AI Models for Predicting Readmission of Pneumonia Patients within 30 Days after Discharge
Abstract
:1. Introduction
1.1. Policies for Reducing Readmissions
1.2. Factors Associated with Readmissions and Interventions for High-Risk Patients
1.3. AI Models for Predicting Associated Events of Hospital Admission and Readmission
1.4. State-of-the-Art Models for the Prediction of Pneumonia Readmissions
1.5. Problem Statements and Research Objectives
2. Materials and Methods
2.1. Data Source
2.2. Samples
2.3. Variables
2.4. Statistica Analysis
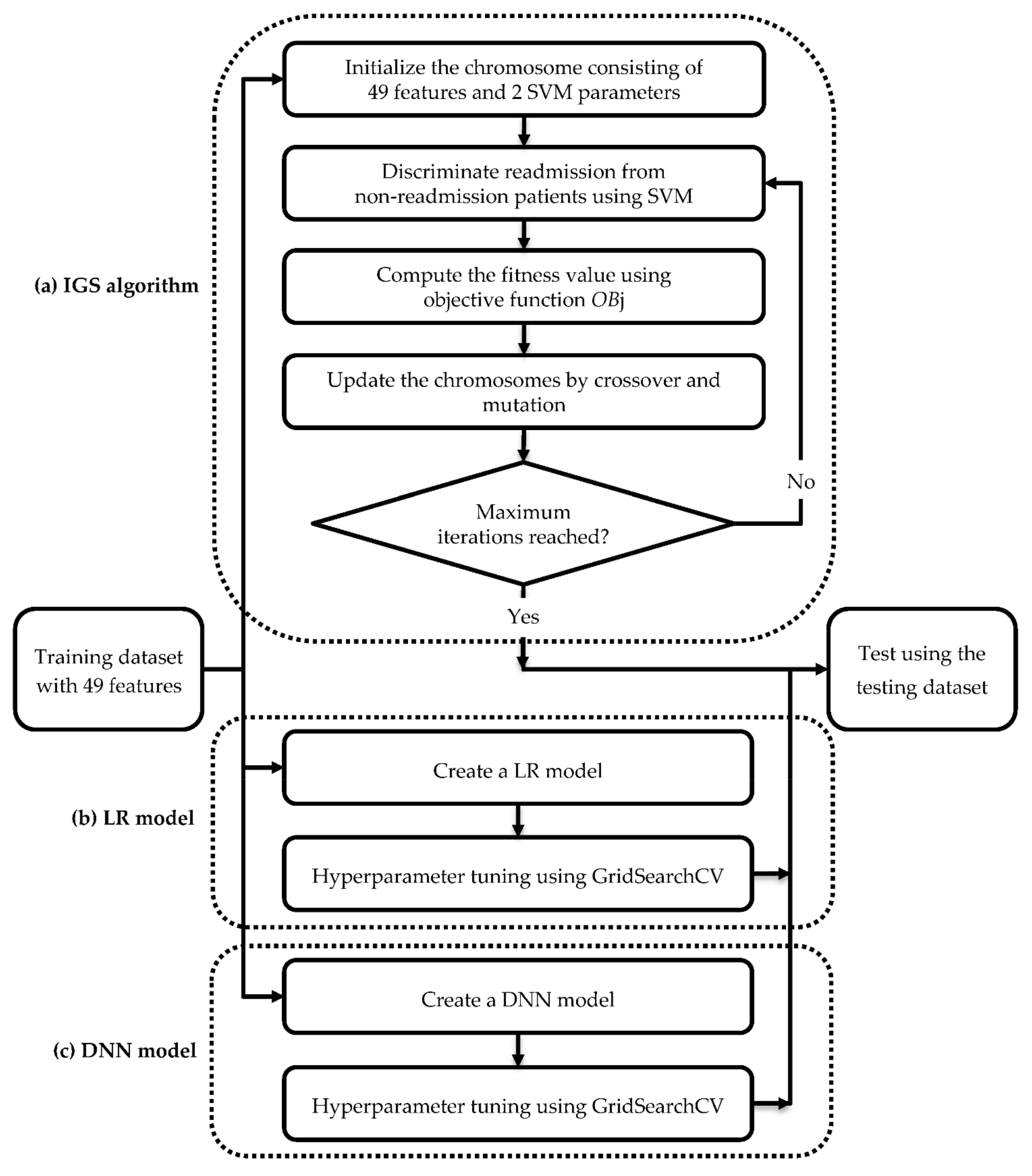
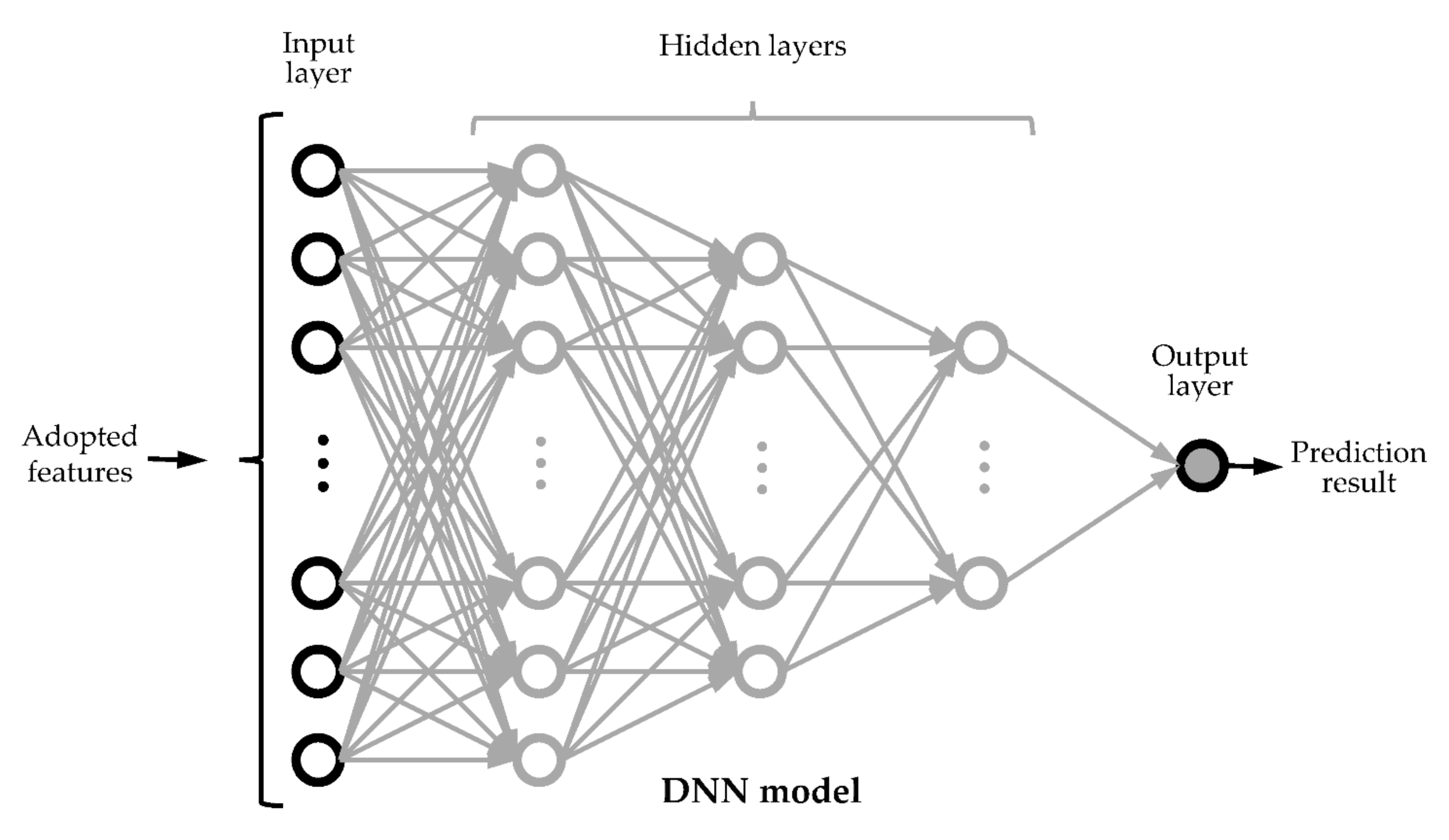
2.5. Design of Prediction Models
3. Results
3.1. Comparisons of Demographic Characteristics, Comorbidities, Inpatient Interventions, and Related Variables between Readmitted and Non-Readmitted Patients
3.2. Predictve Performance
4. Discussions
4.1. Model Explainability
4.2. Performance Comparison
4.3. Future Works
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Kansagara, D.; Englander, H.; Salanitro, A.; Kagen, D.; Theobald, C.; Freeman, M.; Kripalani, S. Risk prediction models for hospital readmission: A systematic review. JAMA 2011, 306, 1688–1698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navathe, A.S.; Zhong, F.; Lei, V.J.; Chang, F.Y.; Sordo, M.; Topaz, M.; Navathe, S.B.; Rocha, R.A.; Zhou, L. Hospital readmission and social risk factors identified from physician notes. Health Serv. Res. 2018, 53, 1110–1136. [Google Scholar] [CrossRef] [PubMed]
- Glans, M.; Ekstam, A.K.; Jakobsson, U.; Bondesson, Å.; Midlöv, P. Risk factors for hospital readmission in older adults within 30 days of discharge—A comparative retrospective study. BMC Geriatr. 2020, 20, 467. [Google Scholar] [CrossRef] [PubMed]
- Dharmarajan, K.; Hsieh, A.F.; Lin, Z.; Bueno, H.; Ross, J.S.; Horwitz, L.I.; Barreto-Filho, J.A.; Kim, N.; Suter, L.G.; Bernheim, S.M. Hospital readmission performance and patterns of readmission: Retrospective cohort study of Medicare admissions. BMJ 2013, 347, f6571. [Google Scholar] [CrossRef] [Green Version]
- Rumball-Smith, J.; Blakely, T.; Sarfati, D.; Hider, P. The mismeasurement of quality by readmission rate: How blunt is too blunt an instrument? A quantitative bias analysis. Med. Care 2013, 51, 418–424. [Google Scholar] [CrossRef]
- Ayabakan, S.; Bardhan, I.; Zheng, Z. Triple Aim and the Hospital Readmission Reduction Program. Health Serv. Res. Manag. Epidemiol. 2021, 8, 2333392821993704. [Google Scholar] [CrossRef]
- Hoffman, G.J.; Yakusheva, O. Association between financial incentives in Medicare’s hospital readmissions reduction program and hospital readmission performance. JAMA Netw. Open 2020, 3, e202044. [Google Scholar] [CrossRef] [Green Version]
- Hogan, T.H.; Lemak, C.H.; Hearld, L.R.; Wheeler, J.; Sen, B.P.; Menachemi, N. Vertical integration into skilled nursing facilities and hospital readmission rates. J. Healthc. Qual. JHQ 2020, 42, 91–97. [Google Scholar] [CrossRef]
- Baechle, C.; Agarwal, A. A framework for the estimation and reduction of hospital readmission penalties using predictive analytics. J. Big Data 2017, 4, 37. [Google Scholar] [CrossRef]
- Pugh, J.; Penney, L.S.; Noël, P.H.; Neller, S.; Mader, M.; Finley, E.P.; Lanham, H.J.; Leykum, L. Evidence based processes to prevent readmissions: More is better, a ten-site observational study. BMC Health Serv. Res. 2021, 21, 189. [Google Scholar] [CrossRef]
- Kash, B.A.; Baek, J.; Davis, E.; Champagne-Langabeer, T.; Langabeer, J.R. II Review of successful hospital readmission reduction strategies and the role of health information exchange. Int. J. Med. Inform. 2017, 104, 97–104. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.D.; Ma, C. Hospital nursing and 30-day readmissions among Medicare patients with heart failure, acute myocardial infarction, and pneumonia. Med. Care 2013, 51, 52–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.W. Selecting the best prediction model for readmission. J. Prev. Med. Public Health 2012, 45, 259. [Google Scholar] [CrossRef] [PubMed]
- Jasti, H.; Mortensen, E.M.; Obrosky, D.S.; Kapoor, W.N.; Fine, M.J. Causes and risk factors for rehospitalization of patients hospitalized with community-acquired pneumonia. Clin. Infect. Dis. 2008, 46, 550–556. [Google Scholar] [CrossRef]
- Donzé, J.; Lipsitz, S.; Bates, D.W.; Schnipper, J.L. Causes and patterns of readmissions in patients with common comorbidities: Retrospective cohort study. BMJ 2013, 347, f7171. [Google Scholar] [CrossRef] [Green Version]
- Baker, M.C.; Alberti, P.M.; Tsao, T.-Y.; Fluegge, K.; Howland, R.E.; Haberman, M. Social Determinants Matter For Hospital Readmission Policy: Insights From New York City: Study examines social determinants and hospital readmissions. Health Aff. 2021, 40, 645–654. [Google Scholar] [CrossRef]
- Melton, L.D.; Foreman, C.; Scott, E.; McGinnis, M.; Cousins, M. Prioritized post-discharge telephonic outreach reduces hospital readmissions for select high-risk patients. Am. J. Manag. Care 2012, 18, 838–844. [Google Scholar]
- Charles, L.; Jensen, L.; Torti, J.M.; Parmar, J.; Dobbs, B.; Tian, P.G.J. Improving transitions from acute care to home among complex older adults using the LACE Index and care coordination. BMJ Open Qual. 2020, 9, e000814. [Google Scholar] [CrossRef]
- Garg, A.X.; Adhikari, N.K.; McDonald, H.; Rosas-Arellano, M.P.; Devereaux, P.J.; Beyene, J.; Sam, J.; Haynes, R.B. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: A systematic review. JAMA 2005, 293, 1223–1238. [Google Scholar] [CrossRef]
- Rajkomar, A.; Oren, E.; Chen, K.; Dai, A.M.; Hajaj, N.; Hardt, M.; Liu, P.J.; Liu, X.; Marcus, J.; Sun, M. Scalable and accurate deep learning with electronic health records. NPJ Digit. Med. 2018, 1, 18. [Google Scholar] [CrossRef]
- Lai, H.J.; Tan, T.H.; Lin, C.S.; Chen, Y.F.; Lin, H.H. Designing a clinical decision support system to predict readmissions for patients admitted with all-cause conditions. J. Ambient. Intell. Humaniz. Comput. 2020, 1–10. [Google Scholar] [CrossRef]
- Wu, F.H.; Lai, H.J.; Lin, H.H.; Chan, P.C.; Tseng, C.M.; Chang, K.M.; Chen, Y.F.; Lin, C.S. Predictive models for detecting patients more likely to develop acute myocardial infarctions. J. Supercomput. 2022, 78, 2043–2071. [Google Scholar] [CrossRef]
- Porat, T.; Kostopoulou, O.; Woolley, A.; Delaney, B.C. Eliciting user decision requirements for designing computerized diagnostic support for family physicians. J. Cogn. Eng. Decis. Mak. 2016, 10, 57–73. [Google Scholar] [CrossRef] [Green Version]
- Horng, S.; Sontag, D.A.; Halpern, Y.; Jernite, Y.; Shapiro, N.I.; Nathanson, L.A. Creating an automated trigger for sepsis clinical decision support at emergency department triage using machine learning. PLoS ONE 2017, 12, e0174708. [Google Scholar] [CrossRef] [Green Version]
- Hsu, J.C.; Chen, Y.F.; Chung, W.S.; Tan, T.H.; Chen, T.S.; Chiang, J.Y. Clinical verification of a clinical decision support system for ventilator weaning. Biomed. Eng. Online 2013, 12, S4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, G.; Nkoy, F.L.; Gesteland, P.H.; Glasgow, T.S.; Stone, B.L. A systematic review of predictive modeling for bronchiolitis. Int. J. Med. Inform. 2014, 83, 691–714. [Google Scholar] [CrossRef] [Green Version]
- Dunn Lopez, K.; Gephart, S.M.; Raszewski, R.; Sousa, V.; Shehorn, L.E.; Abraham, J. Integrative review of clinical decision support for registered nurses in acute care settings. J. Am. Med. Inform. Assoc. 2017, 24, 441–450. [Google Scholar] [CrossRef]
- Scheepers-Hoeks, A.-M.J.; Grouls, R.J.; Neef, C.; Ackerman, E.W.; Korsten, E.H. Physicians’ responses to clinical decision support on an intensive care unit—Comparison of four different alerting methods. Artif. Intell. Med. 2013, 59, 33–38. [Google Scholar] [CrossRef]
- Oluoch, T.; Katana, A.; Kwaro, D.; Santas, X.; Langat, P.; Mwalili, S.; Muthusi, K.; Okeyo, N.; Ojwang, J.K.; Cornet, R. Effect of a clinical decision support system on early action on immunological treatment failure in patients with HIV in Kenya: A cluster randomised controlled trial. Lancet HIV 2016, 3, e76–e84. [Google Scholar] [CrossRef] [Green Version]
- Otto, A.K.; Dyer, A.A.; Warren, C.M.; Walkner, M.; Smith, B.M.; Gupta, R.S. The development of a clinical decision support system for the management of pediatric food allergy. Clin. Pediatrics 2017, 56, 571–578. [Google Scholar] [CrossRef]
- Ammenwerth, E.; Schnell-Inderst, P.; Machan, C.; Siebert, U. The effect of electronic prescribing on medication errors and adverse drug events: A systematic review. J. Am. Med. Inform. Assoc. 2008, 15, 585–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baypinar, F.; Kingma, H.J.; van der Hoeven, R.T.; Becker, M.L. Physicians’ compliance with a clinical decision support system alerting during the prescribing process. J. Med. Syst. 2017, 41, 96. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, É.; Elbattah, M.; Gignon, M.; Dequen, G. Deep learning to predict hospitalization at triage: Integration of structured data and unstructured text. In Proceedings of the 2020 IEEE International Conference on Big Data (Big Data), Atlanta, GA, USA, 10–13 December 2020; pp. 4836–4841. [Google Scholar]
- Sánchez-Salmerón, R.; Gómez-Urquiza, J.L.; Albendín-García, L.; Correa-Rodríguez, M.; Martos-Cabrera, M.B.; Velando-Soriano, A.; Suleiman-Martos, N. Machine learning methods applied to triage in emergency services: A systematic review. Int. Emerg. Nurs. 2022, 60, 101109. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.-M.; Jeon, K.-H.; Lee, M.; Kim, K.-H.; Park, J.; Oh, B.-H. Deep learning algorithm to predict need for critical care in pediatric emergency departments. Pediatric Emerg. Care 2021, 37, e988–e994. [Google Scholar] [CrossRef]
- Raita, Y.; Goto, T.; Faridi, M.K.; Brown, D.F.; Camargo, C.A.; Hasegawa, K. Emergency department triage prediction of clinical outcomes using machine learning models. Crit. Care 2019, 23, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, W.S.; Haimovich, A.D.; Taylor, R.A. Predicting hospital admission at emergency department triage using machine learning. PLoS ONE 2018, 13, e0201016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.W.; Ko, T.; Hong, K.J.; Kim, K.H. Machine learning-based prediction of Korean triage and acuity scale level in emergency department patients. Healthc. Inform. Res. 2019, 25, 305–312. [Google Scholar] [CrossRef]
- Klug, M.; Barash, Y.; Bechler, S.; Resheff, Y.S.; Tron, T.; Ironi, A.; Soffer, S.; Zimlichman, E.; Klang, E. A gradient boosting machine learning model for predicting early mortality in the emergency department triage: Devising a nine-point triage score. J. Gen. Intern. Med. 2020, 35, 220–227. [Google Scholar] [CrossRef]
- Navares, R.; Aznarte, J.L. Deep learning architecture to predict daily hospital admissions. Neural Comput. Appl. 2020, 32, 16235–16244. [Google Scholar] [CrossRef]
- Chung, A.; Famouri, M.; Hryniowski, A.; Wong, A. COVID-Net Clinical ICU: Enhanced Prediction of ICU Admission for COVID-19 Patients via Explainability and Trust Quantification. arXiv 2021, arXiv:2109.06711. [Google Scholar]
- Yu, K.; Yang, Z.; Wu, C.; Huang, Y.; Xie, X. In-hospital resource utilization prediction from electronic medical records with deep learning. Knowl.-Based Syst. 2021, 223, 107052. [Google Scholar] [CrossRef]
- Alsinglawi, B.; Alnajjar, F.; Mubin, O.; Novoa, M.; Alorjani, M.; Karajeh, O.; Darwish, O. Predicting Length of Stay for Cardiovascular Hospitalizations in the Intensive Care Unit: Machine Learning Approach. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 5442–5445. [Google Scholar]
- Xie, J.; Zhang, B.; Ma, J.; Zeng, D.; Lo-Ciganic, J. Readmission prediction for patients with heterogeneous medical history: A trajectory-based deep learning approach. ACM Trans. Manag. Inf. Syst. TMIS 2021, 13, 1–27. [Google Scholar] [CrossRef]
- Choudhury, A.; Greene, C.M. Evaluating patient readmission risk: A predictive analytics approach. arXiv 2018, arXiv:1812.11028. [Google Scholar] [CrossRef]
- Jiang, S.; Chin, K.S.; Qu, G.; Tsui, K.L. An integrated machine learning framework for hospital readmission prediction. Knowl.-Based Syst. 2018, 146, 73–90. [Google Scholar] [CrossRef]
- Du, G.; Zhang, J.; Luo, Z.; Ma, F.; Ma, L.; Li, S. Joint imbalanced classification and feature selection for hospital readmissions. Knowl.-Based Syst. 2020, 200, 106020. [Google Scholar] [CrossRef]
- Du, G.; Zhang, J.; Ma, F.; Zhao, M.; Lin, Y.; Li, S. Towards graph-based class-imbalance learning for hospital readmission. Expert Syst. Appl. 2021, 176, 114791. [Google Scholar] [CrossRef]
- Junqueira, A.R.B.; Mirza, F.; Baig, M.M. A machine learning model for predicting ICU readmissions and key risk factors: Analysis from a longitudinal health records. Health Technol. 2019, 9, 297–309. [Google Scholar] [CrossRef]
- Ryu, B.; Yoo, S.; Kim, S.; Choi, J. Development of Prediction Models for Unplanned Hospital Readmission within 30 Days Based on Common Data Model: A Feasibility Study. Methods Inf. Med. 2021, 60, e65–e75. [Google Scholar]
- Weinreich, M.; Nguyen, O.K.; Wang, D.; Mayo, H.; Mortensen, E.M.; Halm, E.A.; Makam, A.N. Predicting the risk of readmission in pneumonia. A systematic review of model performance. Ann. Am. Thorac. Soc. 2016, 13, 1607–1614. [Google Scholar] [CrossRef] [Green Version]
- Capelastegui, A.; Yandiola, P.P.E.; Quintana, J.M.; Bilbao, A.; Diez, R.; Pascual, S.; Pulido, E.; Egurrola, M. Predictors of short-term rehospitalization following discharge of patients hospitalized with community-acquired pneumonia. Chest 2009, 136, 1079–1085. [Google Scholar] [CrossRef]
- Mather, J.F.; Fortunato, G.J.; Ash, J.L.; Davis, M.J.; Kumar, A. Prediction of pneumonia 30-day readmissions: A single-center attempt to increase model performance. Respir. Care 2014, 59, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Makam, A.N.; Nguyen, O.K.; Clark, C.; Zhang, S.; Xie, B.; Weinreich, M.; Mortensen, E.M.; Halm, E.A. Predicting 30-day pneumonia readmissions using electronic health record data. J. Hosp. Med. 2017, 12, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatipoğlu, U.; Wells, B.J.; Chagin, K.; Joshi, D.; Milinovich, A.; Rothberg, M.B. Predicting 30-day all-cause readmission risk for subjects admitted with pneumonia at the point of care. Respir. Care 2018, 63, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Lai, H.J.; Chan, P.C.; Lin, H.H.; Chen, Y.F.; Lin, C.S.; Hsu, J.C. A web-based decision support system for predicting readmission of pneumonia patients after discharge. In Proceedings of the 2018 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Miyazaki, Japan, 7–10 October 2018; pp. 2305–2310. [Google Scholar]
- Chang, C.C.; Liao, C.C.; Chen, T.L. Perioperative medicine and Taiwan National Health Insurance Research Database. Acta Anaesthesiol. Taiwanica 2016, 54, 93–96. [Google Scholar] [CrossRef]
- Farquad, M.; Bose, I. Preprocessing unbalanced data using support vector machine. Decis. Support Syst. 2012, 53, 226–233. [Google Scholar] [CrossRef]
- Hu, J.; Yang, H.; Lyu, M.R.; King, I.; So, A.M.C. Online nonlinear AUC maximization for imbalanced data sets. IEEE Trans. Neural Netw. Learn. Syst. 2017, 29, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Lin, C.S.; Wang, K.A.; Rahman, L.O.A.; Lee, D.J.; Chung, W.S.; Lin, H.H. Design of a clinical decision support system for fracture prediction using imbalanced dataset. J. Healthc. Eng. 2018, 2018, 9621640. [Google Scholar] [CrossRef] [Green Version]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Lin, W.C.; Tsai, C.F.; Hu, Y.H.; Jhang, J.S. Clustering-based undersampling in class-imbalanced data. Inf. Sci. 2017, 409, 17–26. [Google Scholar] [CrossRef]
- Chang, C.C.; Lin, C.J. LIBSVM: A Library for Support Vector Machines. Available online: https://www.csie.ntu.edu.tw/~cjlin/libsvm/faq.html#f203 (accessed on 29 December 2021).
- Chen, Y.F.; Huang, P.C.; Lin, K.C.; Lin, H.H.; Wang, L.E.; Cheng, C.C.; Chen, T.P.; Chan, Y.K.; Chiang, J.Y. Semi-automatic segmentation and classification of pap smear cells. IEEE J. Biomed. Health Inform. 2013, 18, 94–108. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Abadi, M.; Agarwal, A.; Barham, P.; Brevdo, E.; Chen, Z.; Citro, C.; Corrado, G.S.; Davis, A.; Dean, J.; Devin, M. TensorFlow: Large-scale Machine Learning on Heterogeneous Systems. arXiv Prepr. 2016, arXiv:1603.04467. [Google Scholar]
- Pilgrim, M.; Willison, S. Dive into Python 3; Springer: Berlin/Heidelberg, Germany, 2009; Volume 2. [Google Scholar]
- Chen, Y.F.; Lin, C.S.; Hong, C.F.; Lee, D.J.; Sun, C.M.; Lin, H.H. Design of a clinical decision support system for predicting erectile dysfunction in men using NHIRD dataset. IEEE J. Biomed. Health Inform. 2019, 23, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.T.; Egelund, G.B.; Jensen, A.V.; Andersen, S.B.; Pedersen, M.F.; Rohde, G.; Ravn, P. Associations between biomarkers at discharge and co-morbidities and risk of readmission after community-acquired pneumonia: A retrospective cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1103–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, T.; Cursio, J.F.; Qadir, S.; Lindenauer, P.K.; Ruhnke, G.W. Discharge disposition as an independent predictor of readmission among patients hospitalised for community-acquired pneumonia. Int. J. Clin. Pract. 2017, 71, e12935. [Google Scholar] [CrossRef] [PubMed]
- Danso, S.O.; Zeng, Z.; Muniz-Terrera, G.; Ritchie, C.W. Developing an explainable machine learning-based personalised dementia risk prediction model: A transfer learning approach with ensemble learning algorithms. Front. Big Data 2021, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, F.; Qi, Z.; Duan, K.; Xi, D.; Zhu, Y.; Zhu, H.; Xiong, H.; He, Q. A comprehensive survey on transfer learning. Proc. IEEE 2020, 109, 43–76. [Google Scholar] [CrossRef]
- Feuz, K.D.; Cook, D.J. Collegial activity learning between heterogeneous sensors. Knowl. Inf. Syst. 2017, 53, 337–364. [Google Scholar] [CrossRef]

| Study (Year) | Prediction Event | Method | Dataset | Used Features or Input | Predictive Performance | Issue |
|---|---|---|---|---|---|---|
| [33] (2020) | Hospital admission at ED | MLP with numeric and categorical features + CNN with textual data | 260,000 ED records of a hospital in France collected within 2015–2019 | 28 features of numeric, categorical, and textual data | AUC = 0.83 | |
| [40] (2020) | Daily hospital admission due to respiratory- and circulatory-related disorders | LSTM + CNN | Patients ≥ 65 y due to circulatory or respiratory disorders across the region of Madrid, Spain, within 2001–2013 | 13 locations and 12 features of chemical air pollutants, weather observations, and pollen observations | RMSE = 11.21 and 11.76 for circulatory and respiratory cases, respectively | Patients with age < 65 y were excluded |
| [41] (2021) | ICU admission for COVID-19 patients | COVID-Net Clinical ICU | 1925 COVID-19 patient records retrieved from a hospital in Canada in 2020 | 228 clinical features in fields of demographic information, previous diseases, blood results, and vital signs for each patient | ACC = 96.9% | |
| [42] (2021) | In-hospital cost and LOS of admitted patients | RUP | 750,000 EMRs of discharged patients from 2012 to 2015 collected from a hospital quality monitoring system of China | Patient features, diagnosis texts, operation texts, diagnosis IDs, and operation IDs | RMSE = 7765 CNY and 7.056 days for cost and LOS predictions respectively | |
| [43] (2020) | LOS for cardiovascular hospitalization in ICU | Stacking regression | Health data of 61,532 ICU stays in the MIMIC-III dataset provided by MIT Lab | Demographics, vital signs, laboratory tests, medications, and more clinical variables | MAE = 1.92 days |
| Study (Year) | Prediction Event | Method | Dataset | Categories of Features | AUC | Issue |
|---|---|---|---|---|---|---|
| [44] (2021) | 30-day hospital readmission | TADEL by capturing dynamic medical history | A balanced dataset of 72,668 readmission and 72,663 non-readmission patients acquired from national Medicare claims of all hospitals in the US from 2011 to 2015 | Health status factors, insurance coverage and payment, history of health service utilizations and hospitalizations, and sociodemographic information | 0.884 | Using a balanced dataset for testing is not the real situation in practice, the dataset is usually very imbalanced, which may degrade the predictive performance |
| [45] (2018) | 90-day hospital readmission | GBM + GA | 69,984 encounters retrieved from 10-year dataset of 130 US hospitals | 55 attributes (including HbA1c, gender, discharge disposition, admission source, specialty of the admitting physician, primary diagnosis (9), race, age, time in hospital, etc.) | Not shown, ACC = 97.05% | AUC not shown |
| [46] (2018) | 30-day hospital readmission, etc. | SVM + feature selection algorithm (EMOBPSO), etc. | 2871 and 40,460 readmission and non-readmission cases from the HIS of a hospital in northeast China | 21 fields of 3 databases (outpatient information, EMR, and inpatient information) in the HIS | 0.9038 | Low precision (43.43%) |
| [47] (2020) | hospital readmissions | JICFS (including ℓ1-norm regularization for class-imbalance aware feature selection) | 6 open readmission datasets (all-cause, LACE-score, MIMIC, T-carer, RA, and diabetic) | 15–243 features | 0.733–0.9299 | Low MCC for 2 datasets ranging from 0.5012–0.546 |
| [48] (2021) | hospital readmissions | Graph-CL | 6 open readmission datasets (All-cause, LACE-score, MIMIC, T-carer, RA, and diabetic) | Adopted 15–75 features | 0.776–0.886 | Low MCC for 3 datasets ranging from 0.561–0.617 |
| [49] (2019) | 30-day ICU readmission | MLP | MIMIC-III dataset with 42,307 ICU stays of 31,749 patients from a US hospital in 2001 to 2012 | 12 features | 0.642 | |
| [50] (2021) | 30-day hospital readmission | GBM (AI model) + CDM (for applying trained AI model to multiple institutions) | 106,304 hospitalizations with 32,242 readmissions retrieved from EHR of Seoul National University Hospital in 2017–2018, etc. | Demographics, clinical index score, diagnosis, medication, visit records, surgeries, and clinical examination test. | 0.8414 | (1) Precise features adopted for model creation and prediction are not clear; (2) predictive parameters except AUC are not shown; (3) the predictive performance degrades when applying the model trained in a hospital to another hospital |
| Study (Year) | Prediction Event | Method | Dataset | Adopted Features | AUC | Issue |
|---|---|---|---|---|---|---|
| [52] (2009) | 30-day pneumonia- unrelated readmission #1 | LR #2 | 1117 pneumonia patients discharged at Galdakao Hospital in Basque country, Spain | Age, CCI #3, and decompensated comorbidities | 0.77 | The predictive performances obtained using only 52/29 pneumonia- unrelated/ related readmission cases were less representative |
| 30-day pneumonia- related readmission #1 | Treatment failure and instability factors | 0.65 | ||||
| [53] 2014 | 30-day all-cause readmission | LR | 965 cases (148 readmissions) of pneumonia admission collected at Hartford hospital, Connecticut | 16 significant features (5 demographic items, previous admissions, income, 7 comorbidities, and 2 lab values) selected from 31 variables | 0.71 | Patients with age < 65 y were excluded |
| [54] 2017 | 30-day all-cause readmission | LR | EHRs #4 of 1463 patients (199 readmissions) hospitalized with pneumonia collected from 6 hospitals in northern Texas | Income, platelets, prior hospitalizations in past year, vital sign instabilities #5 on discharge, updated PSI #6, and disposition status at hospital discharge | 0.731 | Readmissions to hospitals beyond 100-mile radius of Dallas were not counted |
| [55] (2018) | 30-day all-cause readmission | LR | EHRs of 1295 hospitalizations (330 readmissions) with pneumonia at the Cleveland clinic main campus in Ohio | 13 significant features (age, cancer, CHD #7, stroke, antibiotics, opioids, temperature, BUN #8, hemoglobin, albumin, sodium, INR #9, and prior admissions within 6 months) | 0.74 | Excluded age < 65 y |
| Pilot study [56] (2018) | 30-day all-cause readmission #1 | IGS | 1103/4331 w/wo readmissions of pneumonia patients retrieved from NHIRD (medical administrative records) in Taiwan | 20 features (demographics, comorbidity no., comorbidity index, events within 1 year before admission, inpatient interventions, category of admitted hospitals, LoA #10, healthcare cost, discharge status, and dosage of antibiotics) | 0.76 | Physiological signals, laboratory test results, and social determinants, were not included in NHIRD and not adopted in our pilot study |
| This study | 30-day all-cause readmission #1 | IGS, DNN, and LR | 1545/6228 w/wo readmissions of pneumonia patients retrieved from NHIRD (medical administrative records) in Taiwan | 49 features listed in Table 4 | 0.7758, 0.7547, and 0.7689 | Physiological signals, laboratory test results, and social determinants were not included in NHIRD and not adopted in this study |
| Variables | Readmission | p-Value | |
|---|---|---|---|
| Yes (n = 1545) | No (n = 6228) | ||
| Gendera, b, c, n(%) | <0.001 | ||
| Men | 1023 (66.2%) | 3495 (56.1%) | |
| Women | 522 (33.8%) | 2733 (43.9%) | |
| Ageb in year, mean (SD) | 74.7 (15.1) | 65.7 (20) | <0.001 |
| Comorbidity, mean (SD) | |||
| No. a, b, c | 3.6 (0.9) | 2.8 (1.4) | <0.001 |
| CCI score | 2.2 (1.9) | 0.9 (1.3) | <0.001 |
| Events within 1 year before admission | |||
| ED visits b, c, n (%) | 1224 (79.2%) | 4621 (74.2%) | <0.001 |
| Hospitalizations a, b, c, mean (SD) | 2.2 (1.9) | 1.5 (1.1) | <0.001 |
| Outpatient visits c, mean (SD) | 20.2 (19.8) | 17 (17.7) | <0.001 |
| Inpatient Interventions | |||
| Surgical operations, mean (SD) | 1.1 (1.4) | 0.7 (1.1) | <0.001 |
| Adm. Medications a, b, c, mean (SD) | 18.2 (8.3) | 15 (7.3) | <0.001 |
| Ventilator use/therapy a, b, c, n (%) | 1149 (74.4%) | 3650 (58.6%) | <0.001 |
| Other interventions a, b, c, n (%) | 820 (53.1%) | 1717 (27.6%) | <0.001 |
| Category of admitted hospitalsa, b, c, n (%) | <0.01 | ||
| Medical center | 333 (21.6%) | 1410 (22.6%) | Chi-square = 9.658; p = 0.008 |
| Regional hospital | 713 (46.1%) | 3056 (49.1%) | |
| District hospital | 499 (32.3%) | 1762 (28.3%) | |
| Length of admissionb, c, mean (SD) days | 11.4 (6.7) | 8.4 (5.5) | <0.001 |
| Total healthcare costa, b, mean (SD) NT$ | 54,268 (46,311) | 36,975 (39,346) | <0.001 |
| Discharge statusb, c | 0.654 | ||
| No follow-up, n (%) | 49 (3.2%) | 184 (3.0%) | |
| Outpatient follow-up, n (%) | 1496 (96.8%) | 6044 (97.0%) | |
| Outpatient visits within 1 year before admission, mean (SD) | |||
| Myocardial infarction a, b, c | 0.2 (2) | 0.2 (1.9) | 0.594 |
| Congestive heart failure | 2.5 (7.8) | 1.7 (6.2) | <0.001 |
| Peripheral vascular disease b, c | 0.4 (2.7) | 0.3 (2.4) | 0.128 |
| Cerebrovascular disease b | 8 (18.1) | 6.3 (17.3) | 0.001 |
| Dementia a | 2.9 (8.4) | 2.3 (8.1) | 0.008 |
| Chronic pulmonary disease b | 0.2 (2.2) | 0.1 (2.1) | 0.408 |
| Rheumatologic disease a, c | 0.5 (5.6) | 0.5 (4.9) | 0.833 |
| Peptic ulcer disease a, b, c | 2.4 (6.3) | 2.1 (6.1) | 0.069 |
| Mild liver disease a | 1.3 (6.4) | 1.3 (5.9) | 0.815 |
| Diabetes w/o chron. compl. | 6.1 (13.8) | 5.6 (13.1) | 0.214 |
| Diabetes w chron. compl. a, b, c | 1.1 (4.9) | 1.3 (6) | 0.25 |
| Hemiplegia or paraplegia a, c | 0.7 (6.7) | 0.6 (5.4) | 0.334 |
| Renal disease a, b, c | 4.1 (14.3) | 3.6 (14.6) | 0.283 |
| Leukemia or lymphoma a, b, c | 5.6 (15.5) | 3.6 (14.4) | <0.001 |
| Moderate/severe liver disease b, c | 0 (0.6) | 0 (0.6) | 0.668 |
| Metastatic solid tumor | 0.2 (2.9) | 0.1 (2) | 0.069 |
| AIDS/HIV b, c | 0.1 (1.7) | 0 (0.9) | 0.298 |
| Hospitalizations within 1 year before admission, mean (SD) | |||
| Myocardial infarction a, b, c | 0.1 (0.3) | 0 (0.2) | <0.001 |
| Congestive heart failure a, b, c | 0.6 (1.4) | 0.2 (0.9) | <0.001 |
| Peripheral vascular disease a, b, c | 0.1 (0.3) | 0 (0.2) | <0.001 |
| Cerebrovascular disease a, b, c | 0.6 (1.4) | 0.3 (1) | <0.001 |
| Dementia b, c | 0.1 (0.6) | 0.1 (0.4) | <0.001 |
| Chronic pulmonary disease a, b | 0 (0.3) | 0 (0.2) | 0.052 |
| Rheumatologic disease b, c | 0.1 (0.7) | 0 (0.3) | 0.007 |
| Peptic ulcer disease a, b, c | 0.3 (0.8) | 0.1 (0.5) | <0.001 |
| Mild liver disease c | 0.3 (1.3) | 0.1 (0.6) | <0.001 |
| Diabetes w/o chron. compl. a, b | 1.1 (2.3) | 0.5 (1.2) | <0.001 |
| Diabetes w chron. compl. a, c | 0.1 (0.7) | 0.1 (0.4) | <0.001 |
| Hemiplegia or paraplegia b, c | 0.1 (0.7) | 0 (0.3) | <0.001 |
| Renal disease a, b, c | 0.5 (1.7) | 0.2 (1) | <0.001 |
| Leukemia or lymphoma a, b, c | 1 (2.9) | 0.3 (1.1) | <0.001 |
| Moderate/severe liver disease a, b, c | 0.1 (0.7) | 0 (0.2) | <0.001 |
| Metastatic solid tumor b | 0.5 (2) | 0.1 (0.6) | <0.001 |
| AIDS/HIV b, c | 0 (0.2) | 0 (0.1) | 0.043 |
| Method | Objective Function | ACC (%) | SEN (%) | SPE (%) | AUC |
|---|---|---|---|---|---|
| Training Phase | |||||
| IGS | OB1 | 70.30 | 70.30 | 70.30 | 0.7536 |
| OB2 | 70.63 | 78.33 | 72.93 | 0.7729 | |
| OB3 | 71.22 | 68.85 | 73.58 | 0.7595 | |
| DNN | ACC | 65.81 | 65.04 | 66.54 | 0.7266 |
| LR | ACC | 69.18 | 65.45 | 72.76 | 0.7543 |
| Testing Phase | |||||
| IGS | OB1 | 68.20 | 74.61 | 66.56 | 0.7727 |
| OB2 | 70.11 | 73.46 | 69.26 | 0.7758 | |
| OB3 | 69.75 | 70.40 | 69.59 | 0.7599 | |
| DNN | ACC | 61.50 | 79.34 | 56.95 | 0.7547 |
| LR | ACC | 65.77 | 78.44 | 62.54 | 0.7689 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, J.-C.; Wu, F.-H.; Lin, H.-H.; Lee, D.-J.; Chen, Y.-F.; Lin, C.-S. AI Models for Predicting Readmission of Pneumonia Patients within 30 Days after Discharge. Electronics 2022, 11, 673. https://doi.org/10.3390/electronics11050673
Hsu J-C, Wu F-H, Lin H-H, Lee D-J, Chen Y-F, Lin C-S. AI Models for Predicting Readmission of Pneumonia Patients within 30 Days after Discharge. Electronics. 2022; 11(5):673. https://doi.org/10.3390/electronics11050673
Chicago/Turabian StyleHsu, Jiin-Chyr, Fu-Hsing Wu, Hsuan-Hung Lin, Dah-Jye Lee, Yung-Fu Chen, and Chih-Sheng Lin. 2022. "AI Models for Predicting Readmission of Pneumonia Patients within 30 Days after Discharge" Electronics 11, no. 5: 673. https://doi.org/10.3390/electronics11050673
APA StyleHsu, J.-C., Wu, F.-H., Lin, H.-H., Lee, D.-J., Chen, Y.-F., & Lin, C.-S. (2022). AI Models for Predicting Readmission of Pneumonia Patients within 30 Days after Discharge. Electronics, 11(5), 673. https://doi.org/10.3390/electronics11050673







