Phytochemical Screening, and In Vitro Evaluation of the Antioxidant and Dermocosmetic Activities of Four Moroccan Plants: Halimium antiatlanticum, Adenocarpus artemisiifolius, Pistacia lentiscus and Leonotis nepetifolia
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Extraction and Sample Preparation
2.4. Determination of the Extraction Yields
2.5. Phytochemical Screening
2.5.1. Qualitative Analysis on Phytochemical Constituents
2.5.2. Quantitative Analysis on Phytochemical Constituents
2.6. Antioxidant Activity
2.6.1. DPPH Radical Scavenging Activity
2.6.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.6.3. ABTS Radical Scavenging Activity
2.7. Dermocosmetic Activities
2.7.1. Collagenase Inhibition Activity
2.7.2. Elastase Inhibition Activity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Extraction Yields
3.2. Phytochemical Screening
3.2.1. Qualitative Analysis on Phytochemical Constituents
3.2.2. Quantitative Analysis on Phytochemical Constituents
3.3. Antioxidant Activity
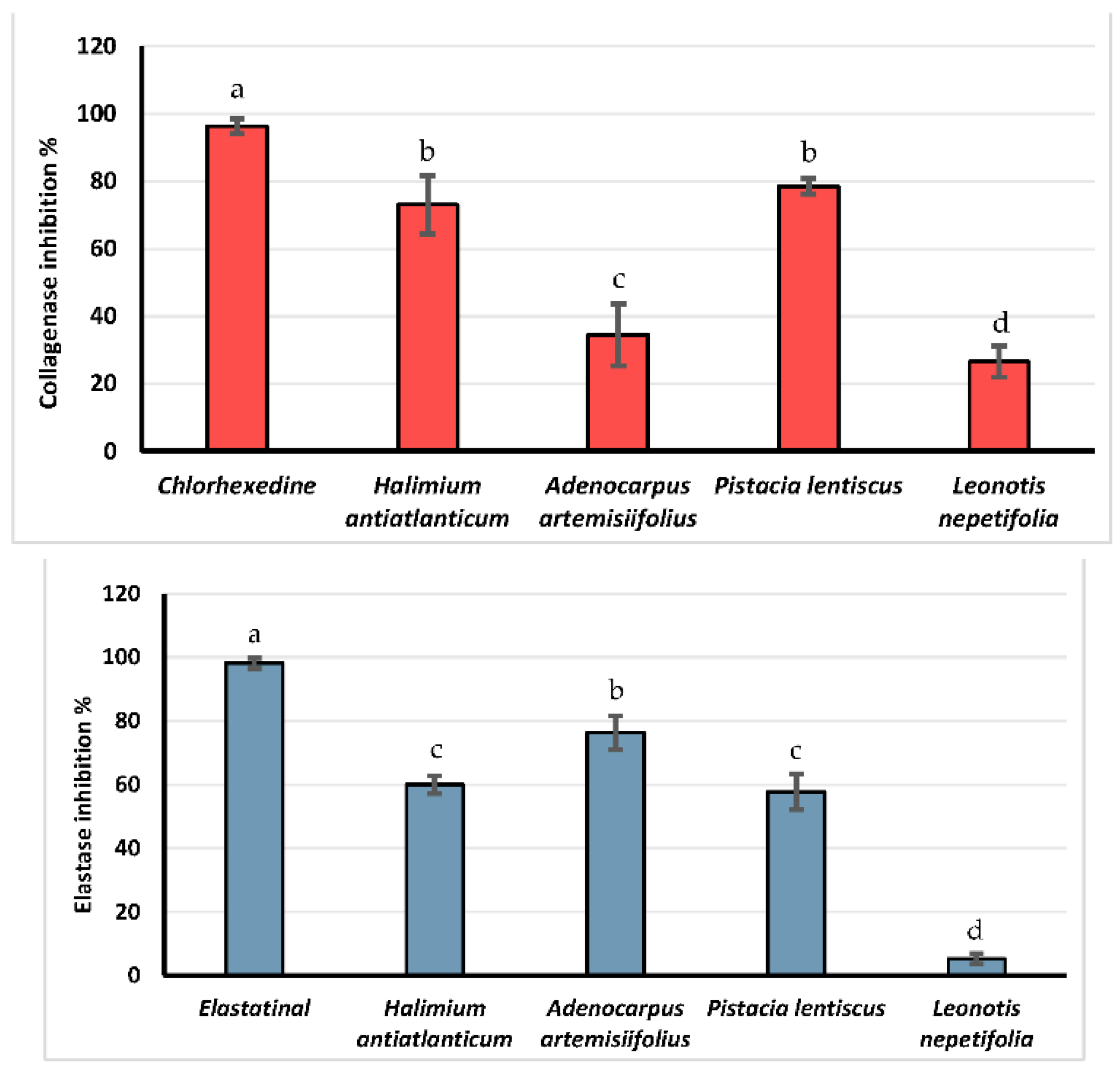
3.4. Dermocosmetic Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, A.R.; Grosso, C.; Delerue-Matos, C.; Rocha, J.M. Comprehensive review on the interaction between natural compounds and brain receptors: Benefits and toxicity. Eur. J. Med. Chem. 2019, 174, 87–115. [Google Scholar] [CrossRef] [PubMed]
- Aboukhalaf, A.; Tbatou, M.; Kalili, A.; Naciri, K.; Moujabbir, S.; Sahel, K.; Rocha, J.M.; Belahsen, R. Traditional knowledge and use of wild edible plants in Sidi Bennour region (Central Morocco). Ethnobot. Res. Appl. 2022, 23, 1–18. [Google Scholar] [CrossRef]
- Gamage, D.G.N.D.; Dharmadasa, R.M.; Chandana Abeysinghe, D.; Saman Wijesekara, R.G.; Prathapasinghe, G.A.; Someya, T. Global Perspective of Plant-Based Cosmetic Industry and Possible Contribution of Sri Lanka to the Development of Herbal Cosmetics. Evid. Based Complement. Altern. Med. 2022, 2022, 9940548. [Google Scholar] [CrossRef]
- Liyanaarachchi, G.D.; Samarasekera, J.K.R.R.; Mahanama, K.R.R.; Hemalal, K.D.P. Tyrosinase, elastase, hyaluronidase, inhibitory and antioxidant activity of Sri Lankan medicinal plants for novel cosmeceuticals. Ind. Crops Prod. 2018, 111, 597–605. [Google Scholar] [CrossRef]
- Samleti, A.; Bumrela, S.; Dhobale, S.; Kekre, V. ‘Herbal Cosmetics’; Indian Streams Research Journal: Solapur, India, 2012; Volume 2. [Google Scholar]
- Melo, R.S.d.; Reis, S.A.G.B.; Guimarães, A.L.; Silva, N.D.d.S.; Rocha, J.M.; El Aouad, N.; Almeida, J.R.G.d.S. Phytocosmetic Emulsion Containing Extract of Morus nigra L.(Moraceae): Development, Stability Study, Antioxidant and Antibacterial Activities. Cosmetics 2022, 9, 39. [Google Scholar] [CrossRef]
- Mechqoq, H.; Hourfane, S.; El Yaagoubi, M.; El Hamdaoui, A.; da Silva Almeida, J.R.G.; Rocha, J.M.; El Aouad, N. Molecular Docking, Tyrosinase, Collagenase, and Elastase Inhibition Activities of Argan By-Products. Cosmetics 2022, 9, 24. [Google Scholar] [CrossRef]
- Fennane, M.; Ibn Tattou, M. Statistiques et commentaires sur l’inventaire actuel de la flore vasculaire du Maroc. Bull. Inst. Sci. Sect. Sci. Vie 2012, 34, 1–9. [Google Scholar]
- Karumi, Y.; Onyeyili, P.; Ogugbuaja, V. Identification of active principles of M. balsamina (Balsam Apple) leaf extract. J. Med. Sci. 2004, 4, 179–182. [Google Scholar] [CrossRef]
- Ayoola, G.; Coker, H.; Adesegun, S.; Adepoju-Bello, A.; Obaweya, K.; Ezennia, E.; Atangbayila, T. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop. J. Pharm. Res. 2008, 7, 1019–1024. [Google Scholar] [CrossRef]
- Khanam, Z.; Wen, C.S.; Bhat, I.U.H. Phytochemical screening and antimicrobial activity of root and stem extracts of wild Eurycoma longifolia Jack (Tongkat ali). J. King Saud Univ. Sci. 2015, 27, 23–30. [Google Scholar] [CrossRef]
- Sayout, A.; Bahi, F.; Ouknin, M.; Arjouni, Y.; Majidi, L.; Romane, A. Phytochemical screening and antioxidant activity of four Moroccan Thymus species: T. leptobotrys Murb., T. pallidus Batt., T. broussonetti Boiss. and T. maroccanus Ball. Arab. J. Med. Aromat. Plants 2015, 1, 117–128. [Google Scholar] [CrossRef]
- Dohou, R.; Yamni, K.; Tahrouch, S.; Hassani, L.I.; Badoc, A.; Gmira, N. Screening phytochimique d’une endémique iberomarocaine, Thymelaea lythroides. Bull. Soc. Pharm. Bord. 2003, 142, 61–78. [Google Scholar]
- Sabri, F.Z.; Belarbi, M.; Sabri, S.; MS Alsayadi, M. Phytochemical screening and identification of some compounds from mallow. J. Nat. Prod. Plant Resour. 2012, 2, 512–516. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Vermerris, W.; Nicholson, R. Phenolic Compound Biochemistry; Springer Science & Business Media: New York, NY, USA, 2007. [Google Scholar]
- Benariba, N.; Djaziri, R.; Bellakhdar, W.; Belkacem, N.; Kadiata, M.; Malaisse, W.J.; Sener, A. Phytochemical screening and free radical scavenging activity of Citrullus colocynthis seeds extracts. Asian Pac. J. Trop. Biomed. 2013, 3, 35–40. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chem. 2000, 68, 81–85. [Google Scholar] [CrossRef]
- Gülçin, İ.; Topal, F.; Sarikaya, S.B.Ö.; Bursal, E.; Bilsel, G.; Gören, A.C. Polyphenol Contents and Antioxidant Properties of Medlar (Mespilus germanica L.). Rec. Nat. Prod. 2011, 5, 158. [Google Scholar]
- Oyaizu, M. Studies on products of browning reaction. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Angelis, A.; Hubert, J.; Aligiannis, N.; Michalea, R.; Abedini, A.; Nuzillard, J.-M.; Gangloff, S.C.; Skaltsounis, A.-L.; Renault, J.-H. Bio-guided isolation of methanol-soluble metabolites of common spruce (Picea abies) bark by-products and investigation of their dermo-cosmetic properties. Molecules 2016, 21, 1586. [Google Scholar] [CrossRef]
- Gardeli, C.; Vassiliki, P.; Athanasios, M.; Kibouris, T.; Komaitis, M. Essential oil composition of Pistacia lentiscus L. and Myrtus communis L.: Evaluation of antioxidant capacity of methanolic extracts. Food Chem. 2008, 107, 1120–1130. [Google Scholar] [CrossRef]
- Bampouli, A.; Kyriakopoulou, K.; Papaefstathiou, G.; Louli, V.; Aligiannis, N.; Magoulas, K.; Krokida, M. Evaluation of total antioxidant potential of Pistacia lentiscus var. chia leaves extracts using UHPLC–HRMS. J. Food Eng. 2015, 167, 25–31. [Google Scholar] [CrossRef]
- Oliveira, D.M.; Melo, F.G.; Balogun, S.O.; Flach, A.; de Souza, E.C.A.; de Souza, G.P.; Rocha, I.d.N.A.; da Costa, L.A.M.A.; Soares, I.M.; da Silva, L.I. Antibacterial mode of action of the hydroethanolic extract of Leonotis nepetifolia (L.) R. Br. involves bacterial membrane perturbations. J. Ethnopharmacol. 2015, 172, 356–363. [Google Scholar] [CrossRef]
- Talibi, I.; Karim, H.; Askarne, L.; Boubaker, H.; Boudyach, E.H.; Msanda, F.; Saadi, B.; Ait Ben Aoumar, A. Antifungal activity of aqueous and organic extracts of eight aromatic and medicinal plants against Geotrichum candidum, causal agent of citrus sour rot. Int. J. Agron. Plant Prod 2013, 4, 3510–3521. [Google Scholar]
- Beghlal, D.; El Bairi, K.; Marmouzi, I.; Haddar, L.; Mohamed, B. Phytochemical, organoleptic and ferric reducing properties of essential oil and ethanolic extract from Pistacia lentiscus (L.). Asian Pac. J. Trop. Dis. 2016, 6, 305–310. [Google Scholar] [CrossRef]
- Villar, A.; Sanz, M.; Paya, M. Hypotensive effect of Pistacia lentiscus L. Int. J. Crude Drug Res. 1987, 25, 1–3. [Google Scholar] [CrossRef]
- Trivedi, A.; Neeraj Sethiya, K.; Mishra, S. Preliminary pharmacognostic and phytochemical analysis of “Granthika” (Leonotis nepetaefolia): An ayurvedic herb. Indian J. Tradit. Knowl. 2011, 10, 682–688. [Google Scholar]
- Sobolewska, D.; Paśko, P.; Galanty, A.; Makowska-Wąs, J.; Padło, K.; Wasilak, W. Preliminary phytochemical and biological screening of methanolic and acetone extracts from Leonotis nepetifolia (L.) R. Br. J. Med. Plant Res. 2012, 6, 4582–4585. [Google Scholar] [CrossRef]
- Imran, S.; Suradkar, S.; Koche, D. Phytochemical analysis of Leonotis nepetifolia (L) R. BR. A wild medicinal plant of Lamiaceae. J. Biosci. Biotechnol. Discv. 2012, 3, 196–197. [Google Scholar]
- Cherbal, A.; Kebieche, M.; Madani, K.; El-Adawi, H. Extraction and valorization of phenolic compounds of leaves of Algerian Pistacia lentiscus. Asian J. Plant Sci. 2012, 11, 131. [Google Scholar] [CrossRef][Green Version]
- Askarne, L.; Talibi, I.; Boubaker, H.; Boudyach, E.; Msanda, F.; Saadi, B.; Serghini, M.; Aoumar, A.A.B. In vitro and in vivo antifungal activity of several Moroccan plants against Penicillium italicum, the causal agent of citrus blue mold. Crop Prot. 2012, 40, 53–58. [Google Scholar] [CrossRef]
- Talibi, I.; Askarne, L.; Boubaker, H.; Boudyach, E.; Msanda, F.; Saadi, B.; Ait Ben Aoumar, A. Antifungal activity of Moroccan medicinal plants against citrus sour rot agent Geotrichum candidum. Lett. Appl. Microbiol. 2012, 55, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Essokne, R.S.; Grayer, R.J.; Porter, E.; Kite, G.C.; Simmonds, M.S.; Jury, S.L. Flavonoids as chemosystematic markers for the genus Adenocarpus. Biochem. Syst. Ecol. 2012, 42, 49–58. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant activity of medicinal and aromatic plants. A review. Flavour Fragr. J. 2010, 25, 291–312. [Google Scholar] [CrossRef]
- Rebaya, A.; Belghith, S.I.; Baghdikian, B.; Leddet, V.M.; Mabrouki, F.; Olivier, E.; Cherif, J.; Ayadi, M.T. Total phenolic, total flavonoid, tannin content, and antioxidant capacity of Halimium halimifolium (Cistaceae). J. Appl. Pharm. Sci. 2014, 5, 52–57. [Google Scholar] [CrossRef]
- Rigane, G.; Ghazghazi, H.; Aouadhi, C.; Ben Salem, R.; Nasr, Z. Phenolic content, antioxidant capacity and antimicrobial activity of leaf extracts from Pistacia atlantica. Nat. Prod. Res 2017, 31, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Abu-Lafi, S.; Al-Rimawi, F.; Abbadi, J.; Naser, S.A.; Qabaha, K. Separation and identification of phenolics and flavonoids from wild Pistacia palaestina extract and its antioxidant activity. J. Med. Plant Res. 2020, 14, 317–325. [Google Scholar] [CrossRef]
- Takeda, T.; Narukawa, Y.; Hada, N. Studies on the constituents of Leonotis nepetaefolia. Chem. Pharm. Bull. 1999, 47, 284–286. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F. Methods for the assessment of antioxidant activity in foods. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 287–333. [Google Scholar]
- Berber, A.; Zengin, G.; Aktumsek, A.; Sanda, M.A.; Uysal, T. Antioxidant capacity and fatty acid composition of different parts of Adenocarpus complicatus (Fabaceae) from Turkey. Rev. Biol. Trop. 2014, 62, 337–346. [Google Scholar] [CrossRef]
- Ghenima, A.I.; Idir, M.; Nadjet, M.G.; Samia, M.A.; Mihoub, Z.M.; Karim, H. In vitro evaluation of biological activities of Pistacia lentiscus aqueous extract. Int. J. Pharm. Pharm. Sci. 2015, 7, 133–139. [Google Scholar]
- Hemma, R.; Belhadj, S.; Ouahchia, C.; Saidi, F. antioxidant activity of Pistacia lentiscus methanolic extracts. Agrobiologia 2018, 8, 845–852. [Google Scholar]
- El Hamdaoui, A.; Msanda, F.; Boubaker, H.; Leach, D.; Bombarda, I.; Vanloot, P.; El Aouad, N.; Abbad, A.; Boudyach, E.H.; Achemchem, F.; et al. Essential oil composition, antioxidant and antibacterial activities of wild and cultivated Lavandula mairei Humbert. Biochem. Syst. Ecol. 2018, 76, 1–7. [Google Scholar] [CrossRef]
- El Guiche, R.; Tahrouch, S.; Amri, O.; El Mehrach, K.; Hatimie, A. Antioxidant activity and total phenolic and flavonoid contents of 30 medicinal and aromatic plants located in the South of Morocco. Int. J. New Technol. Res. 2015, 1, 1151–1157. [Google Scholar]
- Kyriakopoulou, I.; Magiatis, P.; Skaltsounis, A.-L.; Aligiannis, N.; Harvala, C. Samioside, a New Phenylethanoid Glycoside with Free-Radical Scavenging and Antimicrobial Activities from Phlomis s amia. J. Nat. Prod. 2001, 64, 1095–1097. [Google Scholar] [CrossRef]
- Aligiannis, N.; Mitaku, S.; Tsitsa-Tsardis, E.; Harvala, C.; Tsaknis, I.; Lalas, S.; Haroutounian, S. Methanolic extract of Verbascum macrurum as a source of natural preservatives against oxidative rancidity. J. Agric. Food Chem. 2003, 51, 7308–7312. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; Santos, T.C.d.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Structural characteristics of the aging skin: A review. Cutan. Ocul. Toxicol. 2007, 26, 343–357. [Google Scholar] [CrossRef]
- Tortora, G.J.; Funke, B.R.; Case, C.L. Microbiology: An Introduction; Pearson: San Francisco, CA, USA, 2007. [Google Scholar]
- Elloumi, W.; Mahmoudi, A.; Ortiz, S.; Boutefnouchet, S.; Chamkha, M.; Sayadi, S. Wound healing potential of quercetin-3-O-rhamnoside and myricetin-3-O-rhamnoside isolated from Pistacia lentiscus distilled leaves in rats model. Biomed. Pharmacother. 2022, 146, 112574. [Google Scholar] [CrossRef]
- Deniz, F.S.S.; Orhan, I.E.; Duman, H. Profiling cosmeceutical effects of various herbal extracts through elastase, collagenase, tyrosinase inhibitory and antioxidant assays. Phytochem. Lett. 2021, 45, 171–183. [Google Scholar] [CrossRef]
- Chattuwatthana, T.; Okello, E. Anti-collagenase, anti-elastase and antioxidant activities of Pueraria candollei var. mirifica root extract and Coccinia grandis fruit juice extract: An in vitro study. Eur. J. Med. Plants 2015, 5, 318–327. [Google Scholar] [CrossRef]
- Imokawa, G.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging I: Reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef]
- Tanaka, T.; Metori, K.; Mineo, S.; Hirotani, M.; Furuya, T.; Kobayashi, S. Inhibitory effects of berberine-type alkaloids on elastase. Planta Med. 1993, 59, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Samy, M.N.; Le Goff, G.; Lopes, P.; Georgousaki, K.; Gumeni, S.; Almeida, C.; González, I.; Genilloud, O.; Trougakos, I.; Fokialakis, N. Osmanicin, a polyketide alkaloid isolated from Streptomyces osmaniensis CA-244599 inhibits elastase in human fibroblasts. Molecules 2019, 24, 2239. [Google Scholar] [CrossRef]
- Faccio, G. Plant complexity and cosmetic innovation. IScience 2020, 23, 101358. [Google Scholar] [CrossRef]


| Botanical Name | Family | Site | Used Parts |
|---|---|---|---|
| Halimium antiatlanticum Maire and Wilczek | Cistaceae | Souss-Massa Draa valley | Leaves + stems |
| Adenocarpus artemisiifolius Jahandiez, Maire and Weiller | Fabaceae | Ida-ou-Tanane mountain | Leaves + stems |
| Pistacia lentiscus (L.) | Anacardiaceae | Agadir | Leaves |
| Leonotis nepetifolia (L.) R.Br. | Lamiaceae | Agadir | Leaves |
| Botanical Name | Extract Color | Extraction Yield (%) |
|---|---|---|
| Halimium antiatlanticum, Maire and Wilczek | Green | 34.21 |
| Adenocarpus artemisiifolius Jahandiez, Maire and Weiller | Red-green | 27.54 |
| Pistacia lentiscus (L.) | Green | 46.35 |
| Leonotis nepetifolia (L.) R.Br. | Dark green | 13.13 |
| Phytochemical Constituents | Halimium antiatlanticum Maire and Wilczek | Adenocarpus artemisiifolius Jahandiez, Maire and Weiller | Pistacia lentiscus (L.) | Leonotis nepetifolia (L.) R.Br. |
|---|---|---|---|---|
| Alkaloids | − | +++ | − | − |
| Tannins | +++ | ++ | +++ | ++ |
| Polyphenols | +++ | ++ | +++ | + |
| Flavonoids | ++ | + | ++ | + |
| Anthocyanidins | ++ | + | + | − |
| Terpenoids | +++ | +++ | +++ | − |
| Anthraquinons | − | ++ | − | − |
| Carotenoids | − | ++ | − | + |
| Saponins | ++ | ++ | ++ | + |
| Control/Species | DPPH IC50 (µg/mL) | FRAP EC50 (µg/mL) | ABTS EC50 (µg/mL) |
|---|---|---|---|
| Ascorbic acid | 0.836 ± 0.017 e | 32.36 ± 1.306 e | 2.831 ± 0.52 e |
| Halimium antiatlanticum Maire and Wilczek | 5.037 ± 0.122 c | 71.613 ± 1.23 c | 10.98 ± 0.122 c |
| Adenocarpus artemisiifolius Jahandiez, Maire and Weiller | 58.813 ± 4.4 b | 420.88 ± 5.39 b | 67.1 ± 10.75 b |
| Pistacia lentiscus (L.) | 3.705 ± 0.445 d | 65.63 ± 1.41 d | 3.285 ± 0.911 d |
| Leonotis nepetifolia (L.) R.Br. | 779.407 ± 10.57 a | 597.96 ± 20.06 a | 582.3 ± 37.7 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mechqoq, H.; Hourfane, S.; Yaagoubi, M.E.; Hamdaoui, A.E.; Msanda, F.; Almeida, J.R.G.d.S.; Rocha, J.M.; Aouad, N.E. Phytochemical Screening, and In Vitro Evaluation of the Antioxidant and Dermocosmetic Activities of Four Moroccan Plants: Halimium antiatlanticum, Adenocarpus artemisiifolius, Pistacia lentiscus and Leonotis nepetifolia. Cosmetics 2022, 9, 94. https://doi.org/10.3390/cosmetics9050094
Mechqoq H, Hourfane S, Yaagoubi ME, Hamdaoui AE, Msanda F, Almeida JRGdS, Rocha JM, Aouad NE. Phytochemical Screening, and In Vitro Evaluation of the Antioxidant and Dermocosmetic Activities of Four Moroccan Plants: Halimium antiatlanticum, Adenocarpus artemisiifolius, Pistacia lentiscus and Leonotis nepetifolia. Cosmetics. 2022; 9(5):94. https://doi.org/10.3390/cosmetics9050094
Chicago/Turabian StyleMechqoq, Hicham, Sohaib Hourfane, Mohamed El Yaagoubi, Abdallah El Hamdaoui, Fouad Msanda, Jackson Roberto Guedes da Silva Almeida, Joao Miguel Rocha, and Noureddine El Aouad. 2022. "Phytochemical Screening, and In Vitro Evaluation of the Antioxidant and Dermocosmetic Activities of Four Moroccan Plants: Halimium antiatlanticum, Adenocarpus artemisiifolius, Pistacia lentiscus and Leonotis nepetifolia" Cosmetics 9, no. 5: 94. https://doi.org/10.3390/cosmetics9050094
APA StyleMechqoq, H., Hourfane, S., Yaagoubi, M. E., Hamdaoui, A. E., Msanda, F., Almeida, J. R. G. d. S., Rocha, J. M., & Aouad, N. E. (2022). Phytochemical Screening, and In Vitro Evaluation of the Antioxidant and Dermocosmetic Activities of Four Moroccan Plants: Halimium antiatlanticum, Adenocarpus artemisiifolius, Pistacia lentiscus and Leonotis nepetifolia. Cosmetics, 9(5), 94. https://doi.org/10.3390/cosmetics9050094











