Abstract
Tranexamic acid (TXA) has anti-plasmin activity and has been shown when administered orally to be effective against melasma, for which it is considered first-line pharmacotherapy. Several studies have shown that topically applied TXA is also effective against melasma and skin hyperpigmentation caused by sunburn and inflammation. The TXA concentration in the epidermis and dermis/vasculature has been estimated from its distribution in the skin after closed application, and topically applied TXA has thus been shown to act on neutrophils and mast cells in the dermis and on the vascular system. It is unlikely that topically applied TXA acts on dermal neutrophils or mast cells or on the vascular system to form thrombi. As discussed in the present review, studies on the effects of topical TXA on the hyperpigmentation process indicate that the resulting skin-lightening mechanism involves the suppression of cytokine/chemical mediator production, which stimulates melanin production via the keratinocyte-derived urokinase-type plasminogen activator and plasminogen derived from dermal vascular in the basal layer of the epidermis, thereby suppressing the production of excessive melanin to prevent hyperpigmentation.
1. Introduction
Melanin is produced by melanocytes in the basal layer of the epidermis and is constantly supplied to the surrounding keratinocytes. The keratinocytes are regulated to maintain a constant amount of melanin, and skin color therefore should not fluctuate. However, aging, ultraviolet radiation, and hormonal imbalance disrupt the skin’s regulatory mechanisms, resulting in increased melanin and excessive melanin accumulation in keratinocytes, which manifests as pigment spots [1]. The turnover time of the human epidermis varies among individuals, regions, and ages and is approximately 1 month on the forearm of a 37-year-old individual [2]. Hyperpigmentation produced by ultraviolet light fades gradually through epidermal turnover, while melasma fades gradually after menopause when factors that stimulate melanocytes return to normal levels. Senile lentigo, which increases with age, does not fade, and its pathogenic mechanism differs from that of melasma.
To date, approximately 20 quasi-drug whitening active ingredients have been developed for use in Japan [3]. Tranexamic acid (trans-4-aminomethylcyclohexanecarboxylic acid; TXA, Figure 1) is a drug with approximately 10-fold the anti-plasmin action exhibited by ε-aminocaproic acid [4] and has been used for more than 30 years to treat bleeding tendency and abnormal bleeding potentially related to increased fibrinolysis [5]. In the field of dermatology, TXA at 750–2000 mg per day in 3–4 divided doses have been used in the treatment of skin diseases such as eczema and related conditions, urticaria, drug eruptions, and toxic reactions, and its effects on itching, swelling, erythema, and other symptoms have been established [5]. In addition to inhibiting plasmin [6], TXA has recently been shown to readily compete with tyrosinase activity [7] and can inhibit hyperpigmentation by decreasing melanin synthesis [8,9]. TXA is available as a pharmaceutical cosmetic (quasi-drug containing TXA) that is effective against freckles and other pigment spots, and women with freckles can easily use it as part of their skin-lightening skincare regimen. Here, the mechanisms of action through which TXA may exert efficacy against melasma are reviewed.
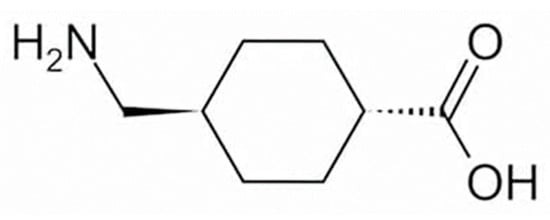
Figure 1.
Structural formula of tranexamic acid (trans-4-aminomethylcyclohexanecarboxylic acid).
2. Effectiveness of TXA for Melasma
Melasma is a common hyperpigmentation disorder, especially in women of reproductive age who are pregnant or using oral contraceptives [10,11]. Increased numbers of mast cells have been observed in the skin at the site of melasma lesions [12]. Mast cells may produce vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), and basic fibroblast growth factor (bFGF), all of which promote vascular growth and therefore contribute to the development of melasma [13,14]. Despite the important role of mast cells and histamine in the pathogenesis of melasma, anti-histamines have failed to show a benefit in the treatment of this condition. Oral TXA (the clinical trial registration number; DH-4243) has been shown to be effective against melasma [15] and has been available in Japan as an over-the-counter treatment for melasma since 2007. Several additional studies have demonstrated the efficacy of oral TXA [16,17,18,19,20,21,22] and its usefulness as a topical agent [23,24,25,26,27], and its ability to reduce epidermal hyperpigmentation of melasma and improve dermal disorders such as vascular and mast cell counts [16] has also been documented. In addition to oral TXA, topical hydroquinone is considered an effective treatment for melasma [28] and is widely used as a 2–5% ointment despite reported skin irritation and formulation instability [29]. Laser treatment, another option, can cause aggravation of lesions. Satisfactory results have been obtained after oral TXA treatment with a combination of oral and topical medications [20,30,31,32]. The toxicity of TXA to melanocytes or adverse effects of the topical application have not been reported.
The clinical efficacy of oral TXA has been well-established by a number of research groups [33]. Higashi et al. reported that oral administration of 750–1500 mg per day of TXA resulted in a significant improvement in hyperpigmentation 2–3 months after treatment initiation and an almost complete resolution in cases where treatment was continued for six months to one year [34]. A previous study evaluated the topical application of TXA as a cream containing 1% vitamin C for twice daily use (in the morning and before bedtime) for more than six months in 10 patients with melasma. Melasma was fully resolved in one patient who had light pigmentation at the start of treatment, while the nine remaining patients showed a reduction in hyperpigmentation, indicating the efficacy of the treatment [34].
The effectiveness against melasma of the topical application of a liposomal lotion containing 2% TXA, 3% ascorbic acid phosphate magnesium salt, and 2% α-tocopherol for at least three months has also been demonstrated [35]. Although quasi-drugs containing TXA take longer to show efficacy than oral TXA, a reduction in hyperpigmentation can nevertheless be observed (Figure 2). However, oral administration of TXA may cause mild gastrointestinal disturbance and irregular menstruation [36]. Therefore, given that melasma can be aggravated by UV light and inflammation, treatment with topical TXA may be beneficial after oral TXA administration.
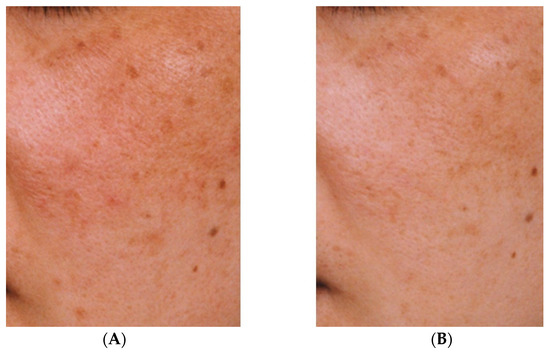
Figure 2.
Improvement of hyperpigmentation after three months (B) of continuous use of an emulsion containing tranexamic acid compared with pre-treatment hyperpigmentation (A) [33].
3. Effectiveness of TXA for Sun-Induced Hyperpigmentation
The effectiveness of an emulsion containing TXA in inhibiting pigmentation caused by UV irradiation was compared with that of an emulsion containing magnesium ascorbate phosphate salt. A significant difference (p < 0.01) was observed between the two emulsions (Figure 3) [33]. No adverse effects were reported in either group. Next, 30 women (aged 33–53 years) who were concerned about spots and dullness were treated with an emulsion containing TXA morning and night, twice a day for 3 months. Skin lightening with the improvement of freckles, dark spots, and dullness was observed, along with improvements in the texture and the appearance of pores [33]. There were no side effects reported during the 3-month treatment period [33].
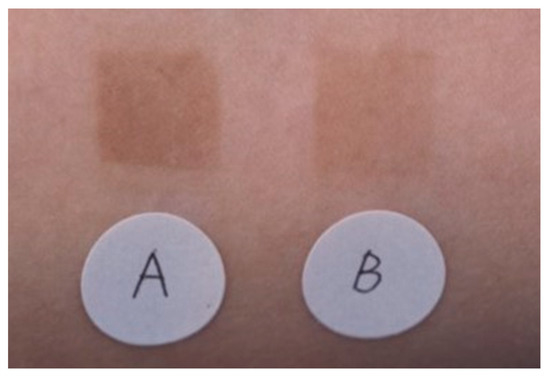
Figure 3.
Superior pigmentation suppression effect of an emulsion containing 2% TXA compared to that of an emulsion containing 3% magnesium ascorbate phosphate salt (APM). A: APM application site, B: TXA application site [33].
Two forms of sun-induced skin hyperpigmentation have been established: immediate pigment darkening (immediate tanning) and delayed darkening (delayed tanning). Immediate pigment darkening is primarily caused by long-wave ultraviolet radiation (UVA) and manifests as a grayish-black color during irradiation that fades within about 30 min and may be attributable to a redox reaction of existing melanin and its intermediates [37,38]. Conversely, delayed-type darkening is primarily caused by medium-wavelength ultraviolet (UVB) radiation, leading to dark brown coloring on approximately the fourth day after sunburn and clearly visible pigmentation on about the sixth day, persisting for weeks to months [39]. This hyperpigmentation is thought to be caused by an increase in tyrosinase activity in melanosomes (premelanosomes) organelles within pigment cells in the basal layer of the epidermis, resulting in an increased synthesis of melanin [39]. Histologically, such hyperactive melanocytes have been shown to have large dendrite extensions [39].
There are two major hypotheses regarding the mechanism of melanocyte activation in sun-induced skin hyperpigmentation (delayed-type darkening). One hypothesis is that UV light directly activates melanocytes and promotes melanin synthesis, while small DNA fragments produced during the repair and removal of DNA in UV-damaged melanocytes have been reported to increase tyrosinase activity, leading to increased melanin synthesis [40]. The other hypothesis is that epidermal cells exposed to UV light release various melanocyte-activating factors (signaling molecules) that target melanocytes to increase melanin synthesis and proliferation. Candidate signaling molecules have been described in the literature and include arachidonic acid metabolites such as prostaglandin E2 (PGE2) [41,42,43,44,45,46,47], various hormones such as melanocyte-stimulating hormone (MSH) [48,49], fibroblast growth factor [50], endothelin [51], and histamine [52]. The observation that these signaling molecules regulate melanocyte activity indicates that UV-induced melanocyte activation may be controlled by a high-level regulatory mechanism.
4. Mechanism of Action of TXA
TXA exhibits hemostatic, anti-inflammatory, and anti-allergic effects through its anti-plasmin activity [5], which results from the strong binding of TXA to the lysine binding site and fibrin affinity site of plasmin and plasminogen. The production by plasmin of kinins and other active peptides responsible for increased vascular permeability, allergy, and inflammatory lesions is subsequently inhibited [5].
In addition to its inhibitory effect on fibrin degradation (anti-fibrinolytic effect) [4], TXA has been reported to inhibit arachidonic acid release [53], prostaglandin and leukotriene production [54,55], neutrophil reactive oxygen species (ROS) release [56], histamine release in mast cells [57], TGF-β1 expression in keratinocytes [58], and vascular VEGF receptor inhibition [59]. In addition, arachidonic acid metabolites such as PGE2 [44,45,46,47] and histamine [52] have been reported to promote dendrite formation, proliferation, and melanogenesis in melanocytes.
Phospholipase A2, which mediates the release of arachidonic acid; activates cultured human melanocytes to promote melanin synthesis, and its metabolites, prostaglandins, and leukotrienes; activate cultured human melanocytes; and induce skin hyperpigmentation [44,45,46,47,60,61,62]. The action of phospholipase A2 on cell membrane phospholipids releases arachidonic acid, and plasmin promotes the conversion of phospholipase A2 from its inactive precursor to the active form [63]. Therefore, TXA may inhibit the release of arachidonic acid and the production of prostaglandins and other substances via its anti-plasmin action at the cell membrane.
The anti-fibrotic effect of TXA, its inhibitory effect on the release of ROS by neutrophils, and its inhibitory effect on the release of histamine by mast cells have been observed in vitro at concentrations of 7.8 μg/mL, 50 μg/mL, and 10 μg/mL, respectively, although the distribution of TXA in guinea pig skin following closed application has been measured at <3.66 μg/g in the epidermis and <0.012 μg/g in the dermis and vascular system [33]. The concentration of TXA in the dermis during topical application is therefore presumed to be lower than the effective concentration, and it is unlikely that TXA acts on dermal neutrophils, mast cells, or on the vascular system to form thrombi.
4.1. Mechanism of Action of TXA on Melasma
TXA shows hemostatic, anti-inflammatory, and anti-allergic effects attributable to its anti-plasmin action [4,5], exerted via strong binding to the lysine binding site and the fibrin affinity site of plasmin and plasminogen, to prevent the binding of plasmin and plasminogen to fibrin. As a result, TXA inhibits the production by plasmin of kinins and other active peptides, which are responsible for increased vascular permeability, allergy, and inflammatory lesions [5].
Histological studies have shown that melanocytes in areas of melasma actively synthesize melanin and that mature melanosomes accumulate abnormally in adjacent epidermal cells [64,65]. In addition to prostaglandins and leukotrienes, biological substances that promote melanogenesis include MSH [45,46], adrenocorticotropic hormone (ACTH) [66], and other factors that promote melanocyte proliferation in vitro, including bFGF [50] and stem cell factor (SCF) [67]. It has also been reported that the epidermis expresses leukotrienes [68], phospholipase A2 [69], VEGF [70,71], and pro-opiomelanocortin (POMC) in melanocytes and keratinocytes [49,72]; that MSH is increased in the epidermis of melasma areas [73,74]; and that dermal SCF and its receptor, c-kit [75], epidermal endothelin-1 [14], Wnt inhibitory factor 1, secreted frizzled-related protein 2, and Wnt5a [76] are increased in the epidermis of areas of melasma. Because plasmin is involved in the processing of POMC to MSH and in the release of bFGF, which promotes melanocyte proliferation [77,78], it is possible that TXA inhibits the processing of POMC to MSH and the release of bFGF and substances in the epidermis (Figure 4). Furthermore, plasmin is involved in the activation of cytokines, the release of active peptides, the promotion of transcription factor phosphorylation, and the promotion of cytokine expression, as shown in Table 1 [79,80,81,82,83]. TXA has also been reported to increase TGF-β in cultured keratinocytes [58] and to promote the autophagy system [84].
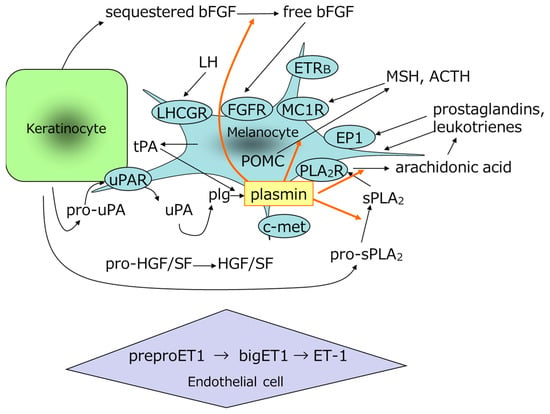
Figure 4.
Model for the generation of melanogenesis-promoting factor and growth factor by plasmin. ACTH, adrenocorticotropic hormone; bFGF, basic fibroblast growth factor; c-met, mesenchymal-epithelial transition factor; EP1, prostaglandin E2 receptor; ET-1, endothelin-1; ETRB, endothelin receptor B; FGFR, fibroblast growth factor receptor; HGF, hepatocyte growth factor; LH, luteinizing hormone; LHCGR, luteinizing hormone/choriogonadotropin receptor; MC1R, melanocortin receptor; MSH, melanocyte-stimulating hormone; PLA2R, phospholipase A2 receptor; PLG, plasminogen; POMC, pro-opiomelanocortin; SF, scatter factor; sPLA2, secretory phospholipase A2; tPA, tissue plasminogen activator; uPA, urokinase-type plasminogen activator; uPAR, uPA receptor.

Table 1.
Factors released or activated by plasmin.
4.2. Mechanism of Action of TXA on Sun-Induced Skin Hyperpigmentation
In addition to increasing tyrosinase in the melanocyte culture system, arachidonic acid metabolites modify cell morphology and increase the number of dendrites, thereby activating melanocytes [85,86,87]. Prostaglandin E2 and its precursor, arachidonic acid, have been used to induce hyperpigmentation in vivo by continuous application of arachidonic acid [45,62], while topical application of indomethacin (which inhibits the production of PGE2 from arachidonic acid) has been shown to suppress sunburn-induced skin hyperpigmentation [88]. These findings suggest that arachidonic acid metabolites such as PGE2, which have been shown to increase in the skin after UV irradiation [89,90,91], are important activators in the development of sun-induced skin hyperpigmentation. Therefore, we hypothesized that suppressing the production of arachidonic acid metabolites represents an effective means to prevent sunspots and freckles caused by sunburn and searched for active ingredients to prevent these conditions. We concluded that TXA, which is known to inhibit prostaglandin production [54], represented an appropriate active ingredient for formulation as a pharmaceutical cosmetic (quasi-drug) to prevent sunspots and freckles caused by sunburn as a result of its established effectiveness, safety, and concentration in the skin.
Based on changes in PGE2 levels [90] and tyrosinase activity [39] observed in the skin after UV irradiation and the concentrations of TXA that accumulate in cultured cells and in vitro [54,92], and in the skin, we evaluated the onset time, site, and mechanism of TXA action in the development of sunburn-induced hyperpigmentation. UV irradiation in humans increases both arachidonic acid and PGE2 in the skin, with a 2.8-fold increase after 24 h [90]. In vivo, UV-induced skin hyperpigmentation was suppressed by the application of 2% TXA [62], suggesting that TXA suppressed hyperpigmentation by inhibiting the production of PGE2, a key activator in hyperpigmentation formation that increases in the skin after UV irradiation [62]. Additionally, injection of TXA into guinea pig skin damaged by UV light has been shown to modulate melanocyte function and suppress melanin expression [93].
Tyrosinase activity increases 2–3 days after UV irradiation, increases more rapidly 3–4 days after irradiation, reaches its maximum at around 6 days, and then declines [39]. The increase in tyrosinase activity on the second to the third day after UV irradiation is thought to be attributable to the protein synthesis of tyrosinase in pigment cells after irradiation [39]. In vitro, TXA antagonistically inhibits tyrosinase with its substrate tyrosine (a competitive inhibitory effect). Therefore, relatively low concentrations of TXA can be expected to inhibit tyrosinase activity when the concentration of the substrate in vivo is sufficiently low [92].
The efficacy of an emulsion containing 2% TXA on sun-induced skin hyperpigmentation was evaluated with respect to its in vitro inhibitory effect on PGE2 production [54] and tyrosinase activity [92], as well as the changes in PGE2 levels [90] and tyrosinase activity in the skin after UV irradiation [39]. Application of TXA from the day of irradiation, when the amount of PGE2 starts to increase, to approximately 6 days later, at maximum tyrosinase activity, appears to suppress melanin formation caused by UV irradiation, resulting in suppression of hyperpigmentation. In a 24-week continuous-use test of an emulsion containing 5% TXA on normal skin in the absence of UV irradiation, neither depigmentation nor pigmentation was observed. TXA is effective when PGE2 and tyrosinase activity are increased by UV irradiation but does not affect normal skin in which these activities are not elevated.
TXA exerts a weak direct melanogenic effect on melanocytes but a moderate inhibitory effect on post-inflammatory hyperpigmentation, and thus may be effective against UV-induced suntanning and micro-inflammatory hyperpigmentation.
4.3. uPA/Plasminogen System in the Epidermis
The effectiveness of topical TXA application for melasma and sun-induced hyperpigmentation may in part attributable to its anti-plasmin action in the skin, especially in the epidermis. Urokinase-type plasminogen activator (uPA), which is implicated in the conversion of plasminogen to plasmin, is a double-stranded enzyme, and cultured human epidermal keratinocytes produce pro-uPA, a single-stranded precursor of uPA with no enzymatic activity. Because cultured human melanocytes produce little or no pro-uPA, melanocytes are thought to be activated by the epidermal keratinocyte-derived uPA and plasminogen derived from dermal vascular (uPA/plasminogen system) in the basal layer of the epidermis [94]. Tyrosinase mRNA and pro-uPA mRNA are co-expressed in pigmented areas, suggesting the involvement of the uPA/plasminogen system in the basal layer of the epidermis in the hyperpigmentation process. Given that melasma and sun-induced skin hyperpigmentation are thought to arise from an increase in melanin in the epidermal basal layer resulting from an abnormal increase in melanin synthesis centered on melanocyte activation, drugs that inhibit melanocyte activation may be effective against melasma and sun-induced skin hyperpigmentation. The effectiveness of topical TXA application in these conditions may be in part attributable to its mechanism of action, whereby melanocyte activation is inhibited by the anti-plasmin action of TXA in the skin.
Plasmin promotes the release of arachidonic acid, a precursor of prostaglandins [53], and arachidonic acid release by plasmin is inhibited by TXA [53]. This action of TXA, indicative of an anti-plasmin effect, is mediated by the formation of a reversible and very specific complex between TXA and the lysine binding sites of plasminogen and plasminogen activator [95], thereby inhibiting their binding to the cell membrane. This may be attributable to the inhibition of activation (conversion to plasmin) by plasminogen activator [96,97,98]. In skin, the presence of plasminogen [99] and uPA produced by epidermal cells [100,101] has been reported in the epidermal basal layer where melanocytes are located and where UV irradiation increases plasmin activity [102,103,104]. TXA is thought to suppress UV-induced increases in arachidonic acid and PGE2 [105,106] and cytokine production through its anti-plasmin effect. Cytokine modulates the generation ofα-MSH from the POMC system of the epidermis [77], the conversion of phospholipase A2 precursors to active [63], and the withdrawal of bFGF [78] upon UVB irradiation. Plasmin has also been reported to stimulate the expression of interleukin-8 (IL-8) mRNA, which is involved in inflammation [80] (Table 2).

Table 2.
Potential mechanisms of hyperpigmentation and involvement of the uPA/plasminogen system in the basal layer of the epidermis.
The proposed involvement of the epidermal uPA/plasminogen system in mechanisms of hyperpigmentation is summarized in Table 2 and includes activation of phospholipase A2; release of arachidonic acid; increased levels of PGE2 and leukotriene; activation of TGF-β; bFGF release; and processing of MSH from POMC. However, the increased number of vascular and mast cells in the dermis, and increased levels of preproendothelin-1 in the endothelial cells are not related to the uPA/plasminogen system in the basal layer of the epidermis. It remains unclear whether increased VEGF, increased autophagy systems, and increased Wnt inhibitory factors 1 and Wnt5a in the epidermis are associated with the uPA/plasminogen system in the basal layer of the epidermis. In addition to the reported effectiveness of TXA against hyperpigmentation after laser treatment, its efficacy in post-inflammatory hyperpigmentation has also been suggested, given the ability of TXA to suppress the increase in interleukin-1α (IL-1α), IL-8, and PGE2 in the culture supernatant of CO2 laser-treated keratinocytes [8].
TXA has also been reported to suppress melanogenesis [59] and proliferation of cultured human melanocytes, decrease mature Stage IV melanosomes [107], and inhibit the activity of tyrosinase, a melanogenic enzyme [7]. Tyrosinase activity appears to be suppressed via antagonistic inhibition, in which the substrate and enzyme compete for the active site [7]. In vivo, when the substrate concentration is sufficiently low, the inhibition of tyrosinase activity is expressed at lower concentrations and is thought to inhibit melanin synthesis [7].
Melasma often presents symmetrically on the forehead and cheeks, can vary in size with indistinct borders, and is thought to be related to levels of sex hormones given its appearance in pregnancy and alongside oral contraceptive treatment [108]. Serum plasminogen levels are elevated in women taking oral contraceptives and in women in their third trimester of pregnancy [109], suggesting that melanocytes are easily stimulated in these situations. Estrogen may cause persistent hyperpigmentation by releasing melanocyte-activating leukotriene C4 [110]. In young women, ovarian hormones, specifically estradiol, modulate endothelin receptor B (ETRB) function and contribute to the regulation of microvascular endothelial function [111]. Orally, TXA administration inhibits the plasmin/plasminogen pathway, thus disrupting the melanocyte and keratinocyte interaction [94], dermal vascular associated with melasma such as erythema [16], increased production and release of endothelin-1 in vascular endothelial cells, and mast cell activity [16]. Endothelial cells promote pigmentation through ETRB activation [112]. Increased levels of luteinizing hormone (LH) [113,114,115] and higher progesterone levels during the luteal phase [116] have been reported in the blood of patients with melasma compared with healthy individuals. The presence of plasminogen (plasmin) in the epidermal basal layer where melanocytes reside [99] has been reported, and LH has been shown to increase plasminogen activator [117], promote the release of arachidonic acid [118], and activate cultured human melanocytes in vitro [119].
5. Conclusions
Oral administration of TXA is effective in many cases of melasma, but recurrence of melasma is observed after discontinuation. Given that melasma and other pigment spots can be aggravated by ultraviolet rays, inflammation, and hormonal imbalance, treatment with pharmaceutical cosmetics (quasi-drugs) containing TXA, which has an anti-plasmin effect, can be beneficial in reducing susceptibility to hyperpigmentation by inhibiting the uPA/plasminogen system in the basal layer of the epidermis.
Funding
This review received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
I thank Clare Cox from Edanz (https://jp.edanz.com/) (accessed on 16 September 2022) for editing a draft of this manuscript.
Conflicts of Interest
The author declares no conflict of interest.
References
- Maeda, K. Large melanosome complex is increased in keratinocytes of solar lentigo. Cosmetics 2017, 4, 49. [Google Scholar] [CrossRef]
- Maeda, K. New method of measurement of epidermal turnover in humans. Cosmetics 2017, 4, 47. [Google Scholar] [CrossRef]
- Maeda, K. Timeline of the development of skin-lightening active ingredients in Japan. Molecules 2022, 27, 4774. [Google Scholar] [CrossRef] [PubMed]
- Abiko, Y.; Iwamoto, M. Plasminogen-plasmin system: VII. Potentiation of antifibrinolytic action of a synthetic inhibitor, tranexamic acid, by α2-macroglobulin antiplasmin. Biochim. Biophys. Acta 1970, 214, 411–418. [Google Scholar]
- Japanese Pharmacopoeia and Related Informations. The Japanese Pharmacopoeia 18th Edition, Tranexamic Acid, 1850~1851. Available online: https://jpdb.nihs.go.jp/kyokuhou/indexe.html (accessed on 15 September 2022).
- Dai, L.; Bevan, D.; Rangarajan, S.; Sørensen, B.; Mitchell, M. Stabilization of fibrin clots by activated prothrombin complex concentrate and tranexamic acid in FVIII inhibitor plasma. Haemophilia 2011, 17, e944–e948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Yang, X.H.; Yang, H.; Yang, Y.P. Study of inhibitory effect of acidum tranexamicum on melanin synthesis. Chin. J. Dermatovenerol. Int. Tradit. West. Med. 2003, 2, 227–229. [Google Scholar]
- Kim, M.S.; Bang, S.H.; Kim, J.H.; Shin, H.J.; Choi, J.H.; Chang, S.E. Tranexamic acid diminishes laser-induced melanogenesis. Ann. Dermatol. 2015, 27, 250–256. [Google Scholar] [CrossRef]
- Lindgren, A.L.; Austin, A.H.; Welsh, K.M. The Use of Tranexamic acid to prevent and treat post-inflammatory hyperpigmentation. J. Drugs Dermatol. 2021, 20, 344–345. [Google Scholar] [CrossRef]
- Passeron, T. Melasma pathogenesis and influencing factors—An overview of the latest research. J. Eur. Acad. Dermatol. Venereol. 2013, 27 (Suppl. 1), 5–6. [Google Scholar] [CrossRef]
- Lee, A.Y. Recent progress in melasma pathogenesis. Pigment Cell Melanoma Res. 2015, 28, 648–660. [Google Scholar] [CrossRef]
- Hernández-Barrera, R.; Torres-Alvarez, B.; Castanedo-Cazares, J.P.; Oros-Ovalle, C.; Moncada, B. Solar elastosis and presence of mast cells as key features in the pathogenesis of melasma. Clin. Exp. Dermatol. 2008, 33, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Kim, Y.C.; Lee, E.S.; Kang, H.Y. The vascular characteristics of melasma. J. Dermatol. Sci. 2007, 46, 111–116. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, J.Y.; Shibata, T.; Fujiwara, R.; Kang, H.Y. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin. Exp. Dermatol. 2016, 41, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, M.; Kawada, A.; Takiwaki, H.; Mizuno, A.; Torii, H.; Hayashi, N.; Nogita, T.; Akiyoshi, E.; Yoshikawa, N.; Watanabe, C.; et al. Clinical efficacy of DH-4243 for Chloasma: A multi-center randomized controlled trial. Jpn. J. Clin. Dermatol. 2007, 61, 735–743. (In Japanese) [Google Scholar]
- Na, J.I.; Choi, S.Y.; Yang, S.H.; Choi, H.R.; Kang, H.Y.; Park, K.C. Effect of TXA on melasma: A clinical trial with histological evaluation. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Thing, T.G.; Goh, C.L. Oral Tranexamic acid (TA) in the treatment of melasma: A retrospective analysis. J. Am. Acad. Dermatol. 2016, 75, 385–392. [Google Scholar] [CrossRef]
- Del Rosario, E.; Florez-Pollack, S.; Zapata, L., Jr.; Hernandez, K.; Tovar-Garza, A.; Rodrigues, M. Randomized, placebo-controlled, double-blind study of oral tranexamic acid in the treatment of moderate to severe melasma. J. Am. Acad. Dermatol. 2018, 78, 63–369. [Google Scholar] [CrossRef]
- Tse, T.W.; Hui, E. Tranexamic acid: An important adjuvant in the treatment of melasma. J. Cosmet. Dermatol. 2013, 12, 57–66. [Google Scholar] [CrossRef]
- Cho, H.H.; Choi, M.; Cho, S.; Lee, J.H. Role of oral tranexamic acid in melasma patients treated with IPL and low fluence QS Nd: YAG laser. J. Dermatolog. Treat. 2013, 24, 292–296. [Google Scholar] [CrossRef]
- Wu, S.; Shi, H.; Wu, H.; Yan, S.; Guo, J.; Sun, Y.; Pan, L. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast. Surg. 2012, 36, 964–970. [Google Scholar] [CrossRef]
- Kato, H.; Araki, J.; Eto, H.; Doi, K.; Hirai, R.; Kuno, S.; Higashino, T.; Yoshimura, K. A prospective randomized controlled study of oral tranexamic acid for preventing postinflammatory hyperpigmentation after Q-switched ruby laser. Dermatol. Surg. 2011, 37, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Okada, Y.; Tomita, Y. Clinical study of effect of tranexamic acid emulsion on melasma and freckles. Skin Res. 2007, 6, 309–315. (In Japanese) [Google Scholar]
- Ebrahimi, B.; Naeini, F.F. Topical tranexamic acid as a promising treatment for melasma. J. Res. Med. Sci. 2014, 19, 753–757. [Google Scholar] [PubMed]
- Banihashemi, M.; Zabolinejad, N.; Jaafari, M.R.; Salehi, M.; Jabari, A. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J. Cosmet. Dermatol. 2015, 14, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Na Ayuthaya, P.K.; Niumphradit, N.; Manosroi, A.; Nakakes, A. Topical 5% tranexamic acid for the treatment of melasma in Asians: A double-blind randomized controlled clinical trial. J. Cosmet. Laser Ther. 2012, 14, 150–154. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, R.; Juliandri, J.; Wang, X.; Xu, B.; Wang, D.; Lu, Y.; Zhou, B.; Luo, D. Efficacy of functional microarray of microneedles combined with topical tranexamic acid for melasma: A randomized, self-controlled, split-face study. Medicine 2017, 96, e6897. [Google Scholar] [CrossRef]
- Shihab, N.; Prihartono, J.; Tovar-Garza, A.; Agustin, T.; Legiawati, L.; Pandya, A.G. Randomised, controlled, double-blind study of combination therapy of oral tranexamic acid and topical hydroquinone in the treatment of melasma. Australas. J. Dermatol. 2020, 61, 237–242. [Google Scholar] [CrossRef]
- Igarashi, M.; Tomita, Y.; Seiji, M. Hydroquinone therapy for chloasma. Rinsho Derma 1977, 19, 761–765. (In Japanese) [Google Scholar]
- Shin, J.U.; Park, J.; Oh, S.H.; Lee, J.H. Oral tranexamic acid enhances the efficacy of low-fluence 1064-nm Q-switched neodymium-doped yttrium aluminum garnet laser treatment for melasma in Koreans: A randomized, prospective trial. Dermatol. Surg. 2013, 39, 435–442. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, F.; Liu, J.; Xia, X. Clinical observation and dermoscopy evaluation of fractional CO2 laser combined with topical tranexamic acid in melasma treatments. J. Cosmet. Dermatol. 2021, 20, 1110–1116. [Google Scholar] [CrossRef]
- Agamia, N.; Apalla, Z.; Salem, W.; Abdallah, W. A comparative study between oral tranexamic acid versus oral tranexamic acid and Q-switched Nd-YAG laser in melasma treatment: A clinical and dermoscopic evaluation. J. Dermatolog. Treat. 2021, 32, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K. Tranexamic acid. Mon. Book Derma 2005, 98, 35–42. (In Japanese) [Google Scholar]
- Higashi, N. Treatment of melasma with oral tranexamic acid. Ski. Res. 1988, 30, 676–680. (In Japanese) [Google Scholar]
- Kita, Y.; Sugai, T. Effect of bleach agents on chloasma. Ski. Res. 1992, 34, 142–146. (In Japanese) [Google Scholar]
- Zhu, C.Y.; Li, Y.; Sun, Q.N.; Takada, A.; Kawada, A. Analysis of the effect of different doses of oral tranexamic acid on melasma: A multicentre prospective study. Eur. J. Dermatol. 2019, 29, 55–58. [Google Scholar]
- Miescher, G.; Minder, H. Untersuchungen über die durch langwelliges Ultraviolett hervorgerufene Pigmetdunkelung. Strahlentherapie 1939, 66, 6–23. [Google Scholar]
- Pathak, M.A.; Stratton, K. Free radicals in human skin before and after exposure to light. Arch. Biochem. Biophys. 1968, 123, 468–476. [Google Scholar] [CrossRef]
- Mizuno, N. Behavior of melanocyte after single ultraviolet irradiation. Jpn. J. Clin. Dermatol. 1968, 22, 131–143. (In Japanese) [Google Scholar]
- Eller, M.S.; Yaar, S.M.; Gilchrest, B.A. DNA damage and melanogenesis. Nature 1994, 372, 413–414. [Google Scholar] [CrossRef]
- Abdel-Malek, Z.A. Endocrine factors as effectors of integumental pigmentation. In Dermatologic Clinics; Nordlund, J.J., Ed.; W.B. Saunders: New York, NY, USA, 1988; Volume 6, pp. 175–183. [Google Scholar]
- Nordlund, J.J.; Abdel-Malek, Z.A.; Boissy, R.E.; Rheins, L.A. Pigment cell biology: An historical review. J. Invest. Dermatol. 1989, 92, 53S–60S. [Google Scholar] [CrossRef]
- Gilchrest, B.A.; Park, H.-Y.; Eller, M.S.; Yaar, M. Mechanisms of ultraviolet light-induced pigmentation. Photochem. Photobiol. 1996, 63, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Maeda, K.; Tagami, H. Mechanisms for hyperpigmentation in postinflammatory pigmentation, ulticaria pigmentosa and sunburn. Dermatologica 1989, 179, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Nordlund, J.J.; Collins, C.E.; Rheins, L.A. Prostaglandin E2 and D2 but not MSH stimulate the proliferation of pigment cells in the pinnal epidermis of the DBA/2 mouse. J. Invest. Dermatol. 1986, 86, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Maeda, K.; Tagami, H. Melanocyte-stimulating properties of arachidonic acid metabolites: Possible role in postinflammatory pigmentation. Pigment. Cell Res. 1992, 5, 357–361. [Google Scholar] [CrossRef]
- Maeda, K.; Naganuma, M. Melanocyte-stimulating properties of secretory phospholipase A2. Photochem. Photobiol. 1997, 65, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Birchall, N.; Orlow, S.J.; Kupper, T.; Pawelek, J. Interactions between ultraviolet light and interleukin-1 on MSH binding in both mouse melanoma and human sequamous carcinoma cells. Biochem. Biophys. Res. Commun. 1991, 175, 839–845. [Google Scholar] [CrossRef]
- Schauer, E.; Trautinger, F.; Köck, A.; Schwarz, A.; Bhardwaj, R.; Simon, M.; Ansel, J.C.; Schwarz, T.; Luger, T.A. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J. Clin. Invest. 1994, 93, 2258–2262. [Google Scholar] [CrossRef]
- Halaban, R.; Langdon, R.; Birchall, N.; Cuono, C.; Baird, A.; Scott, G.; Moellmann, G.; McGuire, J. Basic fibroblast growth factor from human keratinocytes is a natural mitogen for melanocytes. J. Cell Biol. 1988, 107, 1611–1619. [Google Scholar] [CrossRef]
- Imokawa, G.; Yada, Y.; Miyagishi, M. Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J. Biol. Chem. 1992, 267, 24675–24680. [Google Scholar] [CrossRef]
- Tomita, Y.; Maeda, K.; Tagami, H. Stimulatory effect of histamine on normal human melanocytes in vitro. Tohoku J. Exp. Med. 1998, 155, 209–210. [Google Scholar] [CrossRef]
- Chang, W.C.; Shi, G.Y.; Chow, Y.H.; Chang, L.C.; Hau, J.S.; Lin, M.T.; Jen, C.J.; Wing, L.Y.; Wu, H.L. Human plasmin induces a receptor-mediated arachidonate release coupled with G proteins in endothelial cells. Am. J. Physiol. 1993, 264, C271–C281. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, Y.; Kitamura, S.; Kosaka, K.; Harasawa, M. Tranexamic acid no prostaglandin gouseisogai ni kansuru kenkyu. Jpn. Pharmacol. Ther. 1978, 6, 398–402. (In Japanese) [Google Scholar]
- Weide, I.; Tippler, B.; Syrovets, T.; Simmet, T. Plasmin is a specific stimulus of the 5-lipoxygenase pathway of human peripheral monocytes. Thromb. Haemost. 1996, 76, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Akamatsu, H.; Matoba, Y.; Ri, S.; Ito, A.; Asada, Y. Effects of Tranexamic Acid on Neutrophil Chemotaxis, Phagocytosis and Reactive Oxygen Species Generation in vitro. Jpn. Pharmacol. Ther. 1994, 22, 1429–1435. (In Japanese) [Google Scholar]
- Toki, N.; Takasugi, S.; Fujii, K. Basic research of histaminergic drugs and antihistaminergic drugs. Med. Consult. New Remedies 1981, 18, 1195–1202. (In Japanese) [Google Scholar]
- Xing, X.; Xu, Z.; Chen, L.; Jin, S.; Zhang, C.; Xiang, L. Tranexamic acid inhibits melanogenesis partially via stimulation of TGF-β1 expression in human epidermal keratinocytes. Exp. Dermatol. 2022, 31, 633–640. [Google Scholar] [CrossRef]
- Zhu, J.W.; Ni, Y.J.; Tong, X.Y.; Guo, X.; Wu, X.P.; Lu, Z.F. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int. J. Med. Sci. 2020, 17, 903–911. [Google Scholar] [CrossRef]
- Tomita, Y.; Maeda, K.; Tagami, H. Leukotrienes and thromboxane B2 stimulate normal human melanocytes in vitro: Possible inducers of postinflammatory pigmentation. Tohoku J. Exp. Med. 1988, 156, 303–304. [Google Scholar] [CrossRef]
- Morelli, J.G.; Hake, S.S.; Murphy, R.C.; Norris, D.A. Leukotriene B4-induced human melanocyte pigmentation and leukotriene C4-induced human melanocyte growth are inhibited by different isoquinolinesulfonamides. J. Invest. Dermatol. 1992, 98, 55–58. [Google Scholar] [CrossRef]
- Maeda, K.; Naganuma, M. Topical trans-4-aminomethylcyclohexanecarboxylic acid prevents ultraviolet radiation-induced pigmentation. J. Photochem. Photobiol. B Biol. 1998, 47, 136–141. [Google Scholar] [CrossRef]
- Nakano, T.; Fujita, H.; Kikuchi, N.; Arita, H. Plasmin converts pro-form of group I phospholipase A2 into receptor binding, active forms. Biochem. Biophys. Res. Commun. 1994, 198, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, N.P.; Pathak, M.A.; Sato, S.; Fitzpatrick, T.B.; Sanchez, J.L.; Mihm, M.C., Jr. Melasma: A clinical, light microscopic, ultrastructural, and immunofluorescence study. J. Am. Acad. Dermatol. 1981, 4, 698–710. [Google Scholar] [CrossRef]
- Kang, W.H.; Yoon, K.H.; Lee, E.S.; Kim, J.; Lee, K.B.; Yim, H.; Sohn, S.; Im, S. Melasma: Histopathological characteristics in 56 Korean patients. Br. J. Dermatol. 2002, 146, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Hunt, G.; Donatien, P.D.; Lunec, J.; Todd, C.; Kyne, S.; Thody, A.J. Cultured human melanocytes respond to MSH peptides and ACTH. Pigment Cell Res. 1994, 7, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Grabbe, J.; Welker, P.; Dippel, E.; Czarnetzki, B.M. Stem cell factor, a novel cutaneous growth factor for mast cells and melanocytes. Arch. Dermatol. Res. 1994, 287, 78–84. [Google Scholar] [CrossRef]
- Iversen, L.; Kristensen, P.; Grøn, B.; Ziboh, V.A.; Kragballe, K. Human epidermis transforms exogenous leukotriene A4 into peptide leukotrienes: Possible role in transcellular metabolism. Arch Dermatol. Res. 1994, 286, 261–266. [Google Scholar] [CrossRef]
- Man, M.Q.; Lin, T.K.; Santiago, J.L.; Celli, A.; Zhong, L.; Huang, Z.M.; Roelandt, T.; Hupe, M.; Sundberg, J.P.; Silva, K.A.; et al. Basis for enhanced barrier function of pigmented skin. J. Invest. Dermatol. 2014, 134, 2399–2407. [Google Scholar] [CrossRef]
- Yan, B.X.; Zheng, Y.X.; Li, W.; Chen, J.Q.; Zhou, J.; Cai, S.Q.; Zheng, M.; Man, X.Y. Comparative expression of PEDF and VEGF in human epidermal keratinocytes and dermal fibroblasts: From normal skin to psoriasis. Discov. Med. 2018, 25, 47–56. [Google Scholar]
- Khunger, N.; Kandhari, R.; Singh, A.; Ramesh, V. A clinical, dermoscopic, histopathological and immunohistochemical study of melasma and facial pigmentary demarcation lines in the skin of color. Dermatol. Ther. 2020, 33, e14515. [Google Scholar] [CrossRef]
- Bhardwaj, R.S.; Luger, T.A. Proopiomelanocortin production by epidermal cells: Evidence for an immune neuroendocrine network in the epidermis. Arch. Dermatol. Res. 1994, 287, 85–90. [Google Scholar] [CrossRef]
- Miot, L.D.; Miot, H.A.; Polettini, J.; Silva, M.G.; Marques, M.E. Morphologic changes and the expression of alpha-melanocyte stimulating hormone and melanocortin-1 receptor in melasma lesions: A comparative study. Am. J. Dermatopathol. 2010, 32, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Im, S.; Kim, J.; On, W.Y.; Kang, W.H. Increased expression of alpha-melanocyte-stimulating hormone in the lesional skin of melasma. Br. J. Dermatol. 2002, 146, 165–167. [Google Scholar] [PubMed]
- Kang, H.Y.; Hwang, J.S.; Lee, J.Y.; Ahn, J.H.; Kim, J.Y.; Lee, E.S.; Kang, W.H. The dermal stem cell factor and c-kit are overexpressed in melasma. Br. J. Dermatol. 2006, 154, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.Y.; Suzuki, I.; Lee, D.J.; Ha, J.; Reiniche, P.; Aubert, J.; Deret, S.; Zugaj, D.; Voegel, J.J.; Ortonne, J.P. Transcriptional profiling shows altered expression of wnt pathway- and lipid metabolism-related genes as well as melanogenesis-related genes in melasma. J. Invest. Dermatol. 2011, 131, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, L.; Miles, L.; Hoover-Plow, J. Plasminogen regulates pro-opiomelanocortin processing. J. Thromb. Haemost. 2004, 2, 785–796. [Google Scholar] [CrossRef]
- Falcone, D.J.; McCaffrey, T.A.; Haimovitz-Friedman, A.; Vergilio, J.A.; Nicholson, A.C. Macrophage and foam cell release of matrix-bound growth factors. Role of plasminogen activation. J. Biol. Chem. 1993, 268, 11951–11958. [Google Scholar] [CrossRef]
- Syrovets, T.; Jendrach, M.; Rohwedder, A.; Schüle, A.; Simmet, T. Plasmin-induced expression of cytokines and tissue factor in human monocytes involves AP-1 and IKKbeta-mediated NF-kappaB activation. Blood 2001, 97, 3941–3950. [Google Scholar] [CrossRef]
- Kamio, N.; Hashizume, H.; Nakao, S.; Matsushima, K.; Sugiya, H. Plasmin is involved in inflammation via protease-activated receptor-1 activation in human dental pulp. Biochem. Pharmacol. 2008, 75, 1974–1980. [Google Scholar] [CrossRef]
- Burysek, L.; Syrovets, T.; Simmet, T. The serine protease plasmin triggers expression of MCP-1 and CD40 in human primary monocytes via activation of p38 MAPK and janus kinase (JAK)/STAT signaling pathways. J. Biol. Chem. 2002, 277, 33509–33517. [Google Scholar] [CrossRef]
- Matsumura, Y.; Takada, K.; Murakami, A.; Takaoka, M.; Morimoto, S. Plasmin stimulates expression of endothelin-1 mRNA and endothelin-1 release in vascular endothelial cells. Life Sci. 1996, 58, 1067–1074. [Google Scholar] [CrossRef]
- Naldini, L.; Tamagnone, L.; Vigna, E.; Sachs, M.; Hartmann, G.; Birchmeier, W.; Daikuhara, Y.; Tsubouchi, H.; Blasi, F.; Comoglio, P.M. Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth factor/scatter factor. EMBO J. 1992, 11, 4825–4833. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.H.; Park, J.E.; Lim, D.S.; Lee, J.S. Tranexamic acid inhibits melanogenesis by activating the autophagy system in cultured melanoma cells. J. Dermatol. Sci. 2017, 88, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Iwamoto, M.; Masuda, T.; Tagami, H. Stimulatory effect of prostaglandin E2 on the configulation of normal human melanocytes in vitro. J. Invest. Dermatol. 1987, 89, 299–301. [Google Scholar] [CrossRef]
- Abdel-Malek, Z.A.; Swope, V.B.; Amornsiripanitch, N.; Nordlund, J.J. In vitro modulation of proliferation and melanization of S91 melanoma cells by prostaglandins. Cancer Res. 1987, 47, 3141–3146. [Google Scholar] [PubMed]
- Imokawa, G.; Motegi, I. Skin organ culture model for examining epidermal melanization. J. Invest. Dermatol. 1993, 100, 47–54. [Google Scholar] [CrossRef]
- Takiwaki, H.; Shirai, S.; Kohno, H.; Soh, H.; Arase, S. The degrees of UVB-induced erythema and pigmentation correlate linealy and are reduced in a parallel manner by topical anti-inflammatory agents. J. Invest. Dermatol. 1994, 103, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Black, A.K.; Greaves, M.W.; Hensby, C.N.; Plummer, N.A. Increased prostaglandins E2 and F2 alpha in human skin at 6 and 24 h after ultraviolet B irradiation (290–320 nm). Br. J. Clin. Pharmac. 1978, 5, 431–436. [Google Scholar] [CrossRef]
- Black, A.K.; Fincham, N.; Greaves, M.W.; Hensby, C.N. Time course changes in levels of arachidonic acid and prostaglandins D2, E2, F2 alpha in human skin following ultraviolet B irradiation. Br. J. Clin. Pharmac. 1980, 10, 453–457. [Google Scholar] [CrossRef]
- Hawk, J.L.M.; Black, A.K.; Jaenicke, K.F.; Barr, R.M.; Soter, N.A.; Mallett, A.I.; Gilchrest, B.A.; Hensby, C.N.; Parrish, J.A.; Greaves, M.W. Increased concentrations of arachidonic acid, prostaglandins E2, D2, and 6-oxo-F1 alpha, and histamine in human skin following UVA irradiation. J. Invest. Dermatol. 1983, 80, 496–499. [Google Scholar] [CrossRef]
- Mikoshiba, H.; Takei, M.; Takase, Y.; Nijo, S.; Shimosato, F.; Nomoto, S. Kanpan ni taisuru tranekisamsan naifuku ryouhou. Nishi Nihon Hifuka 1985, 47, 1101–1104. (In Japanese) [Google Scholar] [CrossRef]
- Li, D.; Shi, Y.; Li, M.; Liu, J.; Feng, X. Tranexamic acid can treat ultraviolet radiation-induced pigmentation in guinea pigs. Eur. J. Dermatol. 2010, 20, 89–92. [Google Scholar] [CrossRef]
- Maeda, K.; Tomita, Y. Mechanism of the inhibitory effect of tranexamic acid on melanogenesis in cultured human melanocytes in the presence of keratinocyte-conditioned medium. J. Health Sci. 2007, 53, 389–396. [Google Scholar] [CrossRef]
- Takada, A.; Takada, Y. Inhibition by tranexamic acid of the conversion of single-chain tissue plasminogen activator to its two chain form by plasmin: The presence on tissue plasminogen activator of a site to bind with lysine binding sites of plasmin. Thromb. Res. 1989, 55, 717–725. [Google Scholar] [CrossRef]
- Miles, L.A.; Dahlberg, C.M.; Plescia, J.; Felez, J.; Kato, K.; Plow, E.F. Role of cell-surface lysines in plasminogen binding to cells: Identification of alpha-enolase as a candidate plasminogen receptor. Biochemistry 1991, 30, 1682–1691. [Google Scholar] [CrossRef]
- Plow, E.F.; Herren, T.; Redlitz, A.; Miles, L.A.; Hoover-Plow, J.L. The cell biology of the plasminogen system. FASEB J. 1995, 9, 939–9455. [Google Scholar] [CrossRef]
- Bizik, J.; Stephens, R.W.; Grofova, M.; Vaheri, A. Binding of tissue-type plasminogen activator to human melanoma cells. J. Cell Biochem. 1993, 51, 326–335. [Google Scholar] [CrossRef]
- Isseroff, R.R.; Rifkin, D.B. Plasminogen is present in the basal layer of the epidermis. J. Invest. Dermatol. 1983, 80, 297–299. [Google Scholar] [CrossRef]
- Spiers, E.M.; Lazarus, G.S.; Lyons-Giordano, B. Expression of plasminogen activators in psoriatic epidermis. J. Invest. Dermatol. 1994, 102, 333–338. [Google Scholar] [CrossRef][Green Version]
- Loud, L.R.; Eriksen, J.; Ralfkiaer, E.; Romer, J. Differential expression of urokinase plasminogen activator, its receptor, and inhibitors in mouse skin after exposure to a tumor-promoting phorbol ester. J. Invest. Dermatol. 1996, 106, 622–630. [Google Scholar]
- Ichikawa, K.; Takashima, A.; Yasuda, S.; Mizuno, N. Enhanced rabbit skin plasmin activity by UV irradiation. Dermatologica 1989, 179 (Suppl. 1), 132. [Google Scholar] [CrossRef]
- Takashima, A.; Yasuda, S.; Mizuno, N. Determination of the action spectrum for UV-induced plasminogen activator synthesis in mouse keratinocytes in vitro. J. Dermatol. Sci. 1992, 4, 11–17. [Google Scholar] [CrossRef]
- Rotem, N.; Axelrod, J.H.; Miskin, R. Induction of urokinase-type plasminogen activator by UV light in human fetal fibroblasts is mediated through a UV-induced secreted protein. Mol. Cell Biol. 1987, 7, 622–631. [Google Scholar]
- Kang-Rotondo, C.H.; Miller, C.C.; Morrison, A.R.; Pentland, A.P. Enhanced keratinocyte prostaglandin synthesis after UV injury is due to increased phospholipase activity. Am. J. Physiol. 1993, 264, 396–401. [Google Scholar] [CrossRef]
- Grewe, M.; Trefzer, U.; Ballhorn, A.; Gyufko, K.; Henninger, H.; Krutmann, J. Analysis of the mechanism of ultraviolet (UV) B radiation-induced prostaglandin E2 synthesis by human epidermoid carcinoma cells. J. Invest. Dermatol. 1993, 101, 528–531. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Horikoshi, T.; Eguchi, H.; Onodera, H. The effects of tranexamic acid on the growth and melanogenesis of cultured human melanocytes. Jpn. J. Dermatol. 1994, 104, 641–646. [Google Scholar]
- Cario, M. How hormones may modulate human skin pigmentation in melasma: An in vitro perspective. Exp. Dermatol. 2019, 28, 709–718. [Google Scholar] [CrossRef]
- Peterson, R.A.; Krull, P.E.; Finley, P.; Ettinger, M.G. Changes in antithrombin 3 and plasminogen induced by oral contraceptives. Am. J. Clin. Pathol. 1970, 53, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Zaitsu, M.; Narita, S.; Lambert, K.C.; Grady, J.J.; Estes, D.M.; Curran, E.M.; Brooks, E.G.; Watson, C.S.; Goldblum, R.M.; Midoro-Horiuti, T. Estradiol activates mast cells via a non-genomic estrogen receptor-alpha and calcium influx. Mol. Immunol. 2007, 44, 1977–1985. [Google Scholar] [CrossRef]
- Shoemaker, L.N.; Haigh, K.M.; Kuczmarski, A.V.; McGinty, S.J.; Welti, L.M.; Hobson, J.C.; Edwards, D.G.; Feinberg, R.F.; Wenner, M.M. ETB receptor-mediated vasodilation is regulated by estradiol in young women. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H592–H598. [Google Scholar] [CrossRef]
- Regazzetti, C.; De Donatis, G.M.; Ghorbel, H.H.; Cardot-Leccia, N.; Ambrosetti, D.; Bahadoran, P.; Chignon-Sicard, B.; Lacour, J.P.; Ballotti, R.; Mahns, A.; et al. Endothelial cells promote pigmentation through endothelin receptor B activation. J. Invest. Dermatol. 2015, 135, 3096–3104. [Google Scholar] [CrossRef]
- Pérez, M.; Sánchez, J.L.; Aguiló, F. Endocrinologic profile of patients with idiopathic melasma. J. Invest. Dermatol. 1983, 81, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Hassan, I.; Kaur, I.; Sialy, R.; Dash, R.J. Hormonal milieu in the maintenance of melasma in fertile women. J. Dermatol. 1998, 25, 510–512. [Google Scholar] [CrossRef] [PubMed]
- Sialy, R.; Hassan, I.; Kaur, I.; Dash, R.J. Melasma in men: A hormonal profile. J. Dermatol. 2000, 27, 64–65. [Google Scholar] [CrossRef] [PubMed]
- Sato, N. Endocrine environment in adult females with chloasma. Jpn. J. Dermatol. 1987, 97, 937–943. (In Japanese) [Google Scholar]
- Reich, R.; Miskin, R.; Tsafriri, A. Follicular plasminogen activator: Involvement in ovulation. Endocrinology 1985, 116, 516–521. [Google Scholar] [CrossRef]
- Moraga, P.F.; Llanos, M.N.; Ronco, A.M. Arachidonic acid release from rat Leydig cells depends on the presence of luteinizing hormone/human chorionic gonadotrophin receptors. J. Endocrinol. 1997, 154, 201–209. [Google Scholar] [CrossRef]
- Maeda, K.; Naganuma, M.; Fukuda, M.; Matsunaga, J.; Tomita, Y. Effect of pituitary and ovarian hormones on human melanocytes in vitro. Pigment. Cell Res. 1996, 9, 204–212. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).