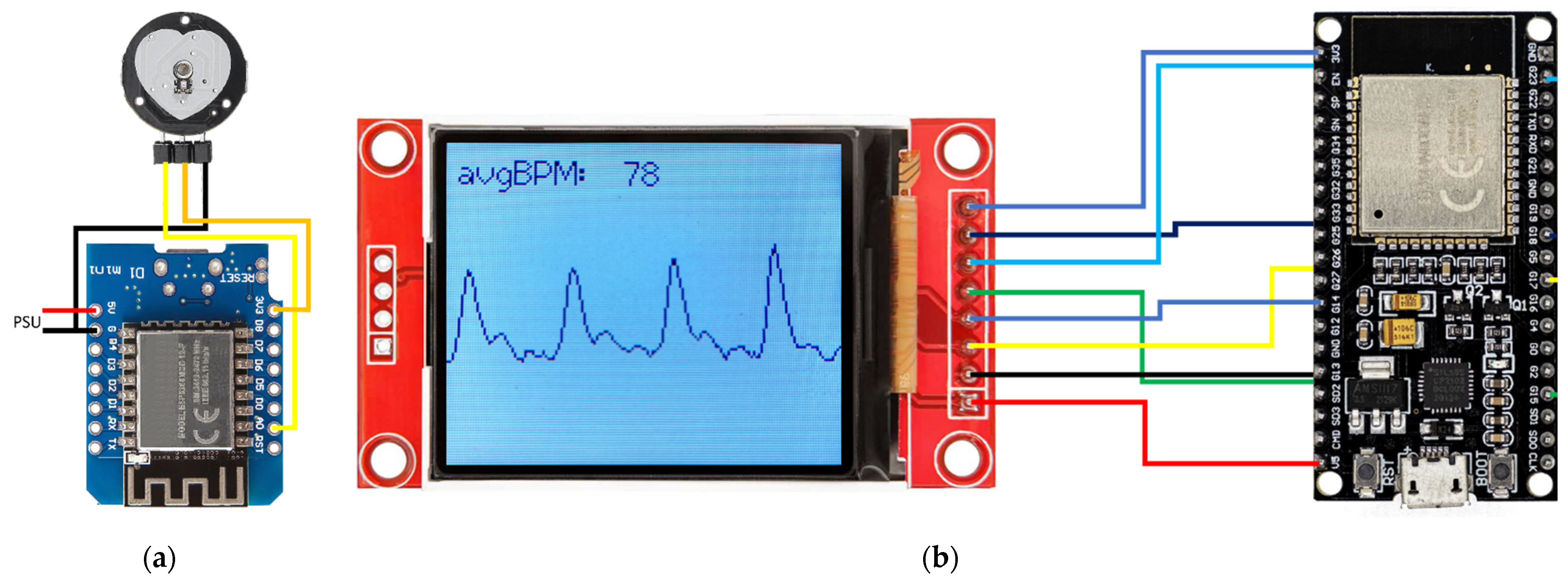
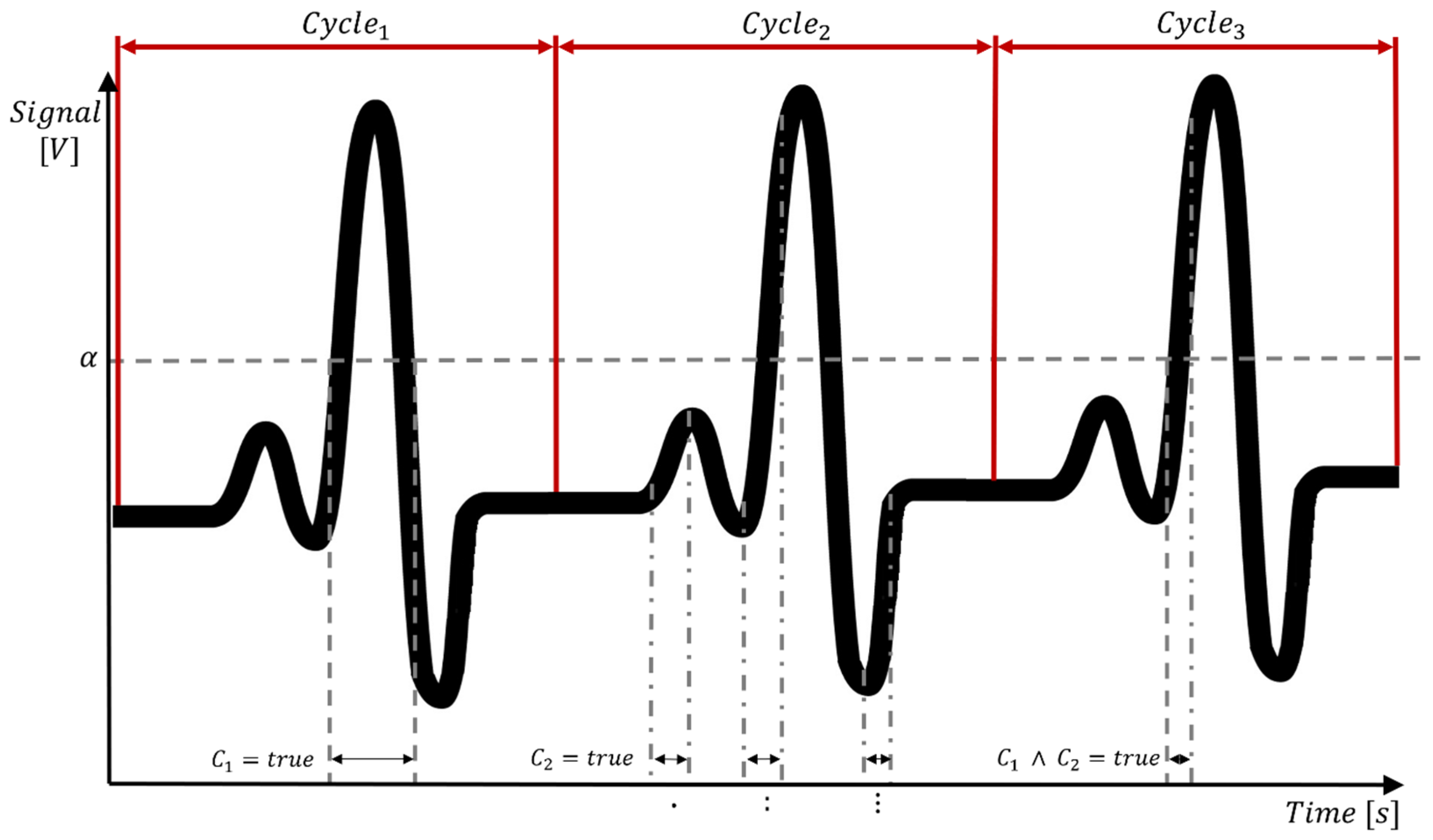
3.3. Hard- and Software Development
4. Future Developments
- Put the sensor it into an energy saving mode;
- Wake up the receiver unit wirelessly;
- Acquire data over a certain period of time;
- Send data to the receiver unit;
- Return to the energy saving mode.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Code for the Sensor Unit


Appendix B. Code for the Receiver Unit


References
- Ritchie, H.; Roser, M. Causes of Death. Our World in Data. 2018. Available online: https://ourworldindata.org/causes-of-death (accessed on 7 January 2022).
- Ahmad, F.B.; Anderson, R.N. The leading causes of death in the US for 2020. JAMA 2021, 325, 1829–1830. [Google Scholar] [CrossRef] [PubMed]
- Aumann, S.; Trummer, S.; Brücken, A.; Ehrmann, A.; Büsgen, A. Conceptual design of a sensory shirt for fire-fighters. Text. Res. J. 2014, 84, 1661–1665. [Google Scholar] [CrossRef]
- Trummer, S.; Ehrmann, A.; Büsgen, A. Development of underwear with integrated 12 channel ECG for men and women. AUTEX Res. J. 2017, 17, 344–349. [Google Scholar] [CrossRef]
- An, X.; Stylios, G.K. A hybrid textile electrode for electrocardiogram (ECG) measurement and motion tracking. Materials 2018, 11, 1887. [Google Scholar] [CrossRef] [PubMed]
- Matsouka, D.; Vassiliadis, S.; Tao, X.; Koncar, V.; Bahadir, S.K.; Kalaoglu, F.; Jevsnik, S. Electrical connection issues on wearable electronics. IOP Conf. Ser. Mater. Sci. Eng. 2018, 459, 012017. [Google Scholar] [CrossRef]
- Acar, G.; Ozturk, O.; Golparvar, A.J.; Elboshra, T.A.; Böhringer, K.; Yapici, M.K. Wearable and Flexible Textile Electrodes for Biopotential Signal Monitoring: A review. Electronics 2019, 8, 479. [Google Scholar] [CrossRef]
- Nigusse, A.B.; Malengier, B.; Mengistie, D.A.; Tseghai, G.B.; van Langenhove, L. Development of Washable Silver Printed Textile Electrodes for Long-Term ECG Monitoring. Sensors 2020, 20, 6233. [Google Scholar] [CrossRef]
- Euler, L.; Guo, L.; Persson, N.-K. Textile Electrodes: Influence of Knitting Construction and Pressure on the Contact Impedance. Sensors 2021, 21, 1578. [Google Scholar] [CrossRef]
- Uz Zaman, S.; Tao, X.Y.; Cochrane, C.; Koncar, V. Smart E-Textile Systems: A Review for Healthcare Applications. Electronics 2022, 11, 99. [Google Scholar] [CrossRef]
- Wang, J.Y.; Liu, K.W.; Sun, Q.Z.; Ni, X.L.; Ai, F.; Wang, S.M.; Yan, Z.J.; Liu, D.M. Diaphragm-based optical fiber sensor for pulse wave monitoring and cardiovascular diseases diagnosis. J. Biophotonics 2019, 12, e201900084. [Google Scholar] [CrossRef]
- Tanima; Saini, I.; Saini, B.S. Physiological Characteristics Classification by Optical Pulse Sensor using Arterial Pulse Waves. In Proceedings of the 2020 2nd International Conference on Innovative Mechanisms for Industry Applications (ICIMIA), Bangalore, India, 5–7 March 2020; pp. 676–679. [Google Scholar]
- Singh, M.; Li, J.K.; Sigel, G.H.; Amory, D. Fiber optic pulse sensor for noninvasive cardiovascular applications. In Proceedings of the Sixteenth Annual Northeast Conference on Bioengineering, State College, PA, USA, 26–27 March 1990; pp. 103–104. [Google Scholar]
- Henning, M.R.; Gerdt, D.W.; Spraggins, T.A. Using a fiber-optic pulse sensor in magnetic resonance imaging. Proc. SPIE 1991, 1420, 34–40. [Google Scholar] [CrossRef]
- Alian, A.A.; Sheeley, K.H. Photoplethysmography. Best Pract. Res. Clin. Anaesthesiol. 2014, 28, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Mark, J.B. Atlas of Cardiovascular Monitoring; Churchill Livingstone Inc.: New York, NY, USA, 1998. [Google Scholar]
- Milner, Q.J.W.; Mathews, G.R. An assessment of the accuracy of pulse oximeters. Anaesthesia 2012, 67, 396–401. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, N.Y.; Kwon, M.J.; Lee, J.H. Attachable Pulse Sensors Integrated with Inorganic Optoelectronic Devices for Monitoring Heart Rates at Various Body Locations. ACS Appl. Mater. Interfaces 2017, 9, 25700–25705. [Google Scholar] [CrossRef]
- Bachmann, A.; Klebsattel, C.; Schankin, A.; Riedel, T.; Beigl, M.; Reichert, M.; Santangelo, P.; Ebner-Priemer, U. Leveraging smartwatches for unobtrusive mobile ambulatory mood assessment. In UbiComp/ISWC′15 Adjunct, Proceedings of the 2015 ACM International Joint Conference on Pervasive and Ubiquitous Computing, Osaka, Japan, 7–11 September 2015; ACM: New York, NY, USA, 2015. [Google Scholar]
- Lee, Y.J.; Shin, H.G.; Choi, H.J.; Kim, C.S. Can pulse check by the photoplethysmography sensor on a smart watch replace carotid artery palpation during cardiopulmonary resuscitation in cardiac arrest patients? A prospective observational diagnostic accuracy study. BMJ Open 2019, 9, e023627. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, G.; Blachowicz, T.; Homburg, S.V.; Ehrmann, A. Measuring biosignals with single circuit boards. Bioengineering 2022, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, G.; Ehrmann, A. Suitability of common single circuit boards for sensing and actuating in smart textiles. Commun. Dev. Assem. Text. Prod. 2020, 1, 170–179. [Google Scholar] [CrossRef]
- Oldfrey, B.; Jackson, R.; Smitham, P.; Miodownik, M. A deep learning approach to non-linearity in wearable stretch sensors. Front. Robot. AI 2019, 6, 27. [Google Scholar] [CrossRef]
- Nuramdhani, I.; Jose, M.; Samyn, P.; Adriaensens, P.; Malengier, B.; Deferme, W.; de Mey, G.; van Langenhove, L. Charge-discharge characteristics of textile energy storage devices having different PEDOT:PSS ratios and conductive yarns configuration. Polymers 2019, 11, 345. [Google Scholar] [CrossRef]
- Li, E.; Lin, X.Y.; Seet, B.-C.; Joseph, F.; Neville, J. Low profile and low cost textile smart mat for step pressure sensing and position mapping. In Proceedings of the IEEE Instrumentation and Measurement Technology Conference 2019, Auckland, New Zealand, 20–23 May 2019; pp. 1564–1568. [Google Scholar]
- Anbalgan, A.; Sundarsingh, E.F.; Ramalingam, V.S. Design and experimental evaluation of a novel on-body textile antenna for unicast applications. Microw. Opt. Technol. Lett. 2020, 62, 789–799. [Google Scholar] [CrossRef]
- Pulsesensor.com. Available online: https://pulsesensor.com/ (accessed on 25 February 2023).
- Tuvshinbayar, K.; Ehrmann, G.; Ehrmann, A. 50/60 Hz Power Grid Noise as a Skin Contact Measure of Textile ECG Electrodes. Textiles 2022, 2, 265–274. [Google Scholar] [CrossRef]



| Washing Cycle No. | Temperature/°C | Softener | Spin Cycle/min−1 |
|---|---|---|---|
| 1 | 60 | Yes | 1200 |
| 2 | 40 | Yes | 1200 |
| 3 | 30 | No | 600 |
| 4 | 60 | Yes | 1200 |
| 5 | 30 | No | 900 |
| 6 | 40 | Yes | 1200 |
| 7 | 40 | Yes | 1200 |
| 8 | 40 | Yes | 1200 |
| 9 | 30 | No | 900 |
| 10 | 60 | Yes | 1200 |
| Washing Cycle No. | Sensor | Sample Number | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| 1 | Keyestudio | ok | ok | - | - | - | |||||
| 1 | Joy-it | ok | ok | ok | ok | ok | |||||
| 1 | Iduino | ok | - | ok | ok | ok | |||||
| 1 | Berrybase | ok | ok | ok | ok | ok | |||||
| 2 | Keyestudio | ok | - | - | - | - | |||||
| 2 | Joy-it | - | ok | ok | - | ok | |||||
| 2 | Iduino | ok | - | ok | ok | ok | |||||
| 2 | Berrybase | ok | ok | ok | ok | ok | |||||
| 3 | Keyestudio | ok | - | - | - | - | |||||
| 3 | Joy-it | - | ok | ok | - | ok | |||||
| 3 | Iduino | - | - | ok | ok | ok | |||||
| 3 | Berrybase | ok | ok | ok | ok | ok | |||||
| 4 | Keyestudio | ok | - | - | - | - | |||||
| 4 | Joy-it | - | ok | - | - | - | |||||
| 4 | Iduino | - | - | ok | - | - | |||||
| 4 | Berrybase | ok | ok | ok | ok | ok | |||||
| 5 | Keyestudio | ok | - | - | - | - | |||||
| 5 | Joy-it | - | ok | - | - | - | |||||
| 5 | Iduino | - | - | - | - | - | |||||
| 5 | Berrybase | ok | ok | ok | ok | ok | |||||
| 6 | Keyestudio | ok | - | - | - | - | |||||
| 6 | Joy-it | - | ok | - | - | - | |||||
| 6 | Iduino | - | - | - | - | - | |||||
| 6 | Berrybase | ok | ok | ok | ok | ok | |||||
| 7 | Keyestudio | ok | - | - | - | - | |||||
| 7 | Joy-it | - | - | - | - | - | |||||
| 7 | Iduino | - | - | - | - | - | |||||
| 7 | Berrybase | ok | ok | ok | ok | ok | |||||
| 8 | Keyestudio | - | - | - | - | - | |||||
| 8 | Joy-it | - | - | - | - | - | |||||
| 8 | Iduino | - | - | - | - | - | |||||
| 8 | Berrybase | ok | ok | ok | ok | ok | |||||
| 9 | Keyestudio | - | - | - | - | - | |||||
| 9 | Joy-it | - | - | - | - | - | |||||
| 9 | Iduino | - | - | - | - | - | |||||
| 9 | Berrybase | ok | ok | ok | ok | ok | |||||
| 10 | Keyestudio | - | - | - | - | - | |||||
| 10 | Joy-it | - | - | - | - | - | |||||
| 10 | Iduino | - | - | - | - | - | |||||
| 10 | Berrybase | ok | ok | ok | ok | ok | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richter, N.; Tuvshinbayar, K.; Ehrmann, G.; Ehrmann, A. Usability of Inexpensive Optical Pulse Sensors for Textile Integration and Heartbeat Detection Code Development. Electronics 2023, 12, 1521. https://doi.org/10.3390/electronics12071521
Richter N, Tuvshinbayar K, Ehrmann G, Ehrmann A. Usability of Inexpensive Optical Pulse Sensors for Textile Integration and Heartbeat Detection Code Development. Electronics. 2023; 12(7):1521. https://doi.org/10.3390/electronics12071521
Chicago/Turabian StyleRichter, Niclas, Khorolsuren Tuvshinbayar, Guido Ehrmann, and Andrea Ehrmann. 2023. "Usability of Inexpensive Optical Pulse Sensors for Textile Integration and Heartbeat Detection Code Development" Electronics 12, no. 7: 1521. https://doi.org/10.3390/electronics12071521
APA StyleRichter, N., Tuvshinbayar, K., Ehrmann, G., & Ehrmann, A. (2023). Usability of Inexpensive Optical Pulse Sensors for Textile Integration and Heartbeat Detection Code Development. Electronics, 12(7), 1521. https://doi.org/10.3390/electronics12071521







