Abstract
The amount and distribution of ceramide, an intercellular lipid, in the fingernails of three Japanese women in their twenties were examined by high-performance liquid chromatography and antibody staining. In addition, the structural changes of fingernail cross sections were examined after fingernails were immersed in an acetone-based nail polish remover solution. The acetone-treated fingernails had a lower water content and higher water evaporation than the inner forearm skin and healthy fingernails, suggesting that they had compromised moisturizing and barrier functions and were more susceptible to roughness and damage. These results also suggest that, compared to healthy fingernails, rough fingernails are more prone to breakage and damage. Furthermore, it was found that the amount of ceramide decreased when fingernails were immersed in nail polish remover solution. The distribution showed ceramide to be present in the ventral and dorsal regions of the free edge of the fingernail plate. After immersion in nail polish remover, the three-layered structure of the free edge of the plate was intact, but the dorsal distal edge of the plate peeled off. Gaps were observed inside the free edge of the plate, which should have been layered. These results show that the frequent use of nail polish remover may worsen condition of fingernails.
1. Introduction
The fingernail plate is formed from the fingernail matrix. The white area, called the lunula, is the developing fingernail that first appears on the surface of the fingernail matrix [1]. The fingernail is a modified keratin skin composed of dense hard keratin with a cystine content exceeding 10% [1]. The function of the fingernail is protecting the tips of fingers and toes, helping the bony tips of the fingers or toes to exert force when working or walking, and helping the fingertips to perform delicate tasks [2]. The nail matrix produces a nail plate consisting of an average of 196 cell layers [3] that are tightly bound and distributed in three anatomical layers: dorsal, intermediate, and ventral.
Nail matrix cells constantly replenish the nail matrix. Nail matrix cells are composed of stratified squamous epithelial cells bound together by a long rete ridge and sparse dermis. Mitosis occurs in the basal layer of the nail matrix, and the matrix is constantly being replaced. Nail matrix cells differentiate and constantly migrate upward, resulting in the abandonment of organelles and the condensation and flattening of cytoplasm. The pressure of this new buildup forces other cells (nail matrix) to move dorsally. Eventually, the cells lose their nuclei and become keratinized [1]. The division of nail matrix cells is continuous so that, unlike hair, which forms in cycles, nails form continuously. On the dorsal surface of the nail matrix, cells overlap to form a smooth surface, while on the ventral surface they are irregular and can interconnect with the nail bed.
Cholesterol is the major lipid component of the nail matrix. The stratum corneum has a lipid content of 10% and a moisture content of 20–40%, whereas the nailfold has a lipid content of 0.1–1% and a moisture content of 7–12% (depending on the relative humidity) [4].
Fingernails have a lower water content [5] and higher water evaporation [6] than other parts of the skin. This may be because intercellular lipids, such as sphingolipids, which play a role in skin barrier function, are less abundant on the fingernail plate surface than on other parts of the skin [5,7].
The free edge of the plate is the most fragile part of the fingernail. In healthy adults, the health of the fingernail is impaired when the surface of the fingernail plate is thinly peeled at the free edge or when the fingernail plate surface is easily peeled, brittle, or cracked due to the excessive use of nail polish remover. A fingernail’s elasticity and flexibility are related to its water content, and it has been reported that fingernails are less likely to break or split into two when the water content in the fingernail plate is 13% to 17% [8], suggesting that fingernails with adequate water content are not too rigid or flexible and are less likely to break or chip. However, if the water content of the fingernail matrix is reduced due to the stripping of the moisturizing components in the fingernail matrix (e.g., due to the excessive use of nail polish remover, frequent contact with detergents, or the dryness of the air), the fingernail becomes brittle. It has also been reported that the thickness of the fingernail plate is inversely related to the amount of water evaporation and that the amount of water evaporation is higher when the fingernails are thin [9], suggesting that fingernails with high water evaporation are more susceptible to cracking and chipping. Therefore, the state of fingernail health can be determined by measuring the water content, water evaporation, and intercellular lipid content of the fingernails.
2. Materials and Methods
2.1. Measurement of Water Content and Water Evaporation
The physiological state of fingernails was examined by measuring the water content and water evaporation in healthy and rough fingernails. Healthy and rough fingernails were identified from images. The water content was measured by a SKICON-200EX (Yayoi Co. Ltd., Tokyo, Japan), and the water evaporation was measured by a Tewameter (Courage + Khazaka Electronic GmbH, Cologne, Germany). We conducted this research following the ethical principles and ethical guidelines for medical research involving human subjects in accordance with the Declaration of Helsinki (revised October 2013). After receiving approval from the ethics committee (E16BS-039; 16 March 2017), we sufficiently explained the aim, details, and methods of this study to the subjects (ten Japanese women between the ages of 21 and 23) and then obtained their written consent to participate in the study.
2.2. Nail Polish Remover Immersion Treatment
A commercially available nail polish remover solution with acetone as its main ingredient (ingredients: acetone, water, butyl acetate, and PG) was used. The free edges of fingernail plates were cut with fingernail clippers, soaked in nail polish solution for 1 day at room temperature, rinsed with tap water, and dried in an incubator at 37 °C.
2.3. Assay of Ceramide in the Stratum Corneum of the Forearm by Thin-Layer Chromatography (TLC)
The stratum corneum of the inner forearm was sampled by applying adhesive tape strips five times, and the resulting stratum corneum was dispersed by adding 200 μL of dispersion solution (0.1 mol/L Tris-HCl buffer (pH 8.5) containing 5 mM EDTA and 2% sodium dodecyl sulfate (SDS)) to an Eppendorf tube and mixing it with 250 μL of lipid extraction solvent (2:1 chloroform/methanol). Then, the mixture was centrifuged, and the lower layer was collected for lipid extraction. After development with a hexane/diethyl ether/acetic acid solution (80:20:1) for lipid extraction on the TLC plate (TLC Silica gel 60 F254 20 Aluminum sheets, Merck & Co., Inc., Rahway, NJ, USA), a copper sulfate phosphate solution (copper sulfate (CuSO4) dissolved in 15% phosphoric acid (H3PO4) to make a 3% (weight/volume) solution) was sprayed, and the sample was heated to stain and image lipids. The spot corresponding to the Rf value of the free fatty acids on the TLC plate was scraped off with a knife and transferred to a microtube. Then, 1 mL of mixed solvent (chloroform/methanol = 2:1) was added, and the mixture was stirred. The supernatant was collected, the solvent was evaporated, and the residue was dissolved in acetonitrile (ACN) and analyzed by high-performance liquid chromatography (HPLC). Cholesterol, oleic acid, and TIC-001 (dihydrosphingolipid; Takasago International Corporation, Tokyo, Japan) were used as positive controls.
2.4. Measurement of Ceramide Content in the Stratum Corneum of the Forearm by HPLC
Adhesive tape strips were applied to the inner forearm stratum corneum five times, and the resulting stratum corneum sample was dispersed by adding 200 μL of dispersion solution (0.1 mol/L Tris-HCl buffer (pH 8.5) containing 5 mmol/L EDTA and 2% sodium dodecyl sulfate (SDS)) to an Eppendorf tube and mixing it with 250 μL of lipid extraction solvent (2:1 chloroform/methanol) and 2 μL of C17 sphingosine. Then, the mixture was centrifuged, and the lower layer was collected. The upper layer was used for the protein content measurement. Subsequent operations were performed as stated above with the fingernail plates.
The surfaces of the fingernail plates were scraped, and the sample (1.0 mg) was incubated with 1.2 mL of lipid extraction solvent (chloroform/methanol = 2:1) for 1 day. Then, 500 μL was dispensed, and 2 μL of 0.1 mmol/L C17 sphingosine (Sigma-Aldrich Co. LLC., St. Louis, MO, USA), an internal standard, was added. CaCl2 (5 mmol/L) and 25 μL of 25 mmol/L sodium acetate buffer (pH 5.5) containing 2% Triton X-100 and 4 μL of sphingolipid ceramide N-deacylase (SCDase; Takara Bio Inc., Shiga, Japan) were added and incubated for 2 h at 37 °C. The reaction was stopped by adding 200 μL of lipid extraction solvent (chloroform/methanol = 2:1), and then 15 μL of pure water was added. After centrifugation, the lower layer of the extraction was collected. The lower layer was evaporated, 120 μL of ethanol and 15 μL of an OPA solution were added, and the solution was incubated at 70 °C for 1 h [10]. The OPA solution was prepared by constantly stirring while adding 1 mg of ortho-phthalaldehyde to 10 μL of ethanol, 2 μL of 2-mercaptoethanol, and 0.99 mL of a 3% boric acid buffer (pH 10.5) and allowing the solution to incubate at 70 °C for 20 min. The upper layer was used to measure the amount of protein.
The enzyme SCDase, which acts on ceramide to produce sphingoids and fatty acids, and an OPA derivative were used to fluorescently label the amino groups of the sphingoids to identify ceramide-derived sphingoids by HPLC [11,12]. Three types of sphingoids, D-sphingosine (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan), D-erythro-dihydrosphingosine (Sigma-Aldrich Co. LLC.), and phytosphingosine (Tokyo Chemical Industry Co., Ltd.) were used at 0.2 μg/mL (Figure 1). Hydroxysphingosine could not be calculated because there was no standard available.
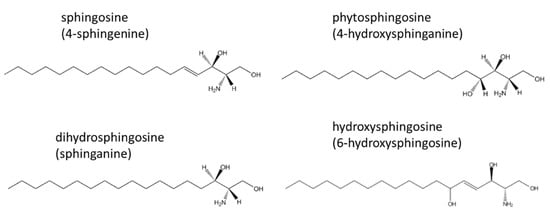
Figure 1.
Four types of sphingoids are produced by the hydrolysis of ceramide by SCDase: sphingosine (4-sphingenine), dihydrosphingosine (sphinganine), phytosphingosine (4-hydroxysphinganine), and hydroxysphingosine (6-hydroxysphingosine).
- HPLC conditions:
- Column used: CAPCELLPAK C18 (UG120; 5 μm, 4.6 mm I.D. × 250 mm, Osaka Soda Co., Ltd., Osaka, Japan);
- Flow rate: 1 mL/min;
- 0~20 min: 80% methanol, 20% 0.1% acetic acid;
- 20~25 min: 100% methanol;
- 25~45 min: 80% methanol, 20% 0.1% acetic acid;
- Detection wavelengths: excitation 335 nm, emission 440 nm.
2.5. Protein Assay
BSA (2 mg/mL stock solution) was diluted with PBS to 0.2, 0.1, 0.05, 0.025, 0.0125, 0.00625, and 0.003125 mg/mL. The reaction solution was prepared by mixing BCA Protein Assay Reagent A and Protein Assay Reagent B from the Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA) at 50:1. Ten microliters each of BSA and the upper layer of fingernail and forearm skin samples were placed in a 96-well plate. After incubation at 37 °C for 2 h, the absorbance at 562 nm was measured.
2.6. Observation of the Free Edge of the Fingernail Plate cross Section and Nuclear Staining/Immunostaining
The isolated free edge of the fingernail plate was embedded in optimal cutting temperature (OCT) compound (Sakura Finetek Japan Co., Ltd., Tokyo, Japan), and 10 μm thick frozen sections were prepared using a cryostat (Leica Biosystems Nussloch GmbH, Wetzlar, Germany) and stained with toluidine blue and DAPI (Dojindo Chemical Research Institute, Kumamoto, Japan). Antibody staining was performed for observation. An anti-ceramide mouse IgM antibody (Glycobiotech GmbH, Hamburg, Germany) and an anti-glucosylceramide rabbit antibody (Glycobiotech GmbH) were used as primary antibodies, and an F(ab’)2-goat anti-mouse IgG/IgM (H + L) secondary antibody with an Alexa Fluor® 488 conjugate (Thermo Fisher Scientific Inc.) and an F(ab’)2-goat anti-rabbit IgG (H + L) cross-adsorbed secondary antibody with Alexa Fluor® 546 (Thermo Fisher Scientific Inc.) were used as secondary antibodies.
2.7. Statistical Analysis
The data obtained from each experiment are expressed as means ± standard deviations for n = 3 to 5. Microsoft Excel (Microsoft, Redmond, WA, USA) was used for statistical analyses, and significant differences are indicated by *: p < 0.05, **: p < 0.01, and ***: p < 0.001.
3. Results
3.1. Measurement of Water Content and Water Evaporation
Figure 2a shows images of healthy and rough fingernails. As shown in Figure 2b, the rough fingernails had a lower water content and higher water evaporation than the healthy fingernails. In addition, the rough fingernails had a lower water content and higher water evaporation than the inner forearm skin (Figure 2c). After the stratum corneum lipids of the inner forearm were separated by TLC (Figure 2d) and treated with SCDase, sphingosine was found to be the main ceramide in the sample, as measured by HPLC (Figure 2d). In the fingernail plate, however, sphingosine was not found since there was little ceramide in the fingernail plate and dyeing during TLC was unsuccessful. Sphingosine-type ceramides and phytosphingosine-type ceramides were less abundant in the surface samples of healthy fingernail plates than in the samples acquired from the inner forearm stratum corneum (Figure 2e). However, there was no difference in the dihydrosphingosine-type ceramides in the healthy fingernail plate and in the inner forearm stratum corneum samples (Figure 2e).
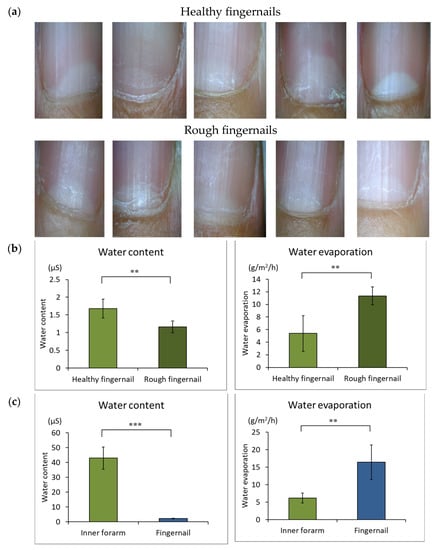
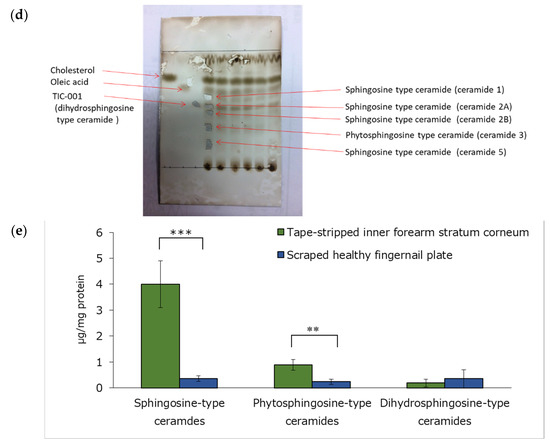
Figure 2.
Fingernail images, water content, and water evaporation. (a): Healthy and rough fingernail images. (b): Fingernail water content and water evaporation of healthy and rough fingernail plates. n = 5, means ± standard deviations, **: p < 0.01. (c): Comparison of water content and water evaporation of fingernail plates and inner forearm skin. n = 4, means ± standard deviations, **: p < 0.01, ***: p < 0.001. (d): The main ceramide in the stratum corneum of the inner forearm was separated by TLC and measured by HPLC after SCDase treatment. (e): Comparison of ceramide types on the surface of healthy fingernail plate and inner forearm stratum corneum samples. n = 3, means ± standard deviations, **: p < 0.01, ***: p < 0.001.
3.2. Ceramide Measurement of Normal Fingernail Plates and Polish-Remover-Soaked Free Edges of Fingernail Plates by HPLC
Figure 3 shows the sphingoid concentration in the normal free edges of fingernail plates and nail polish remover soaked fingernail plates analyzed by HPLC after SCDase treatment. Three types of sphingoids, sphingosine, phytosphingosine, and dihydrosphingosine, were measured. Dihydrosphingosine-type ceramides were the most abundant ceramide in the normal free edges of fingernail plates. The presence of sphingosine-type ceramides and phytosphingosine-type ceramides was decreased in the nail polish remover soaked fingernail plates, showing a significant difference from the normal free edges of fingernail plates.
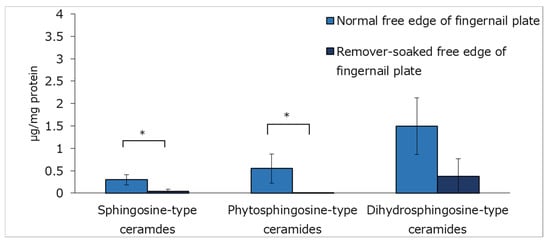
Figure 3.
Ceramide content of the normal free edges of fingernail plates and the nail polish remover soaked free edges of fingernail plates measured by HPLC. n = 3, means ± standard deviations, *: p < 0.05.
3.3. Observation of Cross Sections of the Normal Free Edges of Fingernail Plates and the Nail Polish Remover Soaked Free Edges of Fingernail Plates
Figure 4 shows cross sections of normal free edges of fingernail plates, and nail polish remover soaked free edges of fingernail plates were observed after staining with toluidine blue. There were evident differences in the thicknesses of the normal fingernails and the proportions of the dorsal, middle, and ventral fingernail regions among the three subjects; while the dorsal fingernail region of the fingernail of subject No. 2 was only slightly visible, it was layered in subjects No. 1 and No. 3. A three-layered structure was also observed in the free edges of the fingernail plates immersed in the nail polish remover solution. However, the dorsal free edges of the fingernail plates were peeled off, and gaps were observed on the inside, which, in healthy samples, should have a layered structure.
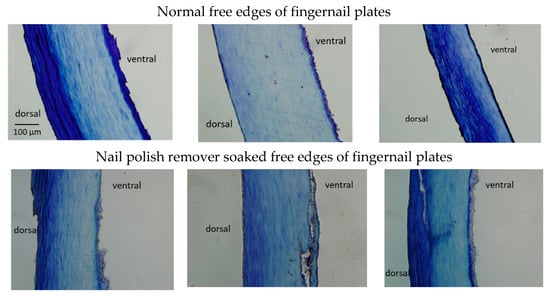
Figure 4.
Cross-sectional observations of the normal free edges of the fingernail plates (left: No. 1, middle: No. 2, right: No. 3) and the nail polish remover soaked free edges of fingernail plates (left: No. 1, middle: No. 2, right: No. 3).
3.4. Anti-Ceramide Antibody Staining and Nuclear Staining of Cross Sections of Normal Free Edges of Fingernail Plates and Nail Polish Remover Soaked Free Edges of Fingernail Plates
The upper row of Figure 5a shows that ceramide was observed in the ventral portions of the normal free edges of the fingernail plates of subjects No. 1, No. 2, and No. 3. Subjects No. 1 and No. 3 showed ceramides in the dorsal free edges of the fingernail plates. The lower row in Figure 5a shows ceramides in the nail polish remover soaked free edges of the fingernail plates. The ceramide levels in the ventral free edges of the fingernail plates were reduced by immersion in the nail polish remover solution in all subjects. Subjects No. 1 and No. 3 showed small amounts of ceramides in the dorsal free edges of the fingernail plates treated by immersion in nail polish remover solution.
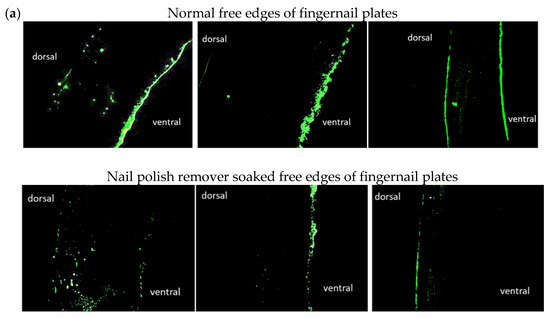
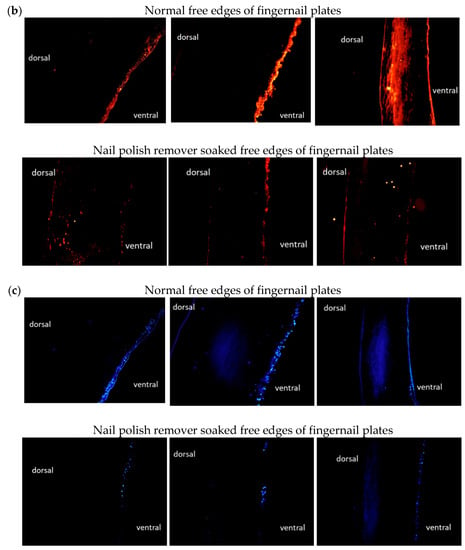
Figure 5.
Cross-sectional anti-ceramide antibody staining and nuclear staining of healthy and nail polish remover soaked free edges of fingernail plates. (a): Cross-sectional anti-ceramide antibody staining of normal free edges of fingernail plates and nail polish remover soaked free edges of fingernail plates (left: No. 1, middle: No. 2, right: No. 3). (b): Cross-sectional anti-glucosylceramide antibody staining of the normal free edges of fingernail plates and nail polish remover soaked free edges of fingernail plates (left: No. 1, middle: No. 2, right: No. 3). (c): Nuclear staining of normal free edges of fingernail plates and nail polish remover soaked free edges of fingernail plates (left: No. 1, middle: No. 2, right: No. 3).
The upper row of Figure 5b shows that all three subjects had glucosylceramides in the ventral free edges of the fingernail plates. Subject No. 3 had glucosylceramide in the entire dorsal free edge of the fingernail plate, in addition to the ventral free edge of the fingernail plate. The lower row of Figure 5b shows that little glucosylceramide was observed in the nail polish remover soaked free edges of the fingernail plates. Only subject No. 3 showed a small amount of glucosylceramide on the dorsal surface of the free edge of the fingernail plate after immersion in nail polish remover solution.
These results indicate that the nail polish remover treatment decreased the ceramide and glucosylceramide levels in the free edges of fingernail plates.
Nuclear staining was also performed on the samples. The upper row in Figure 5c shows that nuclei were observed on the ventral free edges of the fingernail plates in all subjects. Fewer nuclei were observed at the free edges of the fingernail plates immersed in nail polish remover solution than at the free edges of the normal fingernail plates (lower row in Figure 5c).
4. Discussion
Scanning electron microscopy (SEM) has revealed that, regardless of age, fingernails have a three-layered structure consisting of dorsal, middle, and ventral regions and that the surface is lined with keratinocytes similar to the cuticle of a hair [1]. However, it has been reported that, with aging, the dorsal region becomes damaged, and the degree of cuticle-like substance detachment increases [13]. It was reported that there was no significant correlation between the nailfold thickness and the water evaporation from the nail plate [14]. However, Murdan et al. found an inverse linear relationship between transonychial water loss (TOWL) and nail thickness [15]. The factors that cause changes in fingernail morphology have been classified into two categories: internal and external [13]. Internal factors may include age, physical condition, and dietary status, while external factors may include the excessive use of nail polish remover and detergents and seasonal factors. Clearly, fingernails are very delicate parts of the body. The fingernail is much more fragile than the forearm skin because its barrier function is weaker and it is easily roughened. One factor that makes a large difference between the strength of fingernails and forearm skin is ceramide, an intercellular lipid in the stratum corneum.
Ceramide is a general term for compounds consisting of fatty acid amides bonded to the amino groups of sphingoid bases, and they are major intercellular lipids in the outermost layer of the skin. Ceramides form a network of hydrogen bonds with hydrogen molecules in the stratum corneum, construct a lamellar structure containing water molecules as structural units, and are involved in moisture retention and skin barrier functions. Intercellular lipids consist of 50% sphingolipids, 20% cholesterol esters, 10% cholesterol, and 20% fatty acids; 95% of sphingolipids are ceramides [16]. Mammalian sphingoid bases include dihydrosphingosine, sphingosine, phytosphingosine, and 6-hydroxysphingosine, while fatty acids include nonhydroxy fatty acids, α-hydroxy fatty acids, and ester ω-hydroxy fatty acids. As a result of different factorial combinations of these bases, a total of 12 classes of ceramides exist [17,18]. The HPLC fractionation of the major ceramide in the human forearm stratum corneum samples separated by TLC revealed a high percentage of the sphingosine-type ceramide, which was consistent with the report by Ponec et al. [19]. Although van Smeden et al. reported that the phytosphingosine-type ceramide is the major ceramide in the human forearm stratum corneum [20,21], a different extraction solvent, chloroform/MeOH/water (1:2:1/2; 1:1:0; and 2:1:0) may have resulted in the detection of more highly polar phytosphingosine-type ceramides. Here, the dihydrosphingosine-type ceramide was not abundant in the surface samples of the dorsal fingernail plate, but the dihydrosphingosine-type ceramide was abundant in the isolated free edges of the fingernail plates. This suggests that the dihydrosphingosine-type ceramide is more abundant in the ventral than in the dorsal region of the free edge of the fingernail plate. This is supported by reports that cultured keratinocytes have high levels of dihydrosphingosine-type ceramides [22].
According to the results of the ceramide content analyzed by HPLC and the results of anti-ceramide antibody staining, the experimental immersion treatment in the nail polish remover solution reduced the ceramide content in the dorsal and ventral portions of the fingernails, and the amount of ceramide detected by HPLC was also decreased. The glucosylceramide in the ventral fingernail portions was also decreased by immersion in the nail polish remover solution. We hypothesized that ceramide derived from sphingosine was the largest determinant of fingernail condition. The observation of cross sections of the free edges of the fingernail plates cut with a cryomicrotome suggested that soaking with nail polish remover causes delamination, resulting in brittle fingernails on the dorsal and ventral sides of the finger. Fingernail ceramide is important to maintain a normal nail barrier function because water evaporation from the fingernail surface is increased in rough nails and the sphingosine-type ceramide and the phytosphingosine-type ceramide are reduced by immersion in nail polish remover solution.
Antibody staining revealed the presence of nuclei and glucosylceramide in the ventral fingernail area, suggesting that ceramide is produced in the area closest to the fingernail matrix. The presence of nuclei in the ventral fingernail area suggested that ceramide may be related to the matrix cells in the fingernail matrix, where the fingernail is formed. Healthy fingernails have a three-layered structure (dorsal, middle, and ventral fingernail regions). The thickness of the fingernail and the ratio of the three layers vary from person to person. It is thought that acetone penetrates into the fingernail, disrupts ceramide alignment, and reduces the moisture between the layers, which causes the three layers to be disrupted and the fingernails to peel, making them more brittle. These results suggest that there is a relationship between fingernail condition and ceramide.
To achieve healthy fingernails that are less prone to roughness, it is important to improve the barrier function. The first step toward healthy fingernails is to refrain from wearing nail polish, but this can be difficult. Moreover, it is important to minimize the external and internal factors that cause fingernails to become rough. It is important to avoid applying organic solvents such as nail polish remover to the fingernails and to treat and care for the fingernails.
5. Conclusions
The ventral free edge of the fingernail plate area, which is separated from the fingernail bed, contains the nucleus, glucosylceramide, and ceramide, while the dorsal free edge of the fingernail contains ceramide. Experimental studies have shown that nail polish remover decreases the ceramide content of the fingernail, creating gaps in the layered interior. These findings show that the frequent use of nail polish remover may aggravate the condition of the fingernail.
Author Contributions
Conceptualization, K.M.; methodology, K.M.; investigation, N.I. and K.M.; data curation, N.I.; writing—original draft preparation, N.I.; writing—review and editing, K.M.; visualization, N.I.; supervision, K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baswan, S.; Kasting, G.B.; Li, S.K.; Wickett, R.; Adams, B.; Eurich, S.; Schamper, R. Understanding the formidable nail barrier: A review of the nail microstructure, composition and diseases. Mycoses 2017, 60, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Holzberg, M. Nails. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworth-Heinemann: Oxford, UK, 1990; Chapter 108. [Google Scholar]
- de Berker, D.; Mawhinney, B.; Sviland, L. Quantification of regional matrix nail production. Br. J. Dermatol. 1996, 134, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- de Berker, D.A.; Ruben, B.S.; Baran, R. Science of the nail apparatus. In Baran & Dawber’s Diseases of the Nails and their Management, 5th ed.; Baran, R., de Berker, D.A., Holzberg, M., Piraccini, B.M., Richert, B., Tomas, L., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2018; pp. 1–58. [Google Scholar]
- Melnik, B.; Hollmann, J.; Hofmann, U.; Yuh, M.S.; Plewig, G. Lipid composition of outer stratum corneum and nails in atopic and control subjects. Arch. Dermatol. Res. 1990, 282, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Higashi, N. Effects of manicure on water vapor loss through human nail in vivo. Skin Res. 1990, 32, 722–726. (In Japanese) [Google Scholar]
- Imokawa, G. Lipid engineering-now and future. II. Control of signal transmission and mass transport. Structure and function of intercellular lipids in the stratum corneum. J. Jpn. Oil Chem. Soc. 1995, 44, 751–766. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Sugawara, T.; Kawai, M.; Suzuki, T. The relationship between moisture content of human fingernails and the mechanical properties of the fingernail (Part 2). J. Soc. Cosmet. Chem. Jpn. 1999, 33, 364–369. (In Japanese) [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spruit, D. Measurement of water vapor loss through human nails in vivo. J. Invest. Dermatol. 1971, 56, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, Y.; Komorisono, M.; Kuwajima, M.; Yoshikawa, S.; Yoshida, S.; Maeda, K. Anti-skin-aging effects of human ceramides via collagen and fibrillin expression in dermal fibroblasts. Biosci. Biotechnol. Biochem. 2022, 86, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Choi, H.K.; Yoo, J.M.; Choi, K.; Lee, Y.M.; Oh, S.; Kim, T.J.; Yun, Y.; Hong, J.; Okino, N.; et al. N-oleoyl-D-erythro-sphingosine-based analysis of ceramide by high performance liquid chromatography and its application to determination in diverse biological samples. Mol. Cell. Toxicol. 2007, 3, 273–281. [Google Scholar]
- Zama, K.; Hayashi, Y.; Ito, S.; Hirabayashi, Y.; Inoue, T.; Ohno, K.; Okino, N.; Ito, M. Simultaneous quantification of glucosylceramide and galactosylceramide by normal-phase HPLC using O-phtalaldehyde derivatives prepared with sphingolipid ceramide N-deacylase. Glycobiology 2009, 19, 767–775. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hirose, T.; Momota, H.; Kitajima, T.; Okura, S.; Matsuda, T.; Motoyoshi, K. Age difference of human nail components and morphology. J. Soc. Cosmet. Chem. Jpn. 1990, 24, 98–105. (In Japanese) [Google Scholar] [CrossRef]
- Jemec, G.B.; Agner, T.; Serup, J. Transonychial water loss: Relation to sex, age and nail-plate thickness. Br. J. Dermatol. 1989, 121, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Murdan, S.; Hinsu, D.; Guimier, M. A few aspects of transonychial water loss (TOWL): Inter-individual, and intra-individual inter-finger, inter-hand and inter-day variabilities, and the influence of nail plate hydration, filing and varnish. Eur. J. Pharm. Biopharm. 2008, 70, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Mutanu Jungersted, J.; Hellgren, L.I.; Høgh, J.K.; Drachmann, T.; Jemec, G.B.; Agner, T. Ceramides and barrier function in healthy skin. Acta. Derm. Venereol. 2010, 90, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.L.; Blanchette, D.R.; Brogden, K.A.; Dawson, D.V.; Drake, D.R.; Hill, J.R.; Wertz, P.W. The roles of cutaneous lipids in host defense. Biochim. Biophys. Acta 2014, 1841, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Vietzke, J.P.; Brandt, O.; Abeck, D.; Rapp, C.; Strassner, M.; Schreiner, V.; Hintze, U. Comparative investigation of human stratum corneum ceramides. Lipids 2001, 36, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Ponec, M.; Weerheim, A.; Lankhorst, P.; Wertz, P. New acylceramide in native and reconstructed epidermis. J. Invest. Dermatol. 2003, 120, 581–588. [Google Scholar] [CrossRef] [PubMed]
- van Smeden, J.; Janssens, M.; Gooris, G.S.; Bouwstra, J.A. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim. Biophys. Acta 2014, 1841, 295–313. [Google Scholar] [CrossRef] [PubMed]
- van Smeden, J.; Hoppel, L.; van der Heijden, R.; Hankemeier, T.; Vreeken, R.J.; Bouwstra, J.A. LC/MS analysis of stratum corneum lipids: Ceramide profiling and discovery. J. Lipid Res. 2011, 52, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Łuczaj, W.; Wroński, A.; Domingues, P.; Domingues, M.R.; Skrzydlewska, E. Lipidomic analysis reveals specific differences between fibroblast and keratinocyte ceramide profile of patients with psoriasis vulgaris. Molecules 2020, 25, 630. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).