1. Introduction
Aging is a biological process that affects most cells, organisms, and species. Telomeres have been postulated as a universal biological clock that shorten in parallel with the division and aging of cells. The length of telomeres, particularly the abundance of short telomeres, has been proposed as a biomarker of senescence, aging, and general health status [
1]. Telomeres are unique structures at the ends of eukaryotic chromosomes that protect them from degradation. Without a compensatory elongating mechanism, telomeres become shorter with each cell division due to the so-called end-replication problem. Critically short telomeres cannot be sufficiently repaired by any known DNA repair mechanism, triggering a persistent DNA damage response. When telomeres reach a critical length, the cell does not further undergo cell division and becomes senescent or otherwise dysfunctional, contributing to organismal aging [
2].
As the most voluminous body organ, the skin shows evident signs of aging as one becomes older. Both intrinsic and extrinsic factors cause skin aging. Intrinsic aging is an unavoidable physiological process that results in thinning skin, dry skin, fine wrinkles, and progressively shrinking skin. In contrast, extrinsic aging is caused by external environmental factors such as air pollution, smoking, poor diet, and sun exposure, resulting in wrinkles, the loss of skin elasticity, laxity, and a rough-textured appearance [
3]. Skin cells, the turnover cycle of which could be partly driven by telomere replenishment, have a proliferative capacity that varies from a few days in newborns to a few weeks in adults [
4]. Furthermore, facial skin cells are of particular interest. Maintaining fast renewal of skin cells could slow down facial aging and make people look younger. Recent experimental studies have supported a potential causal relationship between longer genetically predicted telomeres and less facial aging [
5].
For many people, especially females, a considerable share of daily expenses is occupied by cosmetics and pharmaceuticals in an attempt to prevent or reverse skin aging. This vast cosmetic need continually promotes research on skin aging and its treatment. In traditional Chinese medicine,
Astragali radix, the dried root of
Astragalus membranaceus, has been used a herbal medicine to counteract oxidative stress, inflammation, and aging since ancient times [
6].
Over 200 components have been isolated from Astragalus-based herbal preparations [
7]. The most important are saponins, polysaccharides, and flavonoids. The main compounds in Astragalus supplements are triterpenoid saponins—more specifically, astragaloside. Among them, astragaloside IV is the main component of the medicinal Astragalus plant. Pure astragaloside IV has a diuretic effect, lowers blood pressure, improves exercise performance, and increases physical activity [
8]. Various researchers have shown that the drug has a wide range of biological activities. It reduces the ability of tumor cells to transition through epithelial cells to mesenchymal cells, and thus, to invade and metastasize [
9]. Astragaloside IV reduces the expression of TLR4 and the rate of generation of reactive oxygen species [
10]. Several studies have shown that the substances that make up traditional Chinese Astragalus-based preparations can have a geroprotective effect. It has been demonstrated that cycloastragenol (CAG) imparts geroprotective properties to plant materials from Astragalus.
This study aimed to determine the effects of an Astragalus-root-containing antiaging face cream on the telomere length and the signs of skin aging.
We first assessed the effects of the Astragalus root extract in vitro on human primary fibroblast cells. The telomere analysis performed using Life Length’s proprietary TAT© technology included three different telomere length variables: the median telomere length, the 20th percentile telomere length, and the percentage of short telomeres (<3 Kbp).
Furthermore, we tested the efficacy of an Astragalus-root-containing face cream on telomeres as part of a clinical study of epidermal biopsies collected from healthy individuals. During this study, the telomere length analysis determined in each sample included the three variables mentioned above (median telomere length, 20th percentile telomere length, and the percentage of short telomeres), as well as an extensive analysis of Telomere-Associated Variables (TAVs), which included a breakdown of the entire telomere length distribution for each sample (please see
Section 2 for a more detailed explanation). Finally, we also measured the signs of facial skin aging as part of the same clinical test in 20 subjects aged 40–70 over 28 days.
2. Materials and Methods
2.1. Test Formulation
The cosmetic product used in this test was a cream containing astragaloside IV and Hylasom EG10 (chemically cross-linked hyaluronic acid) (YouthShots Face Cream, YouthShots GmbH, Düsseldorf, Germany).
2.2. In Vitro Study Cell Culture (Life Length)
Primary cultures of adult human fibroblast cells were established. Cells were seeded at 5 × 10
3 cells/cm
2 in a fibroblast medium kit (Innoprot). Fibroblast medium (FM) is a complete medium designed for the optimal growth of neonatal human fibroblasts in vitro (ATCC PCS-201-010™). It is a sterile, liquid medium that contains essential and nonessential amino acids, vitamins, organic and inorganic compounds, hormones, growth factors, trace minerals, and a low concentration of fetal bovine serum (2%). The medium is HEPES, which is bicarbonate buffered and has a pH of 7.4 when equilibrated in an incubator with an atmosphere of 5% CO
2/95% air. The medium is formulated (quantitatively and qualitatively) to provide a defined and optimally balanced nutritional environment that selectively promotes the proliferation and growth of normal human fibroblasts in vitro. The media were renewed every 2–3 days, and the cells were passaged at subconfluence (70–80%) every seven days. Compounds or a vehicle control was added to the cells in culture. Cell growth was monitored for each condition by counting cell numbers at each passage using a Countess™ cell counter (Invitrogen). Population doubling (PD) was calculated using the formula PD = 3.322(Log(Cf) − Log(Ci)) + x (Cf: Final concentration; Ci: initial concentration; X: PD last passage). One PD is equivalent to one round of cell replication [
11,
12].
Cells were expanded for four weeks under the oxidative (10 μM H2O2) cell culture conditions described previously. Treatment dilutions were prepared fresh in each passage with fresh media. A stock solution of 200 mg/mL of astragaloside IV was prepared in 100% DMSO and sequentially diluted in order to add to the cells. A dilution of 1/200 was made, and the final concentration of DMSO was 0.5% (this was the concentration identified as not negatively affecting the cells). Two different concentrations of the product were tested during the TAT in vitro study.
2.3. Clinical Evaluation of Efficacy and Safety
The astragaloside-IV-containing cream was also tested in vivo. The study was conducted in the Department of Dermatology, University Hospital Essen. For clinical evaluation, a single-center open study was conducted to assess the efficacy and acceptability of the investigational product.
In one group of healthy volunteers, 13 women and 2 men (aged 28–82 years) participated in a placebo-controlled in vivo evaluation of epidermal skin cells. After four weeks of a once-daily application of 0.5 mL of the verum cream (2 mg/mL astragaloside IV) on 1 cm2 of the inner side of the upper arm and the cream vehicle as a placebo on the other side, epidermal cell samples were collected from the treated upper arm sections and analyzed regarding telomere length. The local ethics committee approved the analysis (20–9698-BO).
In the second group, the product was tested on healthy volunteers (20 women aged 40–70 years), for clinical evaluation as a cosmetic agent. After four weeks of twice-daily facial application, participants completed a questionnaire on the cream’s characteristics and effects. Furthermore, the following instrumental and clinical parameters were evaluated: skin moisturization, skin compactness, microwrinkle visibility, skin tonicity, brightness, and smoothness. Self-evaluation (improved skin smoothness, reduced microwrinkle visibility, improved skin tonicity, and improved skin brightness) was performed according to the VNS scale, where 0 is the minimum value and 10 is the maximum value. Wrinkle depth measurements were taken using an Antera 3D® and elaborated with the software Antera 3D®. Skin moisturization was measured using a “Corneometer® CM 825”. Skin compactness measurements were taken using the elastometer “Cutometer® MPA 580”. Due to the study being cosmetic product testing, ethics committee approval was not required. All subjects gave written informed consent for participation and publication.
2.4. Telomere Length Determination Using Telomere Analysis Technology (TAT)
All telomere length measurements were performed using Life Length’s proprietary Telomere Analysis Technology (TAT
©). TAT
© measures telomere length using a high-throughput (HT) quantitative fluorescent in situ hybridization (Q-FISH) technique [
13].
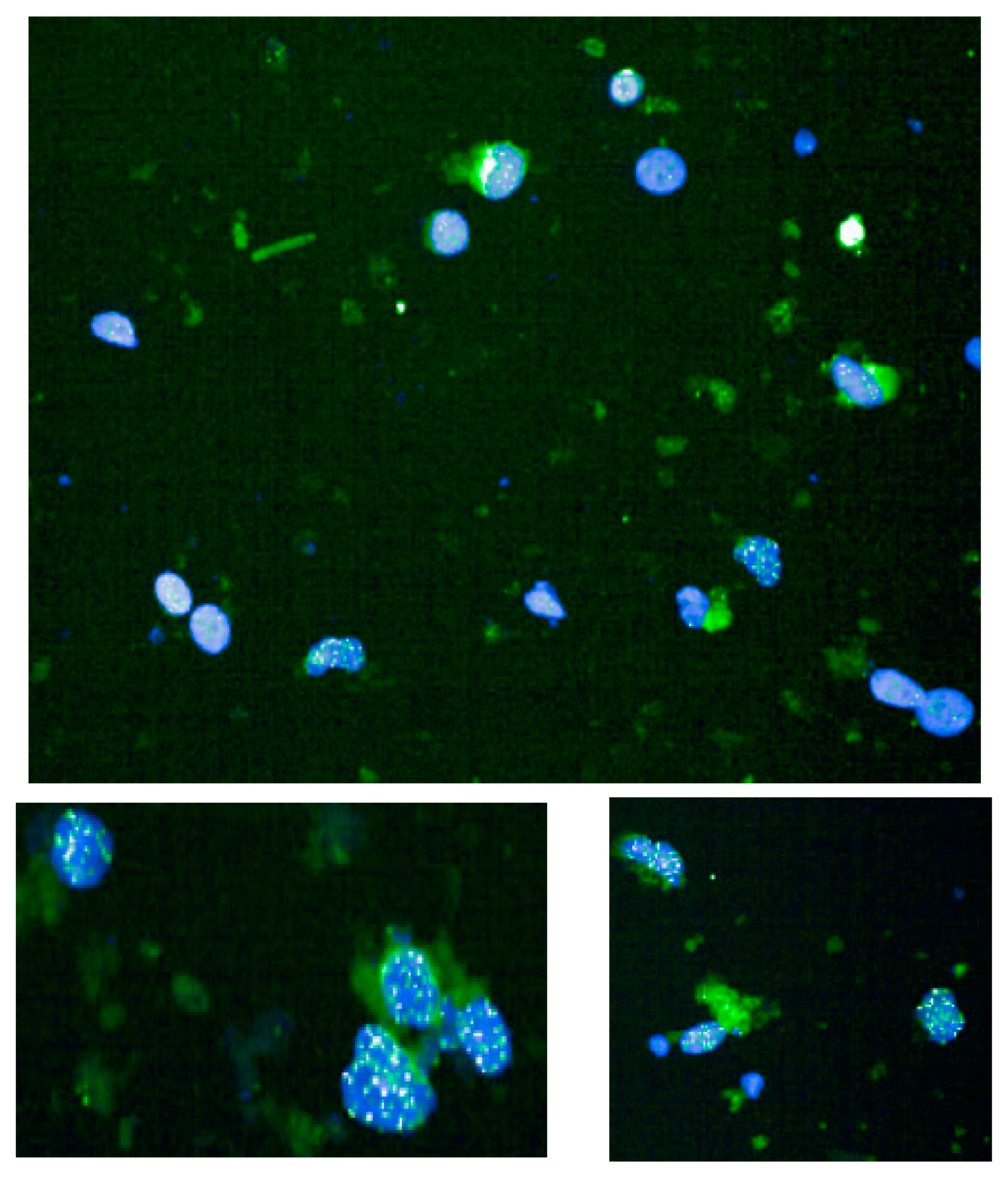
This method is based on a quantitative fluorescence in situ hybridization method modified for cells in interphase. In brief, telomeres are hybridized with a fluorescent peptide nucleic acid (PNA) probe that recognizes three telomere repeats (sequence: Alexa488-OO-CCCTAACCCTAACCCTAA, Panagene). The images of the nuclei and telomeres are captured by a high-content screen system (see below). The intensity of the fluorescent signal from the telomeric PNA probes, which hybridizes to a given telomere, is proportional to the length of that telomere. The fluorescence intensities (representative example in
Figure 1) are translated to base pairs through a standard regression curve generated using control cell lines with known telomere length.
Apart from determining telomere length in absolute base pair (bp) units, TAT© can provide excellent granularity and define multiple variables associated with telomere length, which improves the detection of differences between biological samples, as well as verifying that the differences that are detected in a single variable are representative of the entire telomeric profile of the model, enhancing the robustness of the conclusions. Two distinct analyses can be performed for TAT: the standard analysis and the extended Telomere-Associated Variables (TAVs) analysis.
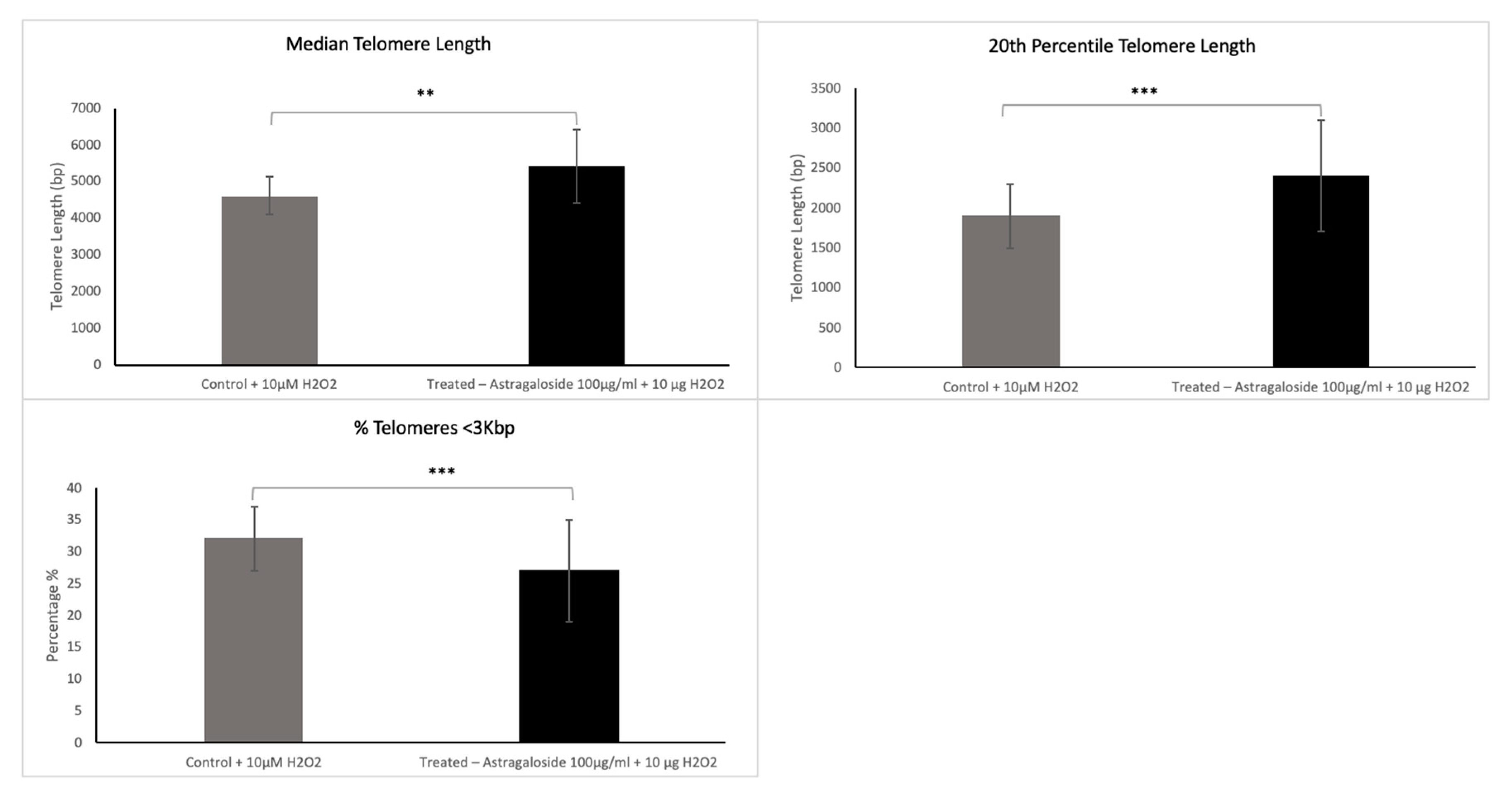
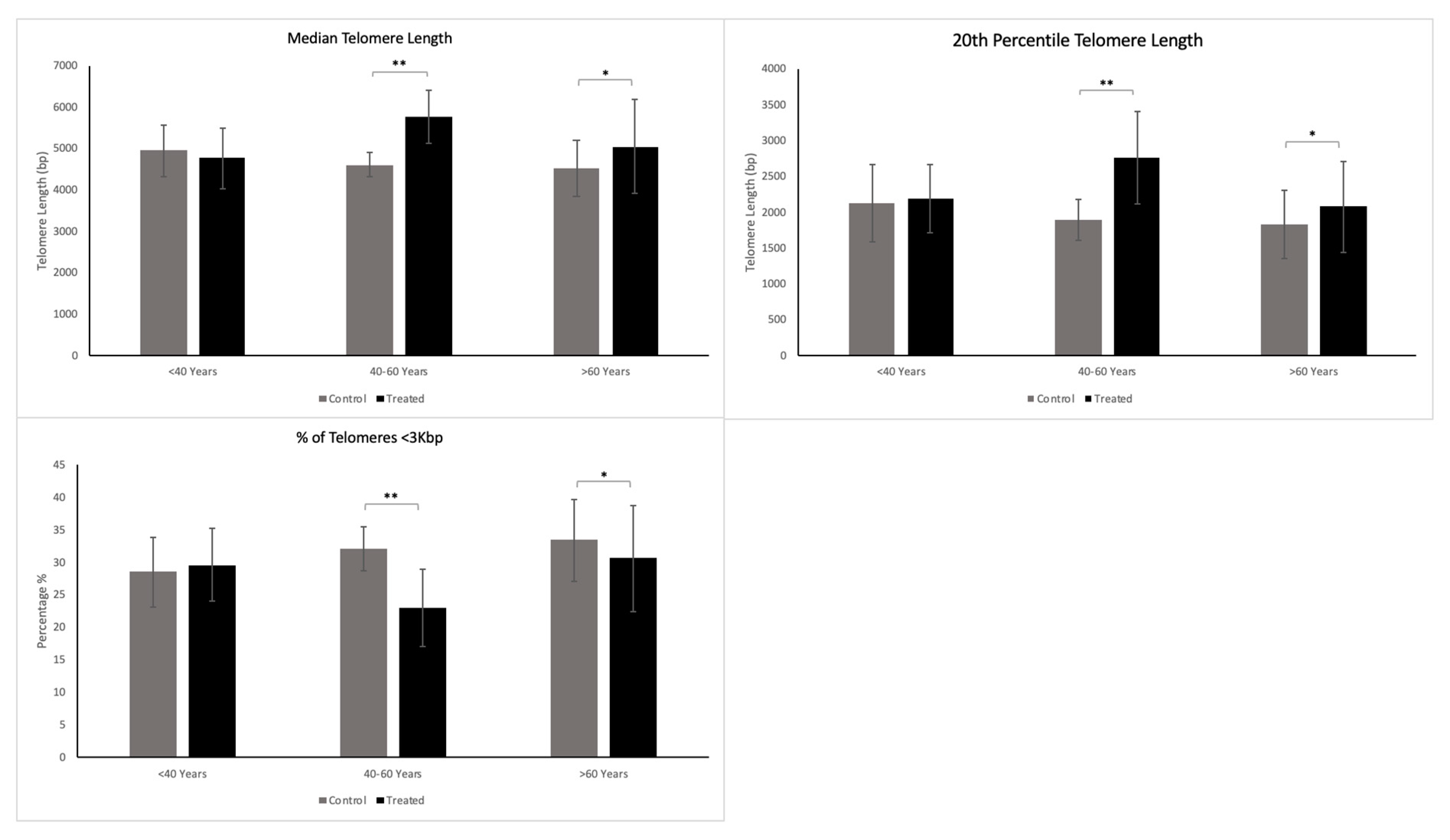
The standard analysis determines three specific variables: the median telomere length; the 20th percentile telomere length (a second telomere length measurement weighted more toward the short telomeres); and the percentage of telomeres < 3 Kbp in length, which is indicative of the critically short telomeres in the sample. The two latter variables allow visibility into the short telomeres of the sample, which are those mainly responsible for cells’ entering senescence.
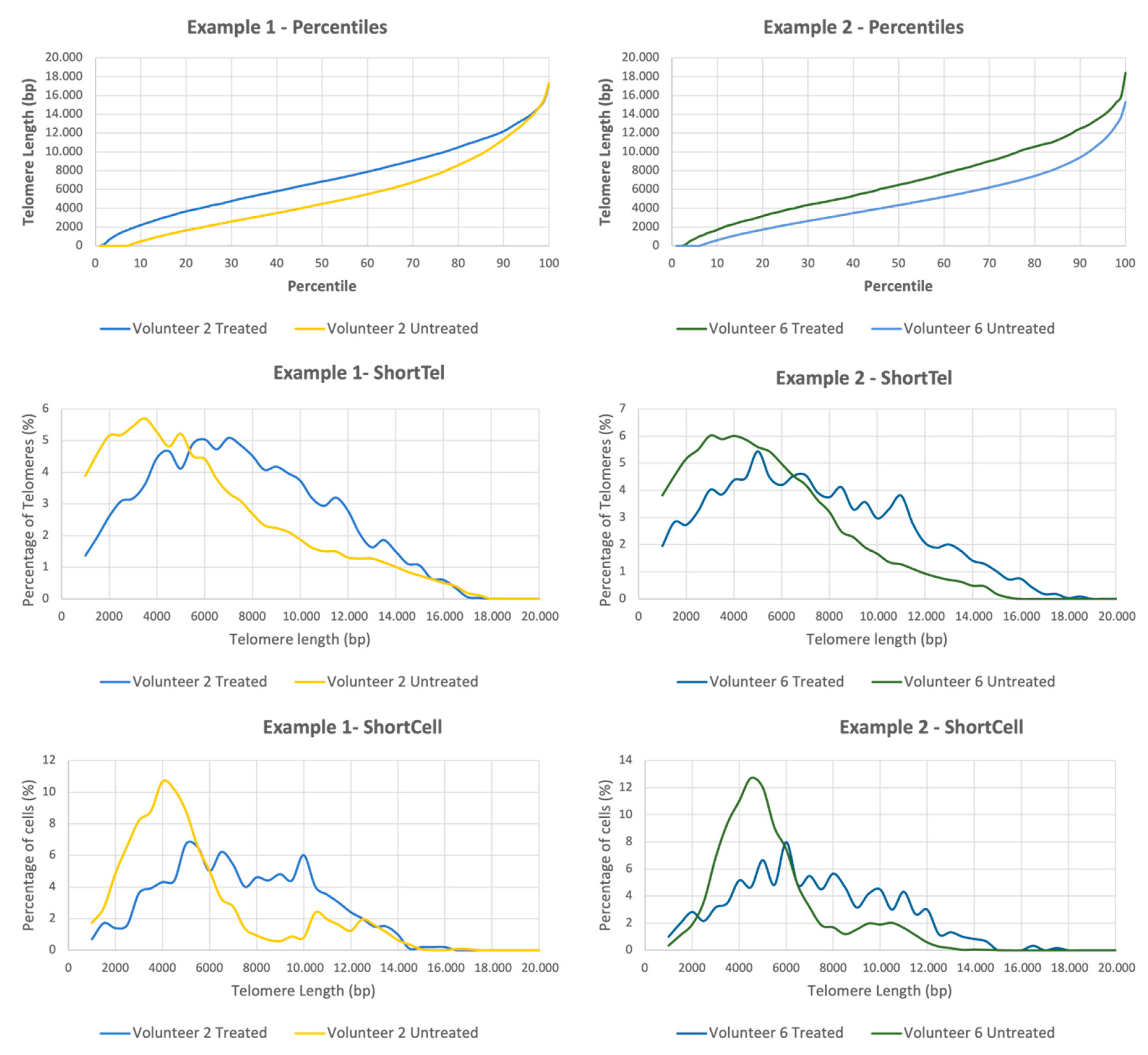
The extended TAVs analysis includes the determination of more than 250 telomere length variables per sample, which translates to a full breakdown of the telomere length distribution. This analysis contains three categories: (1) The telomere length percentiles are one hundred individual telomere length measurements per sample, including the shortest telomeres in the sample (1st percentile length) and the length of the longest telomeres (100th percentile length). The percentiles allow for a comprehensive comparison between all the telomere lengths present in each sample throughout the telomere length distribution. (2) The ShortTel variables are the percentages of telomeres with a specific telomere length. The ShortTel variables help to identify whether one sample has a higher percentage (quantity) of shorter or longer telomeres and to detect differences between samples in a specific distribution area. (3) The ShortCell variables are the percentages of cells with a specific average telomere length. Since senescence and cell apoptosis affect the whole cell, it is essential to address the telomeric profile at a cellular level. The ShortCell variables detect whether a sample presents a higher percentage of cells with shorter telomeres (closer to senescence) or longer ones.
The standard telomere length analysis was performed for the in vitro evaluation of the compound, whereas for the clinical study, both the standard and the extended TAVs analyses were performed.
2.4.1. Instrumental Skin Assessment
The dermatologist measured skin parameters at baseline and after 4 and 8 weeks of test product use. In vivo skin parameters were evaluated using the following methods:
2.4.2. Dermal Assessment by Cutometer MPA580
The elasticity of skin was measured using the Cutometer MPA 580 on the right and left cheek at baseline (day 0) and day 28. The reading was taken independently on each cheek.
2.4.3. Dermal Assessment by Corneometer CM 825
The Corneometer CM 825 is a device that can be used to measure the hydration levels of the superficial layers of the skin. The device works by measuring the dielectric properties of the skin. The assessment was performed by taking three independent readings on the mid-forehead, right cheek, and left cheek at baseline (day 0) and day 28.
2.4.4. Dermal Assessment by Antera 3D
Antera 3D is a tool for measuring the effects of antiaging treatments. The device uses a patent-pending technology to take three-dimensional measurements of the face, recording the width and depth of fine lines, wrinkles, and folds. A filter is used to select the features that are to be measured: 0–1 mm for fine lines, 0–2 mm for medium wrinkles, and 0–3 mm for prominent wrinkles. Three independent readings were taken on each area of the face (right and left forehead, and cheek) on day 0 and day 28.
2.5. Statistical Analysis
Statistical data analysis was performed using IBM SPSS Statistics 25.0 (IBM, Armonk, NY, USA). A paired t-test was used to compare the results before and after treatment.
4. Discussion
As life expectancy rates continue to rise, the global market for functional cosmetics is also flourishing. These products, which are designed to prevent the signs of aging and to promote a healthy appearance, are becoming increasingly popular among consumers of all ages. Skin aging is associated with deep wrinkles, the loss of skin tone and elasticity, and a reduction in the thickness of the epidermis and dermis. Functional cosmetics can help to address these concerns by providing nutrients, moisture, and other important ingredients that help to keep skin looking its best. There are many theories about what causes aging, but from a molecular point of view, it is the result of cumulative internal and external structural and physiological changes. These changes can be caused by genetics, light radiation exposure, the loss of fibrillin-positive structures, and the reduced content of collagen fibers.
Telomere shortening and dysfunction play a significant role in natural aging and age-related diseases [
14]. Genomic instability is a major driver of aging, and telomere dysfunction has been demonstrated to be an environmental factor that causes genomic alterations in older adults [
15,
16]. Counteracting telomere attrition and its consequences has been a primary target of research over the past decade [
17,
18]. Regenerative medicine has also focused on strategies to maintain telomere length [
19].
To date, telomere-protecting compounds and substances have been shown to be essential for antiaging and telomere-dependent disease treatments. In the in vitro study, we characterized the effects of astragaloside IV for its ability to protect telomeres. In agreement with our results, Molgora et al. demonstrated that Astragalus membranaceus extract (TA-65), an aglycone of astragaloside IV, significantly increased telomerase activity in human T-cell cultures. In particular, it has been shown that cycloastragenol activates telomerase, maintains or lengthens telomeres in vitro, and decreases the percentage of critically short telomeres and DNA damage in the cell [
19].
In the present study, we showed that a cream formulation containing astragaloside IV in combination with hyaluronic acid is highly effective in reducing the markers of aging. Astragaloside IV showed significant telomere-protecting activity. This was apparent from both the standard analysis conducted and the extended TAVs analysis in 13 of the 15 participants included in the study, since these treated samples showed an improved telomeric profile.
The combination of the substances can probably achieve a better depth penetration of astragaloside IV. Here, hyaluronic acid was used as a vehicle for astragaloside IV.
The natural moisturizing and water-holding capacities of the skin reduce with age, affecting skin elasticity. Hydration plays a vital role in skin rejuvenation [
20]. Phytochemicals such as astragaloside IV, a saponin from Astragalus membranaceus, can improve skin firmness and elasticity [
21]. The assessment of skin hydration using a corneometer on the forehead and cheek showed a positive change at the measured time points, suggesting that astragaloside IV in combination with hyaluronic acid may have acted as a water content modulator, improving the skin moisture retention of the stratum corneum. As analyzed using a Cutometer, the skin elasticity of the study subjects improved with regular usage of the face cream.
In this study, astragaloside IV face cream revitalized and provided radiance, vibrancy, and skin glow in almost all subjects, probably by protecting telomeres, stimulating collagen production, reducing fine lines and wrinkles, and restoring skin texture. Significant improvements were observed in skin texture and wrinkles at the end of the study, as measured by an Antera 3D.
In this study, we set out to assess the effectiveness of astragaloside IV in improving the appearance of skin. To do this, we used a subjective assessment, and instrumental and image analyses. Our results show that astragaloside IV, when used in combination with hyaluronic acid, significantly improved brightness, elasticity, and the appearance of fine lines and wrinkles. In addition, our image analysis showed a decrease in roughness and an increase in smoothness of the skin of the subjects who used astragaloside IV. All subjects agreed that the test product made their skin moisturized, brighter, younger, and firmer.
No product-related skin irritation, intolerance, adverse events, or serious adverse events were recorded during the study. The application of the astragaloside-IV-containing cream twice daily for 28 days was found to be safe and well-tolerated for consumer application.
Limitations of the Study
Of course, some limitations apply to our exploratory, open-label pilot study. Four weeks may be too short for a long-term follow-up observation. Moreover, studies on larger patient populations should also be performed because of the limited sample size. Nevertheless, the proband population represents the standard population presented to dermatologists, resulting in good external validity.