Abstract
In recent years, telehealthcare systems (TSs) have become more and more widespread, as they can contribute to promoting the continuity of care and managing chronic conditions efficiently. Most TSs and nutrition recommendation systems require much information to return appropriate suggestions. This work proposes an ontology-based TS, namely HeNuALs, aimed at fostering a healthy diet and an active lifestyle in older adults with chronic pathologies. The system is built on the formalization of users’ health conditions, which can be obtained by leveraging existing standards. This allows for modeling different pathologies via reusable knowledge, thus limiting the amount of information needed to retrieve nutritional indications from the system. HeNuALs is composed of (1) an ontological layer that stores patients and their data, food and its characteristics, and physical activity-related data, enabling the inference a series of suggestions based on the effects of foods and exercises on specific health conditions; (2) two applications that allow both the patient and the clinicians to access the data (with different permissions) stored in the ontological layer; and (3) a series of wearable sensors that can be used to monitor physical exercise (provided by the patient application) and to ensure patients’ safety. HeNuALs inferences have been validated considering two different use cases. The system revealed the ability to determine suggestions for healthy, adequate, or unhealthy dishes for a patient with respiratory disease and for a patient with diabetes mellitus. Future work foresees the extension of the HeNuALs knowledge base by exploiting automatic knowledge retrieval approaches and validation of the whole system with target users.
1. Introduction
Telehealthcare systems (TSs) exploit information and communications technologies to provide clinical services remotely, and their advantages have been described in several works since the early 2000s. TSs can extend access to health care in particular geographical conditions such as rural locations and can grant medical consultation to those segments of the population that may not be able to afford traveling [1]. In a context characterized by a global increase of chronic conditions and a growing base of an aging population (also characterized by different comorbidities), healthcare systems are burdened by a growing demand, and in some Western countries, healthcare costs are rising considerably. From the social and economic perspective, it is highlighted how TSs can help reduce the costs of national healthcare systems while extending the provision of services to more of the population [2].
Moreover, TSs represent a promising response in emergency situations such as the recent SARS-CoV-2 pandemic, providing a safer environment both for patients and clinical personnel for triage and first diagnosis [3] and for the management of chronic conditions [4,5]. Chronic patients, in fact, need continuous care and support to monitor the progress of disease in order to reduce the occurrence of secondary conditions and exacerbations, thus increasing the quality of life and reducing mortality rates [6]. TSs represent powerful tools to implement effective continuity of care and therefore improve the management of chronic patients. Moreover, the management of chronic diseases often consists of a multi-domain intervention (e.g., physical training, nutritional advice, and psychological support) and therefore requires an approach that involves different professional experts. Regarding this, TSs may help in strengthening the patient–doctor relationship but also may facilitate the connection between different clinical experts with an overall positive impact on the quality of care.
In addition, with researchers’ attention focusing on different and novel artificial intelligence (AI) techniques in internet of things context, TSs have become smarter, as they have become able to leverage the analysis of data coming from different sources to adapt types of therapy and promptly detect critical situations [7].
However, much research focuses on data-driven AI techniques, which may lack sufficient transparency for clinical personnel. Such an aspect may hinder AI’s adoption in healthcare, as the “black box” model may appear unreliable and unclear for many [8]. Therefore, one of the needs for an explainable AI (xAI) consists of addressing some of the issues arising from the adoption of AI-based tools in diagnosis, recommendations, and predictions, with the aim of creating a more understandable, reliable, and interpretable paradigm [9]. Furthermore, several sources underlined that a patient’s clinical history carries paramount importance in diagnosis [10,11], thus making “black box” and solely data-driven AI approaches to TSs potentially biased. A promising approach aimed at leveraging patient’s clinical history and monitoring his/her disease status via a TS consists in the use of knowledge bases, which can enrich black-box models with transparent ones [9].
In this regard, Semantic Web technologies offer the opportunity to exploit formal knowledge bases to capture relevant domain knowledge and reason over it. In fact, on the one hand, semantic modeling of information can foster data interoperability and provide a shared knowledge base of concepts and their relations [12]. On the other hand, these AI technologies can exploit monotonic reasoning techniques and rules, thus enabling the elicitation of new pieces of information.
This work exploits such advantages of semantics to devise the architecture of a prototypical TS named “Health and Nutrition Active Lifestyle” (HeNuALs), aimed at fostering a healthy diet and an active lifestyle in older adults with chronic conditions by intervening in the “modifiable risk factors”. Both diet and an active lifestyle have been proven to be effective in reducing the effects of all those factors contributing to increased mortality rates and disease incidence among older adults [13]. They are thus fundamental for the appropriate management of several pathologies, including chronic noncommunicable diseases (NCDs). An unhealthy diet and physical inactivity indeed increase the risk of death in patients with NCDs and also negatively influence their quality of life by often inducing a condition of disability. The Global Burden of Disease estimated that in 2017, over 16 million Disability-Adjusted Life Years (DALYs, which is defined by the WHO as “a time-based measure that combines years of life lost due to premature mortality, and years of life lost due to time lived in states of less than full health” [14]) are accounted to unhealthy diets, and over 2.1 million DALYs are due to low physical activity [15]. A lower quality of life and the anticipated occurrence of disabling conditions not only have a direct impact on the patients but also have economic and social impacts on national healthcare systems. For all these reasons, it is crucial to implement solutions such as TSs that are able to reduce such an impact in an effective way.
The remainder of this paper is organized as follows. Section 2 presents some of the relevant research in this field. Section 3 describes the architecture of HeNuALs in each of its components. Section 4 introduces two use cases for testing the inferences produced by the ontology. Section 5 addresses some limitations of the proposed approach, while Conclusions highlights the main contribution of this work.
2. Related Work
As mentioned in the previous section, Semantic Web technologies are adopted in TSs with two main aims. The first consists of easing seamless data interoperability among health stakeholders, in particular in the Electronic Health Record domain [16,17], while the second regards the possibility for ontologies to serve as clinical and expert-based backbones for many Decision Support Systems [18,19]. Moreover, the reasoning processes exploited by this technology has the characteristics envisaged by xAI since they are based on inference [20], thus allowing humans to understand the reasons behind some deductions.
The adoption of ontology-based Decision Support Systems has been widely investigated in many health-related fields, such as Ambient Assisted Living [21,22], continuity of care [23], physical rehabilitation [24], and its extension to the area of supporting the nutrition of different types of patients, and enhancing their physical conditions can potentially help patients in enhancing their quality of life. However, very often, these two sides—nutrition and physical condition enhancement—are treated separately, although in clinical practice, these aspects are complementary and strongly intertwined.
Most of the systems dedicated to nutrition that can be traced in the literature encompass the means to model some nutrient-related information (e.g., [25,26]) to provide end users with insight on what they are eating. Other examples are more focused on prescribing a diet according to user’s preferences and clinical needs [27,28]. Only a limited number of works combine nutritional aspects with physical exercise [29,30]. With regard to systems that exploit ontologies to provide tailored nutritional recommendations in a health context, Espín et al. [31] proposed NutElCare, an ontology-based recommender system that aids older adults in creating their own diet plans according to the needs related to the aging process. NutElCare adds a learning layer that leverages automated inferences to extract users’ behavior patterns and compose tailored recommendations. Similarly, the ProTrip recommender system analyzes users’ preferences and interests to generate personalized food recommendations [32]. Users are compared to other similar users to filter the recommendations together with healthy recommendations. Bianchini et al. [33] shifted the perspective by proposing a set of menus to users, leveraging prescriptions and his or her ideal nutritional behavior. The PREFer system filters recipes according to their features, generates candidate menus, and then refines and ranks them according to the healthiest options for the requesting user. Additionally, Agapito et al. [34] relied on users’ profiles to generate food recommendations, which can also take into account chronic diseases and health conditions.
Different from other semantic-based solutions, HeNuALs does not need to rely on a user’s profile; the system requires the user’s health condition, which is assessed by the clinical personnel using two World Health Organization standards. In this way, the clinical information is made interoperable [19], and the user’s health condition can be generated by leveraging the expertise of different clinical professionals. Moreover, HeNuALs is thought to be a TS able to foster cooperation between patients and clinicians. While it can be adopted by patients as a tool to plan their daily diets (providing reasonable and tailored suggestions), the clinical personnel have the possibility to adjust the diet and check its content. Finally, the proposed system combines the nutritional advice with recommendations for physical activities with the aim of tackling two modifiable risk factors for different chronic conditions.
3. HeNuALs Architecture
The architecture of the HeNuALs system, represented in Figure 1, is composed of the following core elements: the ontological layer stored in a repository, the HeNuALs applications, and the hardware elements for accessing its functionalities and for monitoring physical activity. The ontological layer, which stores the data on the user’s health condition and all related information on diet and physical activity, communicates with the HeNuALs applications in two ways. The first allows for retrieving customized settings on diet and exercise, and the second enables storage of the training results and allows the clinical personnel to modify the settings for diet and exercise for the specific patient. The wearable device(s), needed to monitor physical activity, directly transmit data to the patient application through ad hoc developed communication protocols.
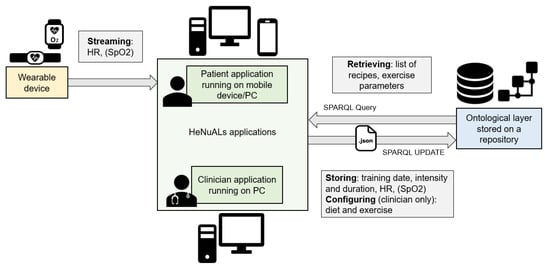
Figure 1.
A graphical representation of the HeNuALs system architecture.
3.1. Ontological Layer
HeNuALs relies on an ontological layer to formalize relevant information related to patients, foods, and exercise. The ontological layer is developed with World Wide Web Consortium-endorsed languages (e.g., Resource Description Framework (RDF) [35] and Ontology Web Language (OWL)) [36]. Following the most common practices for collaborative ontology development in the healthcare domain [37], the HeNuALs ontological layer foresees the reuse of existing models or knowledge sources (as further illustrated). The result of this development process consists of an ontology encompassing three main modules and a set of rules (developed with the Semantic Web Rule Language (SWRL) [38]), each of which is described in the following subsections. The HeNuALs ontology is stored in a semantic repository (Stardog Knowledge Graph [39]), which can be queried with SPARQL [40]. Figure 2 represents the ontological layer by means of the Unified Modeling Language (UML) and illustrates the classes and their attributes (datatype properties) together with the relationships held among classes (object properties), and Figure 3 reproduces a snapshot of the HeNuALs ontology TBox.
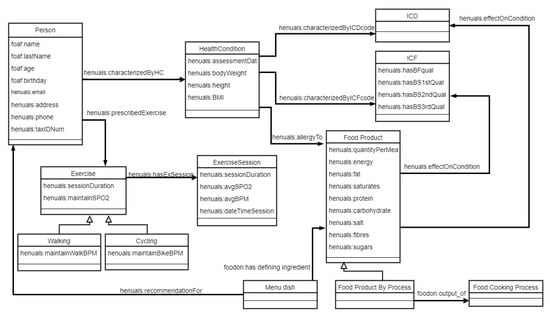
Figure 2.
A UML representation of the HeNuALs ontology layer. OWL classes are represented as UML classes, datatype properties are represented as UML attributes, and object properties are represented as UML associations among classes.
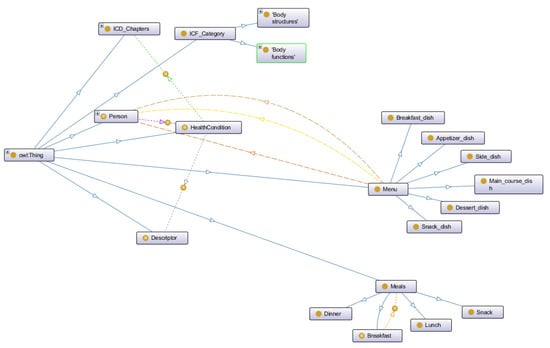
Figure 3.
A graphical representation of an excerpt of the HeNuALs ontology, developed with the Protégé ontology editor [41].
3.1.1. Patients and Health Conditions
Patients are described by means of their personal data (full name, place of birth, age, contacts, etc.), reusing the Friend of A Friend (FOAF) vocabulary [42] with a few additions (namely henuals:TaxIDNum, henuals:address, henuals:email, and henuals:phone). This personal information is stored in a separate module of the ontology. Each patient, represented as an OWL individual, is linked to his or her health condition (also represented as an individual) via an object property. To provide a first protection layer against risks related to personal data security and to ensure patients’ privacy, each patient is identified by a henuals:ID. In this way, clinical personnel can see the patient’s health condition and gender, together with his or her ID, and only when strictly necessary, they can access the ontology module that stores the patient’s personal data using the ID as a key for patient identification.
The description of a health condition takes advantage of two WHO standard classifications: the International Classification of Diseases and Related Health Problems (ICD-11) [43] and the International Classification of Functioning, Disability and Health (ICF) [44]. These standards were developed with the aim of fostering health information interoperability among clinical stakeholders, and they can provide a hierarchy of diseases and a framework for describing the interactions between a person’s health status and the environment in which he or she lives, respectively. While the ICD leverages a classification of illnesses for diagnostic purposes, the ICF is more focused on providing a functional description of a person’s health status. Both classifications share a similar taxonomical structure, as they split their domains of knowledge into chapters. Each chapter is further deepened in categories, which in turn can be further detailed. The ICD consists of more than 10,000 categories (grouped into 24 chapters), each representing a family of diseases, while the ICF adopts more than 1400 categories, each representing either a domain of the body (Body functions and Body structure chapters), the environment (Environmental factors chapter), or a combination of both (Activities and participation chapter), to be evaluated under the functional point of view. Due to their diffusion among health stakeholders, both classifications have been represented into two ontologies, of which this module reuses some chapters and their related categories (5: Endocrine, nutritional and metabolic disorders; 12: Diseases of the respiratory system; 13: Diseases of the digestive system for the ICD; and all of the Body functions and Body structures chapters for the ICF).
This module associates to each health condition relevant information like henuals:bodyWeight, henuals:height, henuals:BMI (body mass index), and physical exercises that are recommended by the clinicians to the patient. Clinical personnel can also use a set of datatype properties to specify the recommended amount of nutrients for a health condition (e.g., the quantities in grams of dietary fibers, carbohydrates, or saturates). Finally, HeNuALs provides the means to list the allergens that may cause a reaction in the person’s health condition (via the henuals:allergyTo object property, whose range is limited to the 14 allergens of food products and their derivatives identified by EU Regulation 1169/2011 [45]).
3.1.2. Foods, Their Effects, and Diets
This second module describes foods by reusing Food Ontology (FoodOn) [46], a model formalizing more than 9600 food products. FoodOn provides the means to describe a food product under different points of view; a bag of potato crisps can be classified as foodon:potato crisps from a potato slice but also as foodon:food(fried), thus representing the food product as the result of the frying cooking process. Moreover, it can be represented as an individual belonging to the foodon:potato crisps class, a subclass of foodon:snack (potato-based). This taxonomical structure allows FoodOn to describe food products while taking into account the transformations to which the ingredients are subjected. In this way, the ontology also enables the representation of more complex food products (i.e., dishes composed of many ingredients). For example, for a rosemary risotto dish, foodon:has the defining ingredient of some foodon:brown rice and is the foondon:output of a foodon:boiling cooking process. This module also provides a few datatype properties to quantify the portion of a dish to be prescribed in a diet (henuals:quantityPerMeal) and the quantities of deriving nutrients (henuals:calories, henuals:fats, henuals:saturates, henuals:carbohydrates, henuals:sugars, henuals:proteins, henuals:fibers, and henuals:salt).
HeNuALs leverages clinical literature knowledge [47,48,49,50] to formalize the relationships between food products and specific diseases. A set of properties (henuals:advisedFor, henuals:positiveEffectOn, henuals:neutralEffectOn, henuals:unhealthyEffectOn, and subproperties of the object property henuals:effectOnCondition) models the effects that foods have on specific health conditions. For example, it can be stated that milk cream has a henuals:negativeEffectOn health conditions characterized by icd:Essential hypertension, while foodon:tomato(raw) henuals:isAdvisedFor the same health condition. The HeNuALs knowledge base currently models 32 dishes (listed in Section 4.3).
Finally, HeNuALs allows for specifying the diet plan for each day. Days, represented as individuals, are linked to henuals:Meals individuals, which are connected through the henuals:selectedDish property to the specific dishes comprising a meal. For example, it is possible to represent a lunch composed of a portion of rosemary risotto, endive salad, grilled chicken breast, and an apple. In its current version, HeNuALs provides the means to represent five main daily meals (breakfast, lunch, dinner, and two snacks), although other meals can be added to the ontology.
3.1.3. Exercise and Its Session
An application ontology models the exercises that the clinicians could prescribe to the patients. The physical activity program is defined considering the American College of Sports Medicine (ACSM) guidelines and focusing in particular on four exercise parameters: frequency, intensity (%HRmax), duration (in minutes), and type [51]. Regarding the type of exercise, HeNuALs currently models two types of exercise: cycling on a cycle-ergometer, which can be performed at home, and walking outdoors. Both exercises require the patient to perform the activity while wearing a sensor device(s) for monitoring some relevant parameters (e.g., oxygen saturation (SpO2) and heart rate (HR)) depending on his or her pathology. The frequency, intensity, and time of the exercise session can also be customized for each patient and modeled in this module. The recommended values can be modified by clinical personnel to optimally match the patient’s needs. The recommended frequency of exercise varies between 3 and 5 days per week, while the time of exercise, which represents the duration of each session, can range from 75 to 150 minutes per week. The exercise intensity HRtarget is defined in HeNuALs as a percentage of the maximum heart rate of the individual (obtained as 208 − (0.7 × age), as specified in [52]). HRtarget is selected in order to elicit light, moderate, or vigorous exercise, depending on the patient’s baseline condition. Moreover, clinical personnel can specify, for each exercise type, the parameters’ thresholds required for a patient to keep safe, and these are summarized as follows. The HR should not exceed more than 30% of HRtarget, and the SpO2 should not go below 90%, thus assuring that a significant exercise-induced desaturation does not occur [53]. Real-time monitoring of the thresholds is performed via a patient application (retrieving thresholds and exercise settings data from the ontology once before starting the exercise session), which receives data regarding HRtarget and SpO2 and provides the average values for each parameter at the end of the session.
Each exercise session is stored in this module as an individual, with datatype properties detailing the date of training, average HR and SpO2 maintained, and the duration of the session. HeNuALs relies on an already-tested solution for supporting older adults in performing physical exercise. This solution adopts commercial devices (a cycle ergometer and a chest band) interfaced with the system as described in [54,55].
3.1.4. Inferences and Querying
Using the SWRL, the HeNuALs ontology layer is able to draw general inferences to help clinicians determine food recommendations based on the effects of foods on specific health conditions and patients to support the implementation of a healthy diet plan [56]. Rule inferences generate triplets, with a dish as subject, a recommendation as an object property (henuals:unhealthySuggestionFor, :adequateSuggestionFor, or :healthySuggestionFor), and a patient as an object. HeNuAL’s SWRL rules also allow for inferring some exercise settings and can be grouped into six sets:
- A set of rules leverages the descriptions of foods and their effects on specific health conditions to draw inferences on more complex foods (dishes). A dish containing at least one ingredient having a negative effect for a specific patient is not suggested as a suitable dietary option. Similarly, a dish composed of healthy ingredients but processed with a cooking method incompatible with the patient’s health condition is also not suggested.
- A second set of rules allows for detecting whether a dish composed of many ingredients is a suitable food option for a person who can experience an allergic reaction to a specific food (e.g., 4A80.1 Bronchospasm provoked by allergy to food substance) by checking its ingredients and matching them with the list of allergies characterizing such an individual.
- The third set of rules leverages the nutrient prescriptions prescribed by the clinicians and the information regarding nutrients for each dish. It is possible to infer as unhealthy options those foods with specific values for some nutrients (e.g., a fruit salad containing raisins and honey is an unsuitable option for patients with diabetes due to the high sugar amount).
- SWRL rules can also provide suggestions regarding the substitution of allergen food products with other products using the foodon:has food substance analog relation.
- SWRL rules also suggest the daily water intake for an individual (formalizing EFSA’s recommendations reported in [57]) and the daily calorie intake (according to [47]).
- SWRL rules determine some thresholds for exercise programs (HRtarget and SpO2) according to the ACSM recommendations detailed in the previous subsection.
By using SPARQL in combination with the inferences produced by the SWRL, it is possible to check whether a daily diet plan foresees unhealthy suggestions or a modeled dietary daily plan foresees a calorie intake higher than the one recommended. Similarly, SPARQL can be used to calculate the total amount of single nutrients (fats, sugars, carbohydrates, fibers, etc.) foreseen in the day and check whether it follows the recommendations provided by clinicians. This feature covers a pivotal role for those conditions that (like type 2 DM) require patients to follow a careful and strict nutrient regime.
3.2. Hardware
The HeNuALs architecture involves the following hardware components: a mobile device or a computer station running the HeNuALs applications and a wearable sensor device for monitoring exercise sessions. As shown in Figure 1, the clinician application is PC-based, while the patient application runs both on mobile devices with the Android OS and on Windows PCs. According to the use case, either one or both of these devices can be part of the system. With respect to the physical activity, those patients who are prescribed walking indoors may prefer using a smartphone for its portability, while those who carry out indoor cycling may rather use a computer station, which has a wider screen.
Optional elements may be added to the system in specific cases. For example, depending on the exercise type a patient is prescribed to perform, he or she may also need a physical device for performing the exercise (e.g., a cycle ergometer in the case of cycling indoors). A second example refers to the “recipe” functionality (described and validated in detail in [22]). In this case, the application can be accessed with any plain surface of the house (e.g., a table, kitchen worktop, or wall) by using a finger-touch projector connected to the computer station running HeNuALs. The possibility to project the GUI offers people the chance of accessing it even while cooking, thus excluding the risk of damaging the electronic devices. Finally, to perform the physical activity in safe conditions, the system includes one or more wearable devices. The device(s) is (are) selected depending on the different use case, with only the requirement of being able to transmit data to a third-party application (i.e., HeNuALs).
3.3. Communication
To send data from HeNuALs applications to the ontological layer, middleware was developed by means of Java scripts [58]. By using a SPARQL UPDATE query pattern, selected data (provided to the middleware in the form of a JSON file) from the applications (e.g., training data produced by the patient application) are sent to the ontology to complete the modeling of an individual representing the exercise session. Using SPARQL, HeNuALs applications (described in Section 3.4 and Section 3.5) can retrieve information to be shown to the end users and the clinicians. Instead, the updating and modification of the information contained in the ontology (especially those pertaining to the health condition) is a prerogative of the clinical personnel.
3.4. Patient Application
The HeNuALs patient application allows the patient to follow the prescribed recommendations on diet and physical activity. The interaction with the HeNuALs patient application is performed via a Graphical User Interface (GUI) developed with Unity 3D [59] as a further development of the home interactive controller described in [60]. This GUI has been developed to be user-friendly and intuitive so as to allow everyone (including older adults with scarce familiarity with technology) to access the HeNuALs data.
Aside from the food-related information mentioned above, HeNuALs integrates the “recipe book” functionality (described in detail in [22]), providing a step-by-step guide to preparing dishes. The first step shows the list of ingredients necessary for preparation, while the following steps are focused on the actions required to complete the dish. Each step is detailed with a textual description and a picture or a short video to support older adults through the most difficult passages of preparation (the video can be stopped, paused, and played back using buttons located under the video frame). Patients can also interact with HeNuALs to model their daily dietary plans.
The list of recipes suitable for a specific person is retrieved via SPARQL query and determined by SWRL rules, according to his or her health condition and the suggestions inferred (Section 3.1.4). These suggestions are illustrated in textual form via the GUI.
Another functionality allows the patient to carry out the physical activity prescribed by the clinicians (with thresholds provided by inferences). The GUI shows the information on the type, frequency, time, and intensity of the exercise. Before starting the exercise, the user wears the monitoring device and, through a button on the GUI, establishes the connection with the sensor. During the session, the heart rate and, where applicable, SpO2 measurements are transmitted and elaborated in order to control the exercise intensity. The value of the HR is displayed on the GUI shown to the user, and a colored circle indicates whether the patient is maintaining the target intensity (green) or not (red). Based on the values retrieved by the wearable device, the application generates a warning, asking the patient to interrupt the exercise when the safety conditions, as formalized in the ontological layer (Section 3.1.3), are not satisfied.
3.5. HeNuALs Clinician Application
The application for clinical personnel allows for inserting and modifying a patient’s data and health condition, as well as prescribing the type of exercise (and its setting) and specifying nutritional recommendations (quantity of nutrients per day). This application also allows clinicians to retrieve data from the exercise sessions so that they can keep track of the patient’s progress, also identifying those not respecting the specified parameters. Based on the data retrieved, clinicians are able to either modify the program or identify potentially dangerous situations in advance. Clinicians can retrieve and control the daily diet plan inserted by their patients (via SPARQL) and modify them if needed.
This application is currently under development. Since this component of HeNuALs requires clinicians’ participation in the different development phases and cannot neglect their direct involvement in the validation, the system currently relies on a prototype.
4. Use Cases
The validation of the HeNuALs semantic layer was performed by considering two different use cases, each one framing an individual with a health issue that would benefit from an appropriate lifestyle intervention. The purpose of this validation was to assess whether or not the inferences produced by reasoners provided safe and sound suggestions for users (according to what was prescribed in the literature for the two specific chronic diseases addressed here) and to verify that daily plans introduced by users fell under the prescriptions modeled into the ontology. The two use cases, provided by clinical partners cooperating in this project, addressed two common chronic conditions: COPD and type 2 Diabetes Mellitus (DM).
4.1. James: A Man with COPD
The first use case was represented by James, a 71-year-old man with moderate COPD (stage II according to the Global Initiative for Obstructive Lung Disease). His health condition was formalized in the ontology layer and described as represented in Table 1.

Table 1.
ICD code and ICF codes and qualifiers describing the health condition of James.
COPD (CA22) had an impact on his respiratory system (s43011) and both the respiratory and limb muscles (s7702). As a consequence of the disease, James badly tolerated exercise (b455) and experienced dyspnea and fatigue (b460) while performing activities of daily living (ADLs) of low or moderate intensity (e.g., going out for shopping or doing housework). James recently lost weight, and his BMI was 20.5 (weight = 62 kg, height = 1.74 m), thus indicating a risky condition that required attention (b530). Unintentional weight loss was due to several reasons, one being the loss of appetite (b1302), which is common in older adults and worsened by the fact that James was living alone and, therefore, not very motivated in preparing meals and following clinicians’ recommendations. The second reason for weight loss was related to increased energy requirements as a consequence of the disease, which was not properly balanced by his dietary intake. A nutritional supplement was, therefore, recommended for James [49]; his daily caloric intake amounted to 2.790 Kcal, and his water intake was estimated to be 2.5 liters/day (inferred via the SWRL rule).
In order to recover his exercise tolerance and strengthen his lower limb muscles, James was prescribed to follow a pulmonary rehabilitation program [61]. The physical training consisted of a 20-minute session of aerobic exercise for 5 days per week. Given his profile, as formalized in the ontology, James was suggested to perform walking outdoors at a light to moderate intensity, defined as 60% of his maximum heart rate [51]. Based on James’s health condition, it was important for therapists to track his exercise-induced heart rate and oxygen saturation levels during exercise. The optimal wearable device for James was, therefore, a wrist-worn pulse oximeter.
4.2. Grace: A Woman with Type 2 Diabetes Mellitus
The second use case depicted Grace, who was 67 years old and had been recently (<1 year prior) diagnosed with type 2 Diabetes Mellitus (DM) (5A11) (Table 2). The disease influenced her cardiovascular system (s410) and urinary system (s6100). As a consequence, Grace suffered from high blood pressure and decreased cardiovascular functionality (b410, b415, and b420). She also reported impaired functionality in metabolic (b450) and urinary excretory functions (b610). As a common effect of DM, Grace, due to the high blood sugar levels, was also more susceptible to developing infections (b435). In addition, her BMI equaled 30.07 (weight = 77 kg, height = 1.60 m), thus indicating a condition of obesity (b530). Her obesity, combined with the other comorbidities, resulted in a reduced exercise tolerance (b455), which prevented Grace from independently performing the more demanding ADLs.

Table 2.
ICD code and ICF codes and qualifiers describing the health condition of Grace.
As suggested by the global guidelines for the management of DM type 2 in older adults, combining physical activity with nutritional therapy can help in promoting weight loss, thus improving physical performance and reducing cardiometabolic risk [48]. Considering her condition, Grace’s nutritionist prescribed a 1.400 Kcal/day intake (with 2 liter/day liquid intake inferred via the SWRL). Regarding physical activity, Grace should have performed cycling on a stationary bike for 40 minutes for 3 days per week at moderate intensity. In order to perform moderate physical exercise, corresponding to 70% of the maximum heart rate, she was equipped with a heart rate chest strap as part of the HeNuALs system.
4.3. Results of Use Case Processing
The two patients’ health conditions were modeled in the HeNuALs ontology layer. Reasoning (performed using Stardog semantic repository reasoning and a query engine) provided lists of dishes classified according to their effect on the specific patient’s health condition (Table 3). The reasoning process was able to successfully identify the suitable and unsuitable foods for both health conditions, according to the respective nutrition guidelines [49]. SWRL rules allowed for determining a patient’s daily caloric intake, which took into account their impairments and their daily water intake.

Table 3.
Inferred classification of the dishes modeled in HeNuALs ontology for each of the use cases presented.
In addition, the ability to infer whether or not a diet plan was suitable for a patient was tested. As presented in Section 3.1, each patient could insert his or her daily diet plan by specifying the dishes he or she wanted to eat. Plans could contain any of the dishes modeled in HeNuALs and therefore could comply to a specific patient’s suggestions or not. Moreover, even a diet plan containing healthy or adequate suggestions may not have respected the caloric intake recommended to the patient by the clinicians. As an example, Figure 4 reports three of James’ plans:
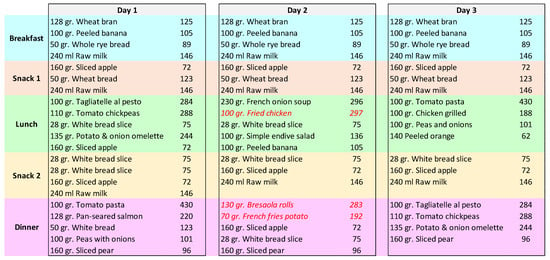
Figure 4.
A schema representing three days modeled by patient James.
- The plan envisioned for Day 1, although containing healthy or adequate food choices, exceeded the prescribed amount for James’ health condition, and therefore it was classified as a henuals:notAppropriateDayPlan;
- The plan for Day 2 contained some dishes that were inferred to be henuals:unhealthySuggestionFor. Therefore, this plan was also classified as a henuals:notAppropriateDayPlan;
- Finally, the plan for Day 3 contained healthy choices for foods, and its caloric amount was adequate to the daily intake prescribed to the patient.
5. Discussion, Limitations of This Work, and Future Work
HeNuALs TS leverages semantic knowledge bases to draw general and user-specific inferences regarding foods and their effects on health conditions and to monitor daily physical exercise.
As illustrated in Section 4, HeNuALs proved able to draw significant inferences to suggest users’ food recommendations and help them in compiling their dietary plans autonomously. Moreover, once the application for clinicians would be completed, the system would also enable clinical personnel to remotely monitor the composition of dietary plans and to intervene if necessary.
In its current state, HeNuALs is limited to only two use cases. This is due to the fact that its knowledge base has been developed mostly manually by deriving nutritional food products’ information from the scientific literature. Given the complexity of the problem (e.g., considering the presence of different metabolic phenotypes in the same patient population) and the heterogeneity of nutrition care guidelines, a future step for improving HeNuALs will consist of the validation and integration of knowledge retrieved from the literature by domain experts (i.e., clinical nutritionists and specific disease specialists). However, to increase the number of food products and consequently of dishes modeled in the ontology and thus extend the possible applications of HeNuALs both vertically (more dishes) and horizontally (more patients), the automatic population of ontology instances can be performed. Though such a process may seem like an immediate approach, its performance is still a debated issue, and many methods are currently being studied (ranging from deep learning for automatic triplet extraction to text extraction [62,63]), since no optimum method has been found. A future work should thus tackle the definition of a method to extract food-related knowledge from clinically approved databases.
With the increase in modeled food and dishes, HeNuALs could be rapidly extended to also include other types of patients with chronic conditions who may benefit from a healthy diet (e.g., patients with all the inflammatory bowel diseases, osteoporosis, or cardiovascular disorders). Moreover, HeNuALs can also be adopted for the prevention of nutrition-related conditions that are dependent on aging (e.g., malnutrition, marginal deficiency of vitamins and trace elements, or too low an intake of vitamin E and calcium [64]), and it can benefit from a healthy diet and physical exercise. Clearly, as the system will become more complex and possibly used in the clinical practice, data protection and security must be improved, taking into account all the recommendations given by European General Data Protection Regulation.
Another aspect that needs to be taken into consideration regards the reuse of the HeNuALs ontology layer. Although the ontologies presented are well-known and often reused, it is fundamental to foster information interoperability by mapping HeNuALs domain ontologies to other relevant existing models. In this way, by determining correspondences between different ontologies’ concepts and relations, information generated by HeNuALs can be made interoperable with other heterogeneous sources.
Finally, a typical drawback of TSs consists of the difficulty of providing the patient with a comprehensive physical examination. In this regard, HeNuALs makes no exception, as the health conditions contained in the ontological layer need to be updated following clinical evaluation (e.g., examinations and tests). In its current state, HeNuALs is unable to automatically acquire new information regarding modifications to the users’ health conditions. However, in the context of full information interoperability (such as the one enabled by Electronic Health Records), HeNuALs can be adapted to automatically update the patients’ conditions stored in the ontology.
6. Conclusions
This work introduces the ontological framework for a telehealthcare system dedicated to enhancing healthy nutrition and active lifestyles in the older adult population. The semantic approach allows avoiding the use of black box models, thus creating a transparent link between the input data (i.e., patient’s characteristics) and the inferred outcomes (i.e., dietary plan and physical exercise program).
In this work, the common approach (highlighted in Section 2) requiring much data, preferences, and profile(s) regarding the users is overturned. Instead of leveraging knowledge extracted from the user, it is argued here that user-specific information is not strictly necessary to provide healthy and tailored dietary suggestions. On the contrary, HeNuALs focuses on the diseases and only needs the users’ health conditions to be formalized using two widely known and adopted WHO standards, thus drawing inferences (nutritional recommendations and physical exercise indications) on the specific clinical situation comprising the users’ health conditions from a functional and pathological perspective. In this way, the proposed framework can be easily adapted for different conditions, thus including a variety of (also complex and comorbid) chronic conditions.
Author Contributions
Conceptualization, methodology, writing—original draft, and software, D.S.; writing—original draft and software, V.C.; investigation and data curation, S.A.; software and visualization, A.M.; supervision, A.T.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Regione Lombardia under the POR FESR 2014–2020 Asse Prioritario I-Call Hub Ricerca e Innovazione, project “sPATIALS3-Miglioramento delle produzioni agroalimentari e tecnologie innovative per un’alimentazione più sana, sicura e sostenibile” ID 1176485.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Weinstein, R.S.; Lopez, A.M.; Joseph, B.A.; Erps, K.A.; Holcomb, M.; Barker, G.P.; Krupinski, E.A. Telemedicine, telehealth, and mobile health applications that work: Opportunities and barriers. Am. J. Med. 2014, 127, 183–187. [Google Scholar] [CrossRef]
- Jennett, P.A.; Hall, L.A.; Hailey, D.; Ohinmaa, A.; Anderson, C.; Thomas, R.; Young, B.; Lorenzetti, D.; Scott, R.E. The socio-economic impact of telehealth: A systematic review. J. Telemed. Telecare 2003, 9, 311–320. [Google Scholar] [CrossRef]
- Smith, A.C.; Thomas, E.; Snoswell, C.L.; Haydon, H.; Mehrotra, A.; Clemensen, J.; Caffery, L.J. Telehealth for global emergencies: Implications for coronavirus disease 2019 (COVID-19). J. Telemed. Telecare 2020, 26, 309–313. [Google Scholar] [CrossRef]
- Jácome, C.; Marques, A.; Oliveira, A.; Rodrigues, L.; Sanches, I. Pulmonary telerehabilitation: An international call for action. Pulmonology 2020, 26, 335. [Google Scholar] [CrossRef] [PubMed]
- Polisena, J.; Coyle, D.; Coyle, K.; McGill, S. Home telehealth for chronic disease management: A systematic review and an analysis of economic evaluations. Int. J. Technol. Assess. Health Care 2009, 25, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.J.P.; Sidaway-Lee, K.; White, E.; Thorne, A.; Evans, P.H. Continuity of care with doctors—A matter of life and death? A systematic review of continuity of care and mortality. BMJ Open 2018, 8, e021161. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.K.; Bonnet, C.; Gyrard, A.; Da Costa, R.P.F.; Boudaoud, K. Applying Internet of Things for personalized healthcare in smart homes. In 2015 24th Wireless and Optical Communication Conference (WOCC); IEEE: Manhattan, NY, USA, 2015; pp. 164–169. [Google Scholar]
- Goodman, B.; Flaxman, S. European Union regulations on algorithmic decision-making and a “right to explanation”. AI Mag. 2017, 38, 50–57. [Google Scholar] [CrossRef]
- Arrieta, A.B.; Díaz-Rodríguez, N.; Del Ser, J.; Bennetot, A.; Tabik, S.; Barbado, A.; Garcia, S.; Gil-López, S.; Molina, D.; Benjamins, R.; et al. Explainable Artificial Intelligence (XAI): Concepts, taxonomies, opportunities and challenges toward responsible AI. Inf. Fusion 2020, 58, 82–115. [Google Scholar] [CrossRef]
- Peterson, M.C.; Holbrook, J.H.; Von Hales, D.; Smith, N.; Staker, L. Contributions of the history, physical examination, and laboratory investigation in making medical diagnoses. West. J. Med. 1992, 156, 163. [Google Scholar] [CrossRef]
- Roshan, M.; Rao, A. A study on relative contributions of the history, physical examination and investigations in making medical diagnosis. J. Assoc. Physicians India 2000, 48, 771–775. [Google Scholar]
- Gruber, T.R. A translation approach to portable ontology specifications. Knowl. Acquis. 1993, 5, 199–220. [Google Scholar] [CrossRef]
- Haveman-Nies, A.; de Groot, L.P.G.M.; Burema, J.; Cruz, J.A.A.; Osler, M.; van Staveren, W.A. Dietary quality and lifestyle factors in relation to 10-year mortality in older Europeans: The SENECA study. Am. J. Epidemiol. 2002, 156, 962–968. [Google Scholar] [CrossRef]
- World Health Organization. Disability-Adjusted Life Years (DALYs)-The Global Health Observatory. Available online: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/158 (accessed on 21 August 2021).
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Adel, E.; El-Sappagh, S.; Barakat, S.; Elmogy, M. Ontology-based electronic health record semantic interoperability: A survey. In U-Healthcare Monitoring Systems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 315–352. [Google Scholar]
- Roehrs, A.; da Costa, C.A.; da Rosa Righi, R.; Rigo, S.J.; Wichman, M.H. Toward a model for personal health record interoperability. IEEE J. Biomed. Health Inform. 2018, 23, 867–873. [Google Scholar] [CrossRef]
- Blomqvist, E. The use of Semantic Web technologies for decision support–A survey. Semant. Web 2014, 5, 177–201. [Google Scholar] [CrossRef]
- Spoladore, D. Ontology-based decision support systems for health data management to support collaboration in ambient assisted living and work reintegration. In Working Conference on Virtual Enterprises; Springer: Berlin/Heidelberg, Germany, 2017; pp. 341–352. [Google Scholar]
- Turhan, A.-Y. Description logic reasoning for semantic web ontologies. In Proceedings of the International Conference on Web Intelligence, Mining and Semantics, Sogndal, Norway, 25–27 May 2011; pp. 1–5. [Google Scholar]
- Chen, L.; Nugent, C.; Mulvenna, M.; Finlay, D.; Hong, X. Semantic smart homes: Towards knowledge rich assisted living environments. In Intelligent Patient Management; Springer: Berlin/Heidelberg, Germany, 2009; pp. 279–296. [Google Scholar]
- Spoladore, D.; Mahroo, A.; Trombetta, A.; Sacco, M. DOMUS: A domestic ontology managed ubiquitous system. J. Ambient Intell. Humaniz. Comput. 2021, 1–16. [Google Scholar] [CrossRef]
- Spoladore, D.; Arlati, S.; Colombo, V.; Modoni, G.; Sacco, M. A semantic-enabled smart home for AAL and continuity of care. In IoT in Healthcare and Ambient Assisted Living; Springer: Berlin/Heidelberg, Germany, 2021; pp. 343–371. [Google Scholar]
- Arlati, S.; Spoladore, D.; Mottura, S.; Zangiacomi, A.; Ferrigno, G.; Sacchetti, R.; Sacco, M. Analysis for the design of a novel integrated framework for the return to work of wheelchair users. Work 2018, 61, 603–625. [Google Scholar] [CrossRef]
- Bailoni, T.; Dragoni, M.; Eccher, C.; Guerini, M.; Maimone, R. Healthy lifestyle support: The perkapp ontology. In OWL: Experiences and Directions–Reasoner Evaluation; Springer: Berlin/Heidelberg, Germany, 2016; pp. 15–23. [Google Scholar]
- Chi, Y.-L.; Chen, T.-Y.; Tsai, W.-T. A chronic disease dietary consultation system using OWL-based ontologies and semantic rules. J. Biomed. Inform. 2015, 53, 208–219. [Google Scholar] [CrossRef]
- Cioara, T.; Anghel, I.; Salomie, I.; Barakat, L.; Miles, S.; Reidlinger, D.; Taweel, A.; Dobre, C.; Pop, F. Expert system for nutrition care process of older adults. Future Gener. Comput. Syst. 2018, 80, 368–383. [Google Scholar] [CrossRef]
- Lee, C.-S.; Wang, M.-H.; Lan, S.-T. Adaptive personalized diet linguistic recommendation mechanism based on type-2 fuzzy sets and genetic fuzzy markup language. IEEE Trans. Fuzzy Syst. 2014, 23, 1777–1802. [Google Scholar] [CrossRef]
- Faiz, I.; Mukhtar, H.; Qamar, A.M.; Khan, S. A semantic rules & reasoning based approach for Diet and Exercise management for diabetics. In 2014 International Conference on Emerging Technologies (ICET); IEEE: Manhattan, NY, USA, 2014; pp. 94–99. [Google Scholar]
- Fudholi, D.H.; Maneerat, N.; Varakulsiripunth, R. Ontology-based daily menu assistance system. In 2009 6th International Conference on Electrical Engineering/Electronics, Computer, Telecommunications and Information Technology; IEEE: Manhattan, NY, USA, 2009; Volume 2, pp. 694–697. [Google Scholar]
- Espín, V.; Hurtado, M.V.; Noguera, M. Nutrition for Elder Care: A nutritional semantic recommender system for the elderly. Expert Syst. 2016, 33, 201–210. [Google Scholar] [CrossRef]
- Subramaniyaswamy, V.; Manogaran, G.; Logesh, R.; Vijayakumar, V.; Chilamkurti, N.; Malathi, D.; Senthilselvan, N. An ontology-driven personalized food recommendation in IoT-based healthcare system. J. Supercomput. 2019, 75, 3184–3216. [Google Scholar] [CrossRef]
- Bianchini, D.; De Antonellis, V.; De Franceschi, N.; Melchiori, M. PREFer: A prescription-based food recommender system. Comput. Stand. Interfaces 2017, 54, 64–75. [Google Scholar] [CrossRef]
- Agapito, G.; Simeoni, M.; Calabrese, B.; Caré, I.; Lamprinoudi, T.; Guzzi, P.H.; Pujia, A.; Fuiano, G.; Cannataro, M. DIETOS: A dietary recommender system for chronic diseases monitoring and management. Comput. Methods Programs Biomed. 2018, 153, 93–104. [Google Scholar] [CrossRef]
- Pan, J.Z. Resource description framework. In Handbook on Ontologies; Springer: Berlin/Heidelberg, Germany, 2009; pp. 71–90. [Google Scholar]
- Antoniou, G.; Van Harmelen, F. Web ontology language: Owl. In Handbook on Ontologies; Springer: Berlin/Heidelberg, Germany, 2004; pp. 67–92. [Google Scholar]
- Spoladore, D.; Pessot, E. Collaborative Ontology Engineering Methodologies for the Development of Decision Support Systems: Case Studies in the Healthcare Domain. Electronics 2021, 10, 1060. [Google Scholar] [CrossRef]
- Horrocks, I.; Patel-Schneider, P.F.; Boley, H.; Tabet, S.; Grosof, B.; Dean, M. SWRL: A semantic web rule language combining OWL and RuleML. W3C Memb. Submiss. 2004, 21, 1–31. [Google Scholar]
- Union, S. Stardog-the Enterprise Knowledge Graph Platform. Available online: https://www.stardog.com/categories/knowledge-graph/ (accessed on 29 August 2021).
- Quilitz, B.; Leser, U. Querying distributed RDF data sources with SPARQL. In European Semantic Web Conference; Springer: Berlin/Heidelberg, Germany, 2008; pp. 524–538. [Google Scholar]
- Tudorache, T.; Noy, N.F.; Tu, S.; Musen, M.A. Supporting collaborative ontology development in Protégé. In International Semantic Web Conference; Springer: Berlin/Heidelberg, Germany, 2008; pp. 17–32. [Google Scholar]
- Graves, M.; Constabaris, A.; Brickley, D. Foaf: Connecting people on the semantic web. Cat. Classif. Q. 2007, 43, 191–202. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Diseases for Mortality and Morbidity Statistics (11th Revision). Available online: https://www.who.int/classifications/classification-of-diseases (accessed on 29 August 2021).
- World Health Organization. International Classification of Functioning, Disability and Health. Available online: https://www.who.int/standards/classifications/international-classification-of-functioning-disability-and-health (accessed on 29 August 2021).
- Fransvea, A.; Celano, G.; Pagliarone, C.N.; Disanto, C.; Balzaretti, C.; Celano, G.V.; Bonerba, E. Food labelling: A brief analysis of European Regulation 1169/2011. Ital. J. Food Saf. 2014, 3, 1703. [Google Scholar] [CrossRef][Green Version]
- Dooley, D.M.; Griffiths, E.J.; Gosal, G.S.; Buttigieg, P.L.; Hoehndorf, R.; Lange, M.C.; Schriml, L.M.; Brinkman, F.S.; Hsiao, W.W. FoodOn: A harmonized food ontology to increase global food traceability, quality control and data integration. NPJ Sci. Food 2018, 2, 1–10. [Google Scholar] [CrossRef]
- Collins, P.F.; Yang, I.A.; Chang, Y.-C.; Vaughan, A. Nutritional support in chronic obstructive pulmonary disease (COPD): An evidence update. J. Thorac. Dis. 2019, 11, S2230. [Google Scholar] [CrossRef]
- Mann, J.; De Leeuw, I.; Hermansen, K.; Karamanos, B.; Karlström, B.; Katsilambros, N.; Riccardi, G.; Rivellese, A.; Rizkalla, S.; Slama, G.; et al. Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 373–394. [Google Scholar] [CrossRef]
- Rawal, G.; Yadav, S. Nutrition in chronic obstructive pulmonary disease: A review. J. Transl. Intern. Med. 2015, 3, 151. [Google Scholar] [CrossRef]
- Schols, A.M.; Ferreira, I.M.; Franssen, F.M.; Gosker, H.R.; Janssens, W.; Muscaritoli, M.; Pison, C.; Rutten-van Mölken, M.; Slinde, F.; Steiner, M.C.; et al. Nutritional assessment and therapy in COPD: A European Respiratory Society statement. Eur. Respir. J. 2014, 44, 1504–1520. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef]
- Kim, C.; Park, Y.B.; Park, S.Y.; Park, S.; Kim, C.-H.; Park, S.M.; Lee, M.-G.; Hyun, I.-G.; Jung, K.-S.; Kim, D.-G. COPD patients with exertional desaturation are at a higher risk of rapid decline in lung function. Yonsei Med. J. 2014, 55, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Arlati, S.; Colombo, V.; Spoladore, D.; Greci, L.; Pedroli, E.; Serino, S.; Cipresso, P.; Goulene, K.; Stramba-Badiale, M.; Riva, G.; et al. A social virtual reality-based application for the physical and cognitive training of the elderly at home. Sensors 2019, 19, 261. [Google Scholar] [CrossRef]
- Baldassini, D.; Colombo, V.; Spoladore, D.; Sacco, M.; Arlati, S. Customization of domestic environment and physical training supported by virtual reality and semantic technologies: A use-case. In 2017 IEEE 3rd International Forum on Research and Technologies for Society and Industry (RTSI); IEEE: Manhattan, NY, USA, 2017; pp. 1–6. [Google Scholar]
- Spoladore, D.; Sacco, M. Towards a collaborative ontology-based decision support system to foster healthy and tailored diets. In Working Conference on Virtual Enterprises; Springer: Berlin/Heidelberg, Germany, 2020; pp. 634–643. [Google Scholar]
- Gandy, J. Water intake: Validity of population assessment and recommendations. Eur. J. Nutr. 2015, 54, 11–16. [Google Scholar] [CrossRef]
- Mahroo, A.; Spoladore, D.; Nolich, M.; Buqi, R.; Carciotti, S.; Sacco, M. Smart cabin: A semantic-based framework for indoor comfort customization inside a cruise cabin. In Fourth International Congress on Information and Communication Technology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 41–53. [Google Scholar]
- Unity 3D Real-Time Development Platform. Available online: https://www.unity.com/ (accessed on 29 August 2021).
- Pizzagalli, S.; Spoladore, D.; Arlati, S.; Sacco, M.; Greci, L. HIC: An interactive and ubiquitous home controller system for the smart home. In 2018 IEEE 6th International Conference on Serious Games and Applications for Health (SeGAH); IEEE: Manhattan, NY, USA, 2018; pp. 1–6. [Google Scholar]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.-C.; et al. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef] [PubMed]
- Celjuska, D.; Vargas-Vera, M. Ontosophie: A semi-automatic system for ontology population from text. In Proceedings of the International Conference on Natural Language Processing (ICON), Hyderabad, India, 19–22 December 2004; Volume 60. [Google Scholar]
- Su, M.-H.; Wu, C.-H.; Shih, P.-C. Automatic ontology population using deep learning for triple extraction. In 2019 Asia-Pacific Signal and Information Processing Association Annual Summit and Conference (APSIPA ASC); IEEE: Manhattan, NY, USA, 2019; pp. 262–267. [Google Scholar]
- Meydani, M. Nutrition interventions in aging and age-associated disease. Ann. N. Y. Acad. Sci. 2001, 928, 226–235. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).