Effect of Coffee Berry Extract on Anti-Aging for Skin and Hair—In Vitro Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction
2.2. Determination of Caffeine and Chlorogenic Acid
2.3. Cell Culture
2.4. Cytotoxicity
2.5. Cell Proliferation
2.6. Antioxidant Activity by Superoxide Dismutase
2.7. Antioxidant Activity by Nitric Oxide Inhibition
2.8. Anti-Collagenase Activity
2.9. 5α-Reductase Inhibition
2.10. Growth Factor Gene Expression
2.11. Statistical Analysis
3. Results and Discussion
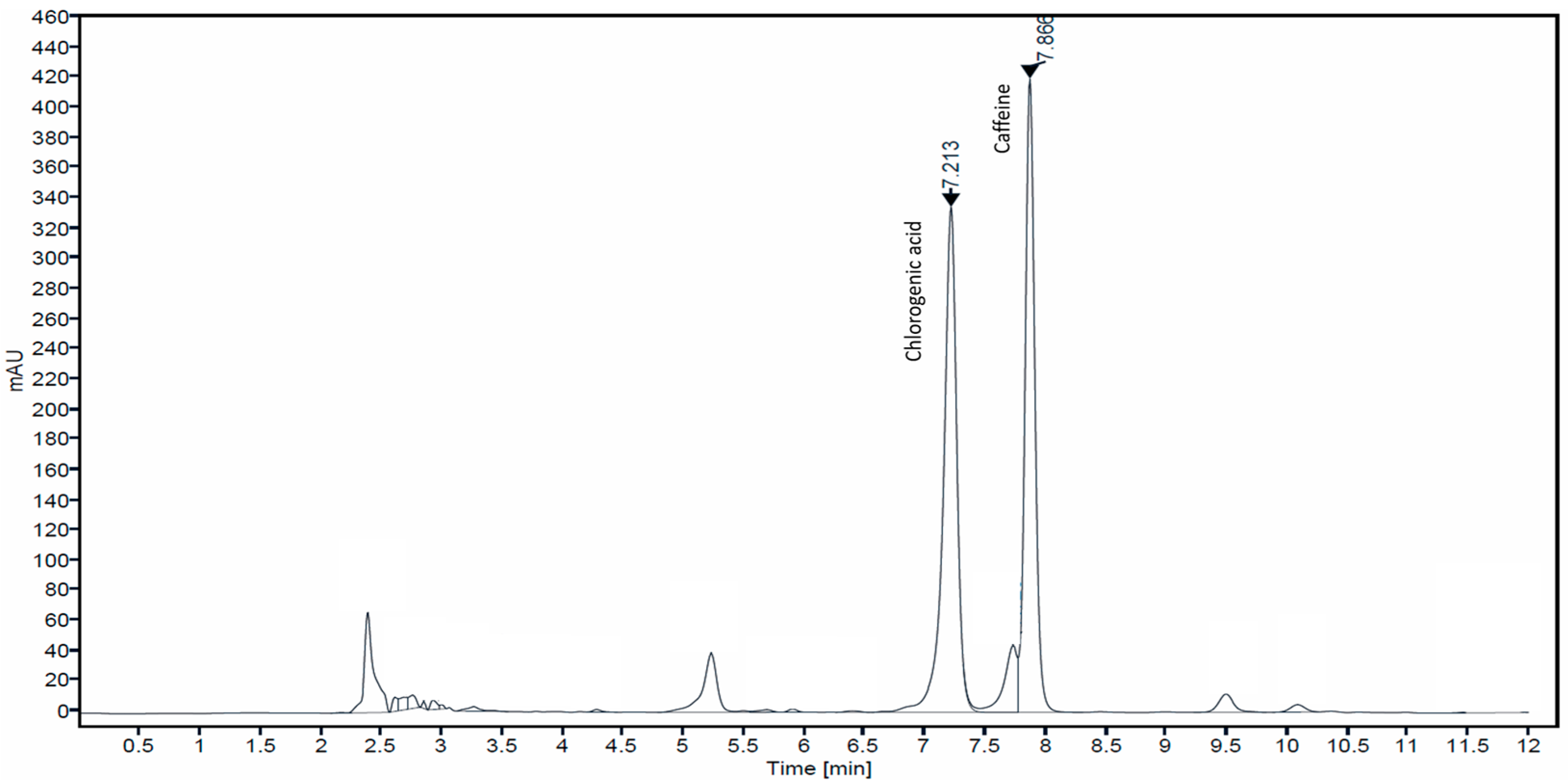
3.1. Determination of Caffeine and Chlorogenic Acid
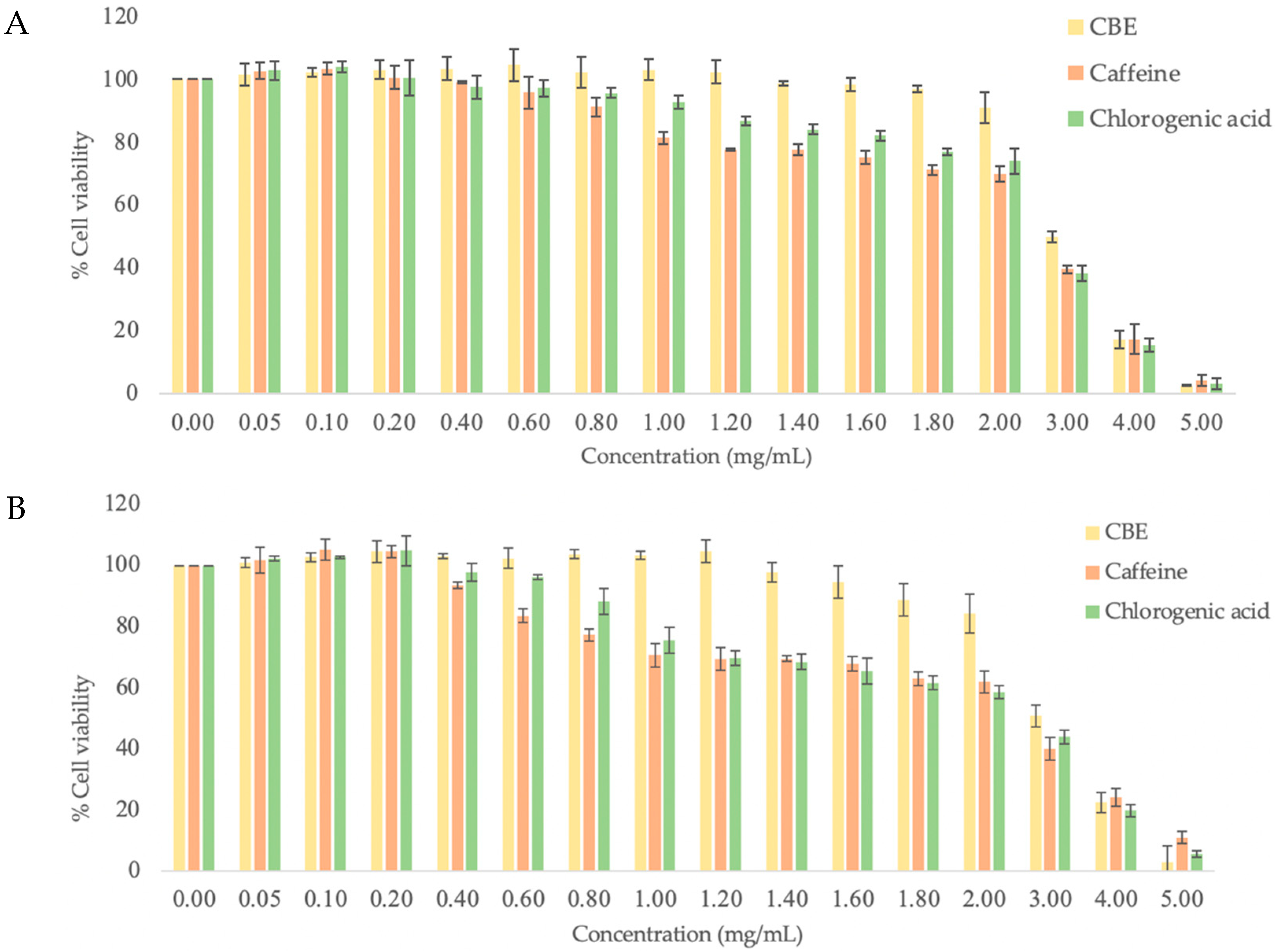
3.2. Cytotoxicity
3.3. Skin Anti-Aging Effect
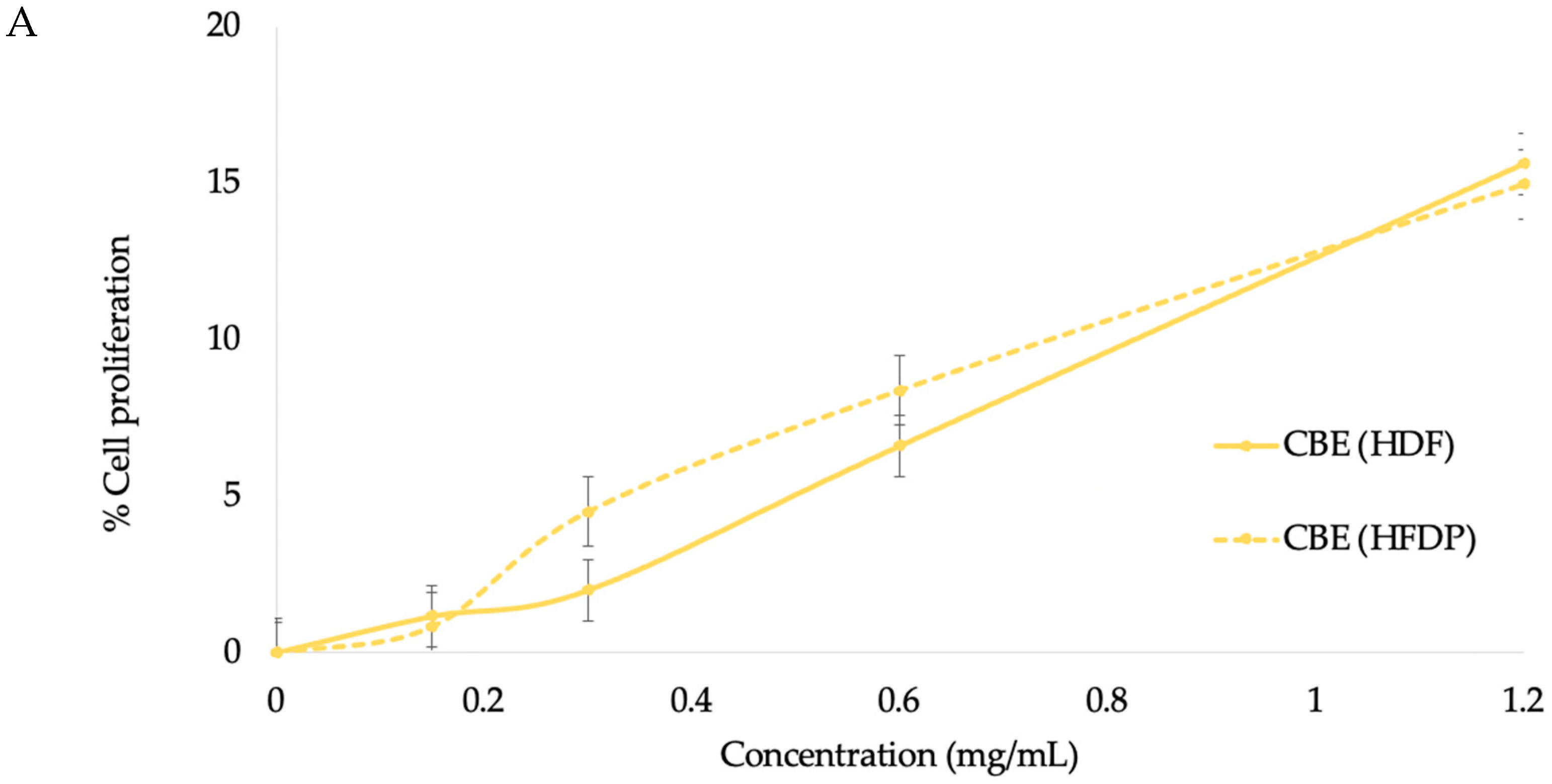
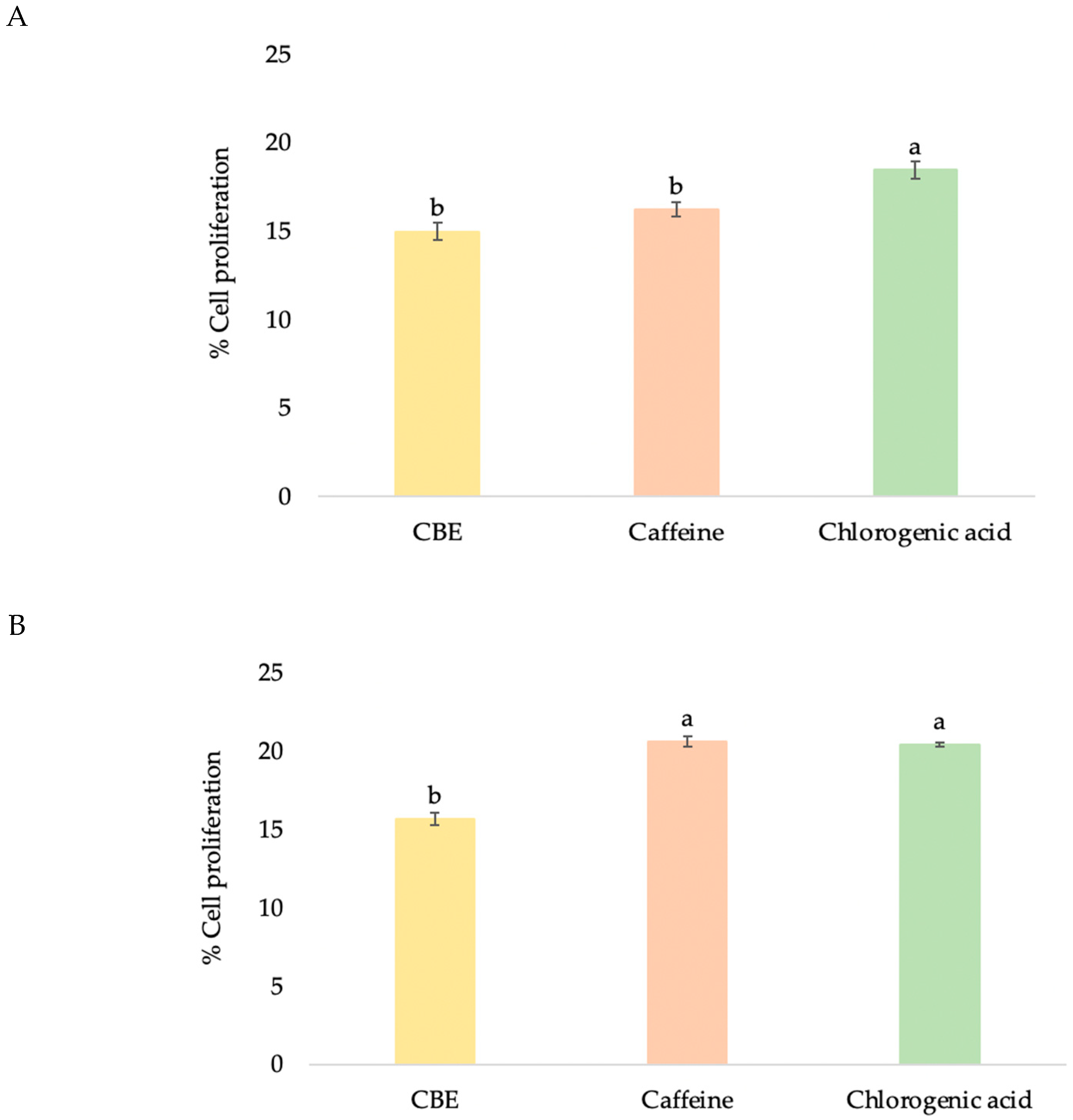
3.3.1. HDF Cell Proliferation
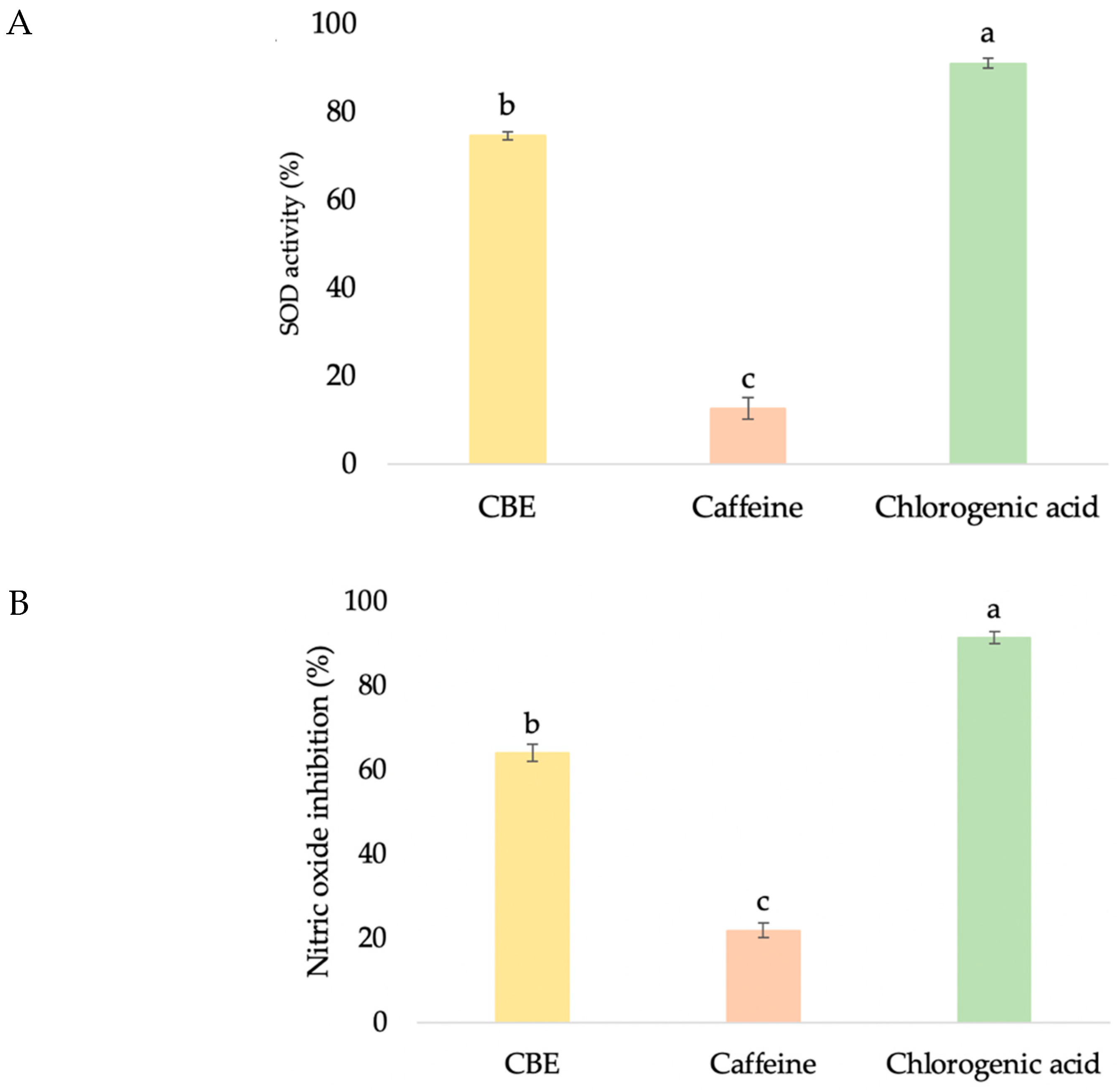
3.3.2. Antioxidant Activities
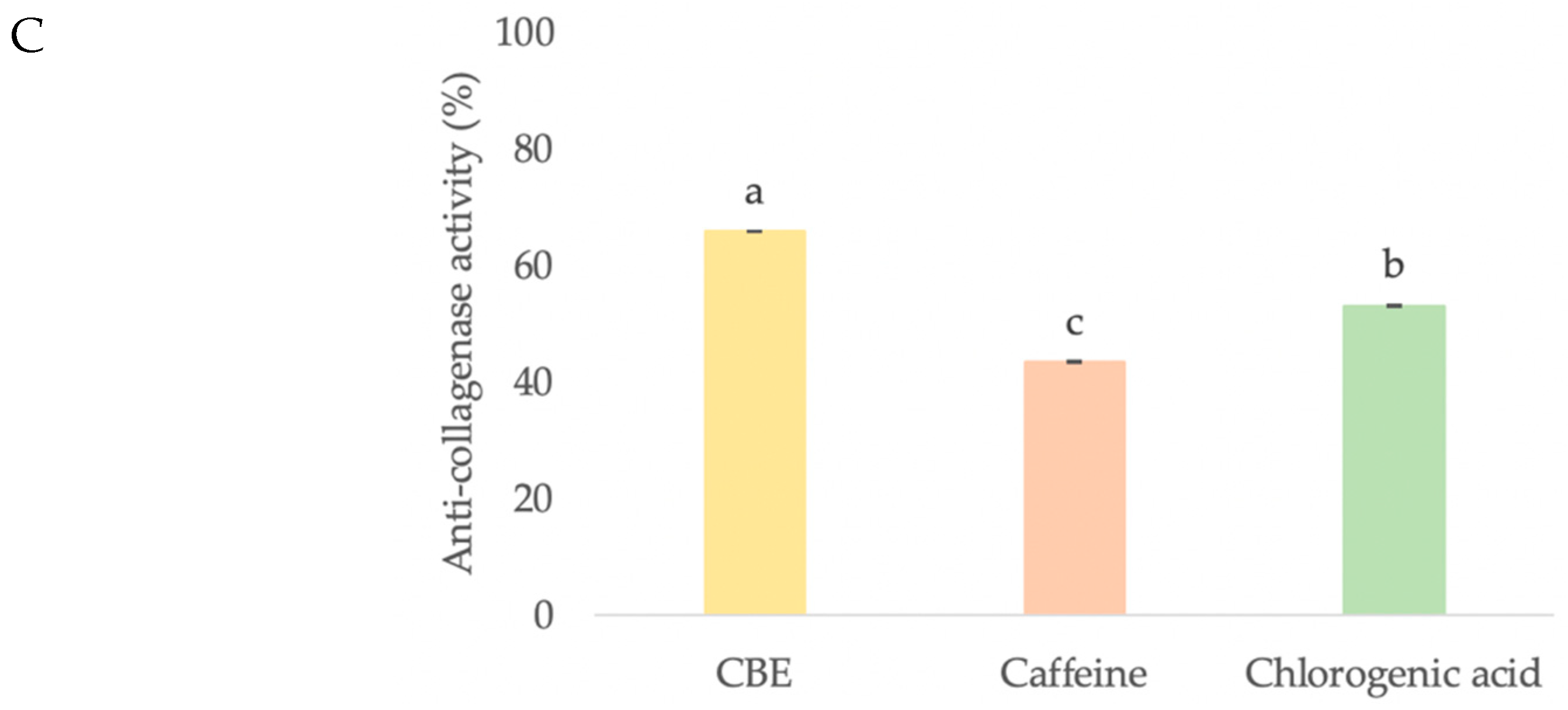
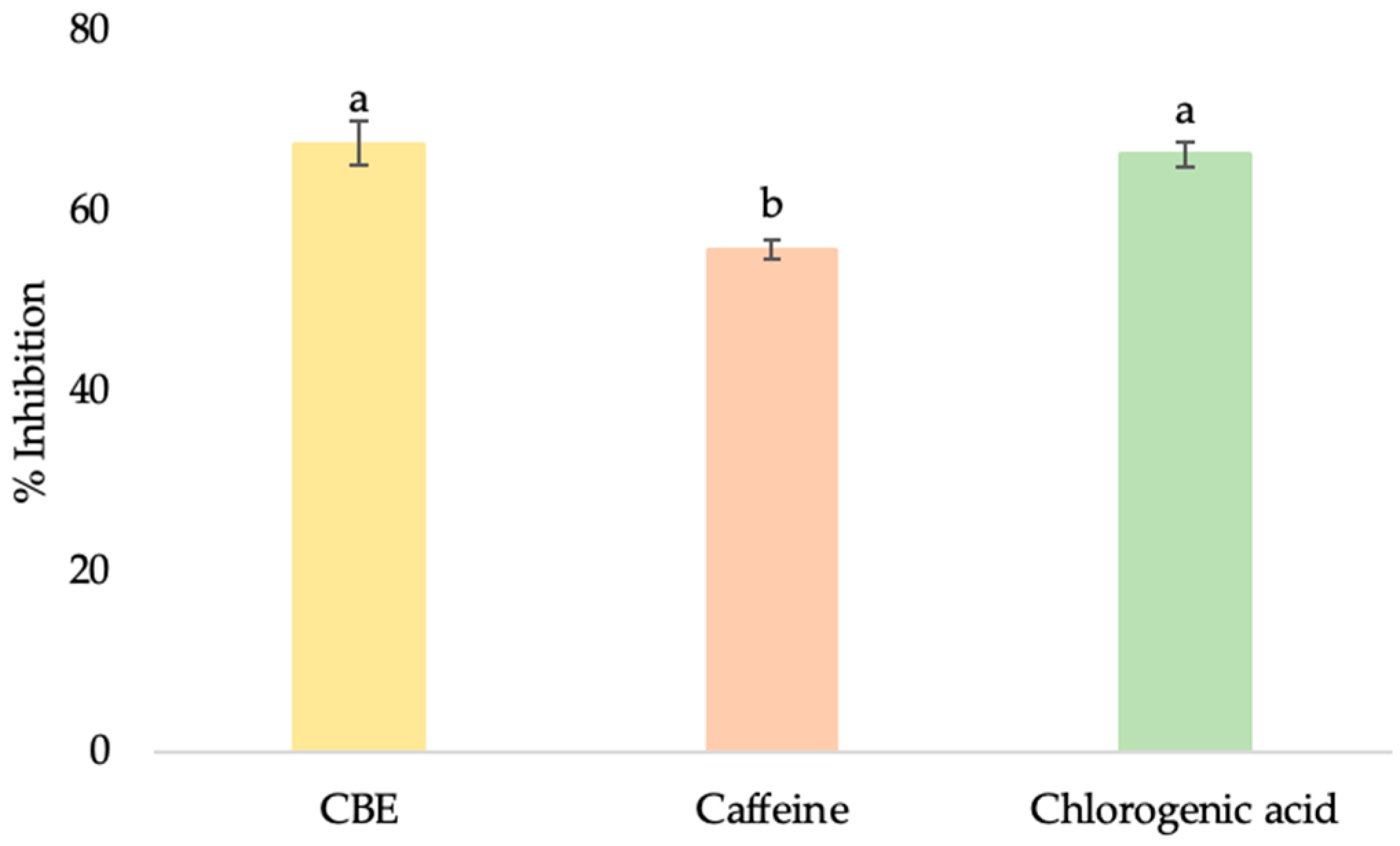
3.3.3. Anti-Collagenase Activity
3.4. Anti-Hair Loss Effect
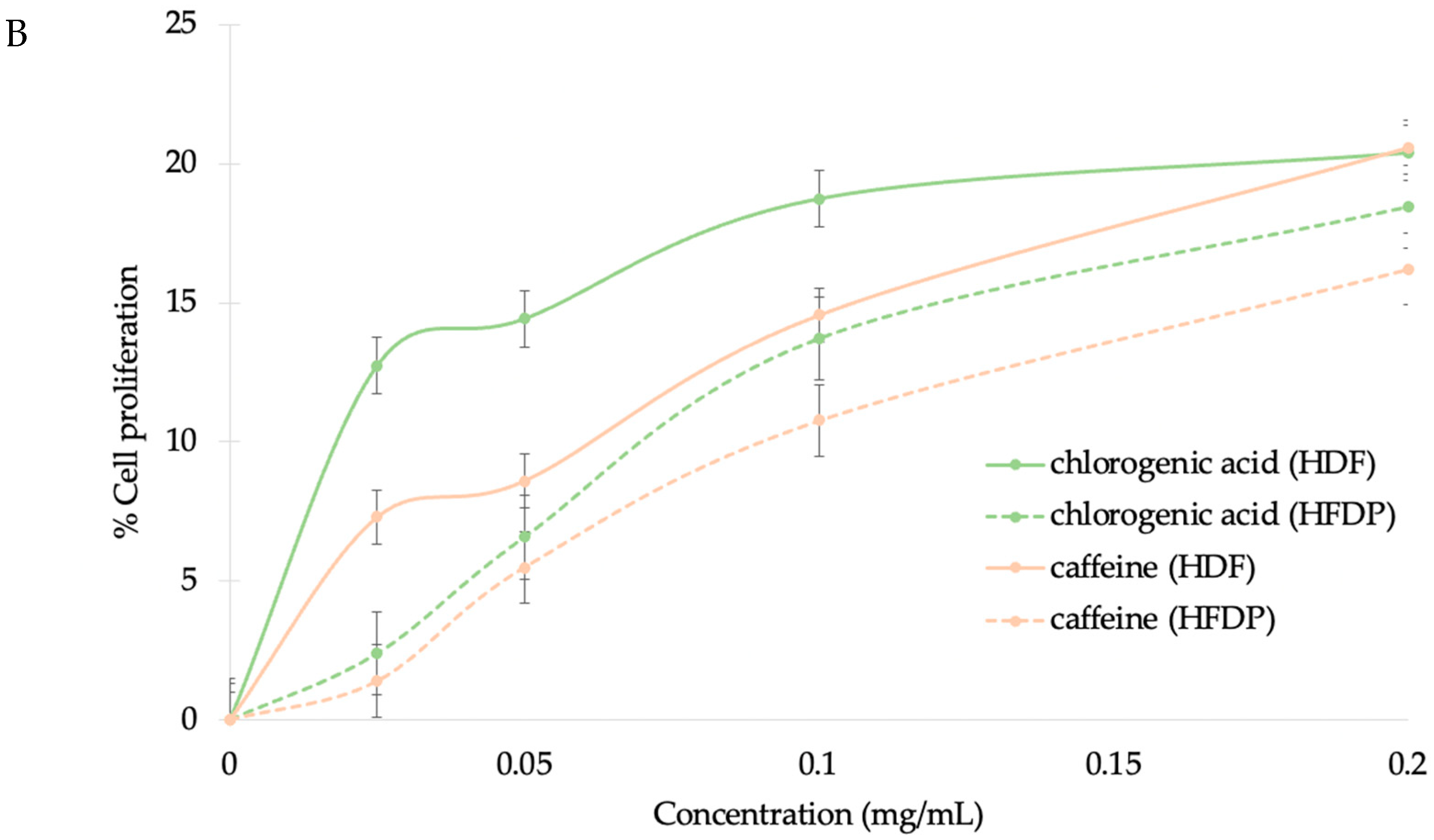
3.4.1. HFDP Cell Proliferation
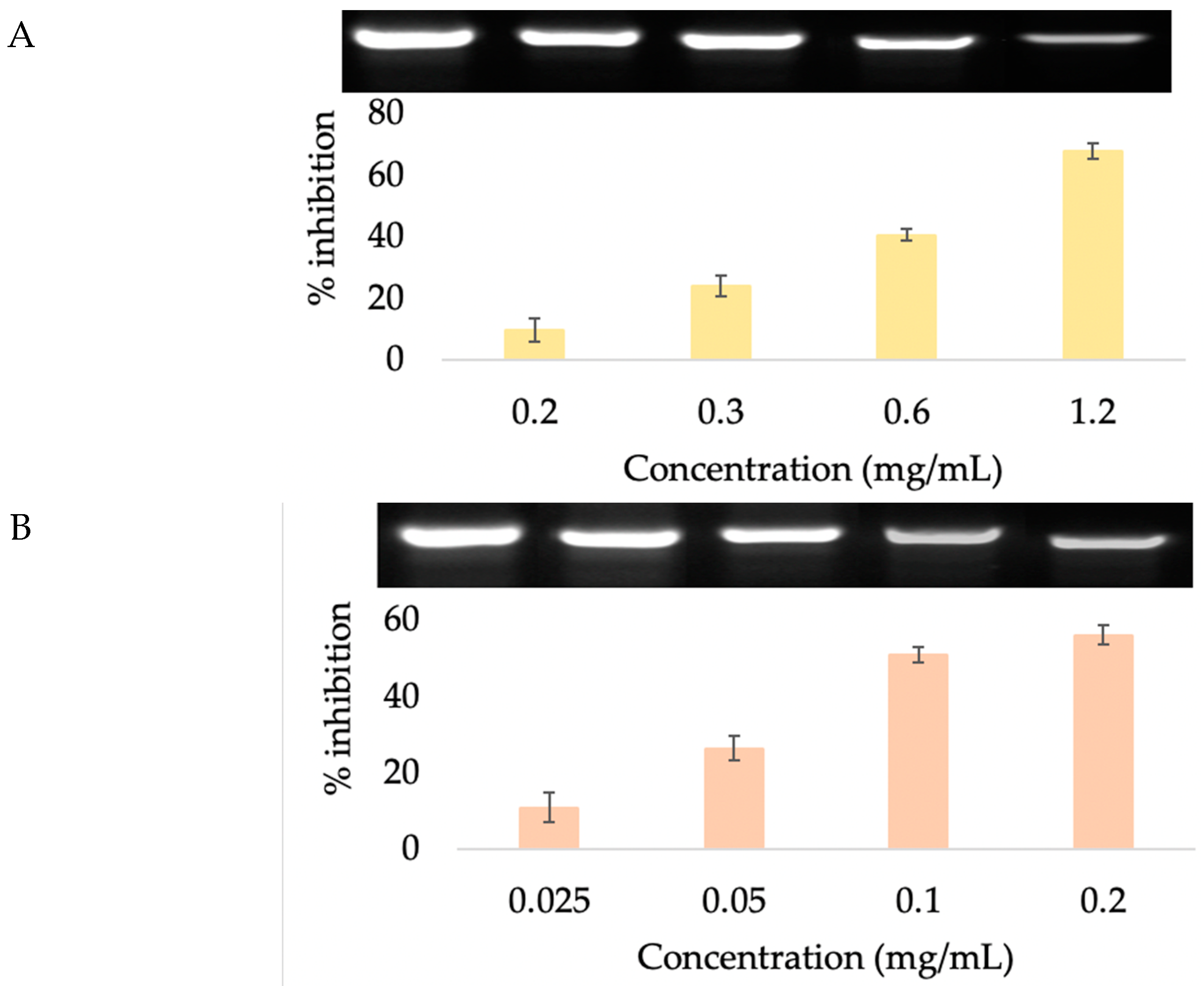
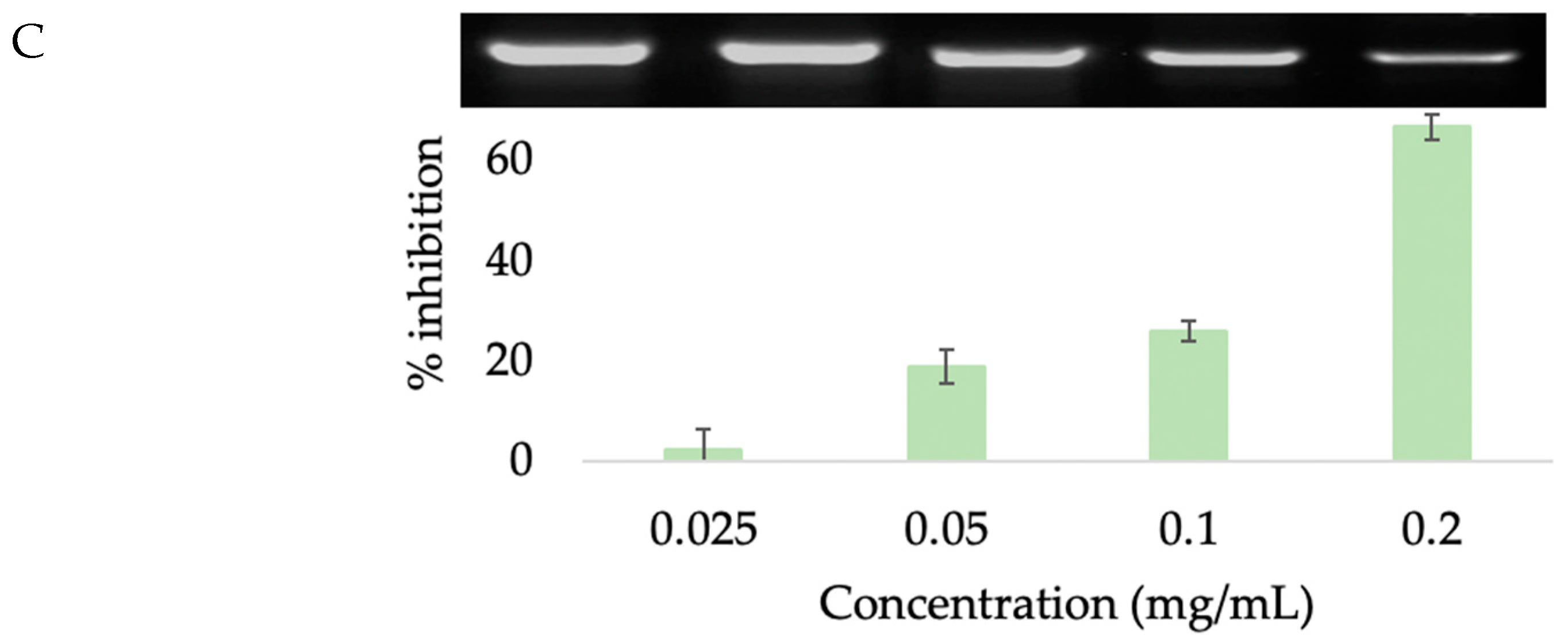
3.4.2. 5α-Reductase Inhibition
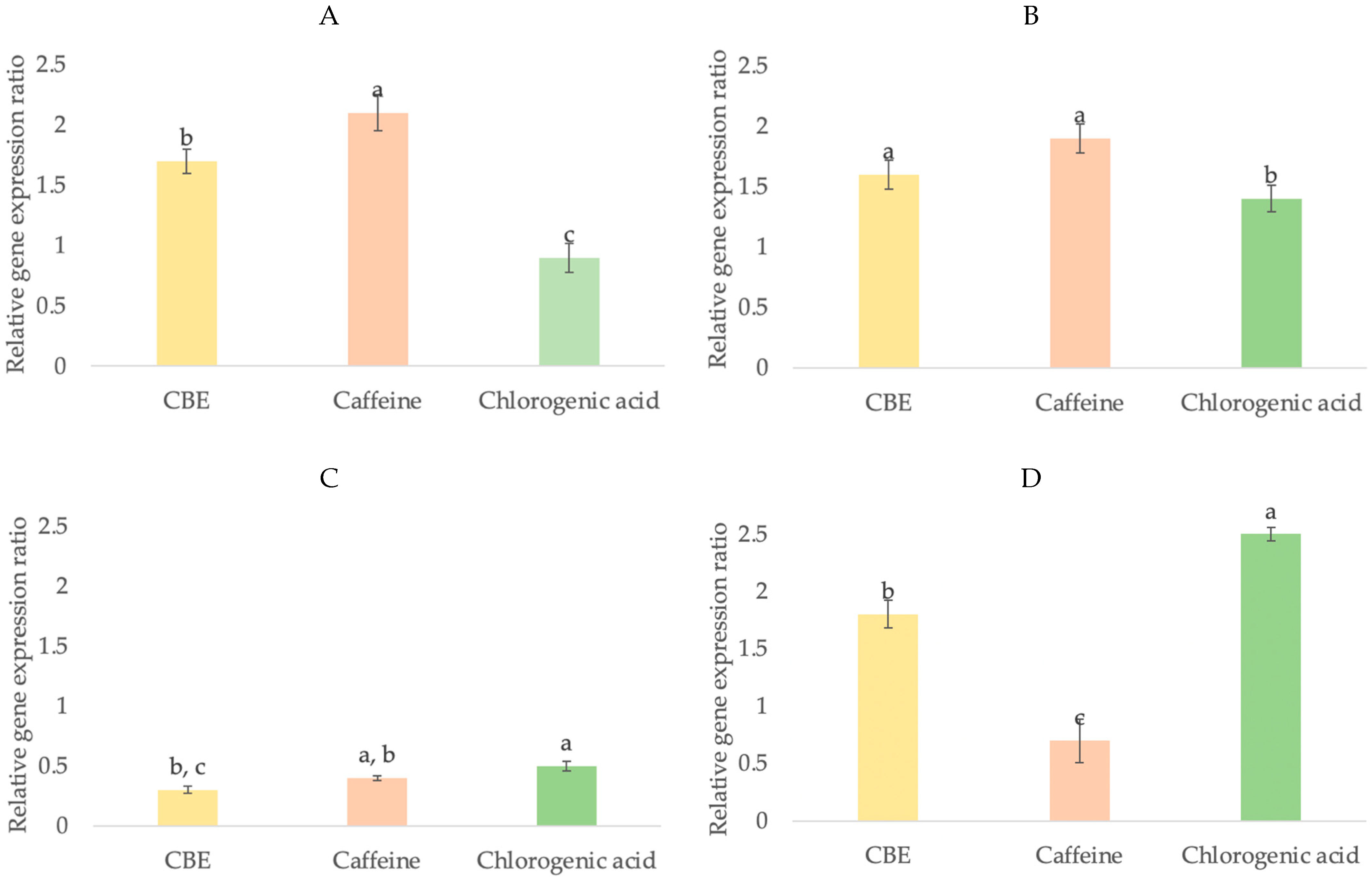
3.4.3. Growth Factor Gene Expression
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiu, P.C.; Chan, C.C.; Lin, H.M.; Chiu, H.C. The clinical anti-aging effects of topical kinetin and niacinamide in Asians: A randomized, double-blind, placebo-controlled, split-face comparative trial. J. Cosmet. Dermatol. 2007, 6, 243–249. [Google Scholar] [CrossRef]
- Williams, R.; Pawlus, A.D.; Thornton, J.M. Getting under the skin of hair aging: The impact of the hair follicle environment. Exp. Dermatol. 2020, 29, 588–597. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef] [Green Version]
- Geromel, C.; Ferreira, L.P.; Guerreiro, S.M.; Cavalari, A.A.; Pot, D.; Pereira, L.F.P.; Leroy, T.; Vieira, L.G.E.; Mazzafera, P.; Marraccini, P. Biochemical and genomic analysis of sucrose metabolism during coffee (Coffea arabica) fruit development. J. Exp. Bot. 2006, 57, 3243–3258. [Google Scholar] [CrossRef] [Green Version]
- Hall, R.D.; Trevisan, F.; de Vos, R.C. Coffee berry and green bean chemistry–Opportunities for improving cup quality and crop circularity. Food Res. Int. 2022, 151, 110825. [Google Scholar] [CrossRef]
- Azam, S.; Hadi, N.; Khan, N.U.; Hadi, S.M. Antioxidant and prooxidant properties of caffeine, theobromine and xanthine. Med. Sci. Monit. 2003, 9, 325–330. [Google Scholar]
- Duangjai, A.; Suphrom, N.; Wungrath, J.; Ontawong, A.; Nuengchamnong, N.; Yosboonruang, A. Comparison of antioxidant, antimicrobial activities and chemical profiles of three coffee (Coffea arabica L.) pulp aqueous extracts. Integr. Med. Res. 2016, 5, 324–331. [Google Scholar] [CrossRef] [Green Version]
- Devasagayam, T.P.; Kamat, J.P.; Mohan, H.; Kesavan, P.C. Caffeine as an antioxidant: Inhibition of lipid peroxidation induced by reactive oxygen species. Biochim. Biophys. Acta 1996, 1282, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Santana-Galvez, J.; Cisneros-Zevallos, L.; Jacobo-Velazquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [Green Version]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic Acid on regulating glucose and lipids metabolism: A review. Evid. Based Complement. Alternat. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef]
- Affonso, R.C.L.; Voytena, A.P.L.; Fanan, S.; Pitz, H.; Coelho, D.S.; Horstmann, A.L.; Pereira, A.; Uarrota, V.G.; Hillmann, M.C.; Varela, L.A.C.; et al. Phytochemical composition, antioxidant activity, and the effect of the aqueous extract of coffee (Coffea arabica L.) bean residual press cake on the skin wound healing. Oxid. Med. Cell. Longev. 2016, 2016, 1923754. [Google Scholar] [CrossRef] [Green Version]
- Desai, J.; Mallya, R. Development of green coffee beans extract loaded anti-aging liposomal gel. Indian J. Pharm. Educ. Res. 2021, 55, 979–988. [Google Scholar] [CrossRef]
- Zofia, N.Ł.; Aleksandra, Z.; Tomasz, B.; Martyna, Z.D.; Magdalena, Z.; Zofia, H.B.; Tomasz, W. Effect of fermentation time on antioxidant and anti-ageing properties of green coffee Kombucha ferments. Molecules 2020, 25, 5394. [Google Scholar] [CrossRef]
- Bessada, S.M.; Alves, R.C.; Oliveira, M.B.P.P. Coffee silverskin: A review on potential cosmetic applications. Cosmetics 2018, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Saewan, N.; Vichit, W.; Jimtaisong, A. Optimization of microwave-assisted extraction of anti-oxidant compound from coffee berry pulp using response surface methodology. In Proceedings of the 6th International Conference on Natural Products for Health and Beauty (NATPRO6), Khon Kaen, Thailand, 21–23 January 2016. [Google Scholar]
- McDaniel, D.H. Clinical safety and efficacy in photoaged skin with coffeeberry extract, a natural antioxidant. Cosmet. Dermatol. Cedark Knollis 2009, 22, 610. [Google Scholar]
- Murthy, P.S.; Naidu, M.M. Recovery of phenolic antioxidants and functional compounds from coffee industry by-products. Food Bioproc. Tech. 2012, 5, 897–903. [Google Scholar] [CrossRef]
- Lachin, T. Effect of antioxidant extract from cherries on diabetes. Recent Pat. Endocr. Metab. Immune Drug. Discov. 2014, 8, 67–74. [Google Scholar] [CrossRef]
- Vichit, W.; Saewan, N.; Jimtaisong, A. Antioxidant and sun protection activities of coffee berry pulp extracts. In Proceedings of the 42nd Congress on Science and Technology of Thailand (STT42), Bangkok, Thailand, 30 November–2 December 2016. [Google Scholar]
- Vichit, W.; Saewan, N. Antioxidant activities and cytotoxicity of Thai pigmented rice. Int. J. Pharm. Pharm. Sci. 2015, 7, 329–334. [Google Scholar]
- Vichit, W.; Saewan, N. Effect of germination on antioxidant, anti-inflammatory and keratinocyte proliferation of rice. Int. Food Res. J. 2016, 23, 2006–2015. [Google Scholar]
- Jain, R.; Monthakantirat, O.; Tengamnuay, P.; De-Eknamkul, W. Identification of a new plant extract for androgenic alopecia treatment using a non-radioactive human hair dermal papilla cell-based assay. BMC Complement. Altern. Med. 2016, 16, 18–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roh, S.S.; Kim, C.D.; Lee, M.H.; Hwang, S.L.; Rang, M.J.; Yoon, Y.K. The hair growth promoting effect of Sophora flavescens extract and its molecular regulation. J. Dermatol. Sci. 2002, 30, 43–49. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Brand, D.; Mohan, R.; Roussos, S. Biotechnological potential of coffee pulp and coffee husk for bioprocesses. Biochem. Eng. J. 2000, 6, 153–162. [Google Scholar] [CrossRef]
- Brezová, V.; Šlebodová, A.; Staško, A. Coffee as a source of antioxidants: An EPR study. Food Chem. 2009, 114, 859–868. [Google Scholar] [CrossRef]
- Fernandez-Gomez, B.; Ramos, S.; Goya, L.; Mesa, M.D.; del Castillo, M.D.; Martín, M.Á. Coffee silverskin extract improves glucose-stimulated insulin secretion and protects against streptozotocin-induced damage in pancreatic INS-1E beta cells. Food Res. Int. 2016, 89, 1015–1022. [Google Scholar] [CrossRef] [Green Version]
- Kiattisin, K.; Intasai, N.; Nitthikan, N.; Nantarat, T.; Lee, K.; Lin, W.C.; Lue, S.C.; Leelapornpisid, L. Antioxidant, anti-tyrosinase, anti-aging potentials and safety of arabica coffee cherry extract. Chiang Mai J. Sci. 2019, 46, 930–945. [Google Scholar]
- Abd, E.; Gomes, J.; Sales, C.C.; Yousef, S.; Forouz, F.; Telaprolu, K.C.; Andréo-Filho, N. Deformable liposomes as enhancer of caffeine penetration through human skin in a Franz diffusion cell test. Int. J. Cosmet. Sci. 2021, 43, 1–10. [Google Scholar] [CrossRef]
- Seifert, A.W.; Monaghan, J.R.; Voss, S.R.; Maden, M. Skin regeneration in adult axolotls: A blueprint for scar-free healing in vertebrates. PLoS ONE 2012, 7, e32875. [Google Scholar] [CrossRef] [Green Version]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermatoendocrinology 2012, 4, 308–319. [Google Scholar] [CrossRef] [Green Version]
- Bonora, M.; Pinton, P. Mitochondrial DNA keeps you young. Cell Death Dis. 2018, 9, 992. [Google Scholar] [CrossRef]
- Mariggio, M.A.; Cassano, A.; Vinella, A.; Vincenti, A.; Fumarulo, R.; Lo Muzio, L.; Maiorano, E.; Ribatti, D.; Favia, G. Enhancement of fibroblast proliferation, collagen biosynthesis and production of growth factors as a result of combining sodium hyaluronate and aminoacids. Int. J. Immunopathol. Pharmacol. 2009, 22, 485–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sriram, G.; Bigliardi, P.L.; Bigliardi-Qi, M. Fibroblast heterogeneity and its implications for engineering organotypic skin models in vitro. Eur. J. Cell Biol. 2015, 94, 483–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.C.; Liou, S.S.; Tzeng, T.F.; Lee, S.L.; Liu, I.M. Effect of topical application of chlorogenic acid on excision wound healing in rats. Planta Medica 2013, 79, 616–621. [Google Scholar] [CrossRef]
- Pieme, C.A.; Penlap, V.N.; Ngogang, J.; Costache, M. In vitro cytotoxicity and antioxidant activities of five medicinal plants of Malvaceae family from Cameroon. Environ. Toxicol. Pharmacol. 2010, 29, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Mohd Esa, N.; Abdul Kadir, K.K.; Amom, Z.; Azlan, A. Antioxidant activity of white rice, brown rice and germinated brown rice (in vivo and in vitro) and the effects on lipid peroxidation and liver enzymes in hyperlipidaemic rabbits. Food Chem. 2013, 141, 1306–1312. [Google Scholar] [CrossRef]
- Serra, V.; von Zglinicki, T.; Lorenz, M.; Saretzki, G. Extracellular superoxide dismutase is a major antioxidant in human fibroblasts and slows telomere shortening. J. Biol. Chem. 2003, 278, 6824–6830. [Google Scholar] [CrossRef] [Green Version]
- Debnath, T.; Park, S.; Kim, D.; Jo, J.; Lim, B. Anti-oxidant and anti-inflammatory activities of Inonotus obliquus and germinated brown rice extracts. Molecules 2013, 18, 9293–9304. [Google Scholar] [CrossRef] [Green Version]
- Aramwit, P.; Kanokpanont, S.; De-Eknamkul, W.; Kamei, K.; Srichana, T. The effect of sericin with variable amino-acid content from different silk strains on the production of collagen and nitric oxide. J. Biomater. Sci. Polym. Ed. 2009, 20, 1295–1306. [Google Scholar] [CrossRef]
- Lemos, M.F.; de Andrade Salustriano, N.; de Souza Costa, M.M.; Lirio, K.; da Fonseca, A.F.A.; Pacheco, H.P.; Scherer, R. Chlorogenic acid and caffeine contents and anti-inflammatory and antioxidant activities of green beans of conilon and arabica coffees harvested with different degrees of maturation. J. Saudi Chem. Soc. 2022, 26, 101467. [Google Scholar] [CrossRef]
- Kiattisin, K.; Nantarat, T.; Leelapornpisid, P. Evaluation of antioxidant and anti-tyrosinase activities as well as stability of green and roasted coffee bean extracts from Coffea arabica and Coffea canephora grown in Thailand. J. Pharmacogn. Phytother. 2016, 8, 182–192. [Google Scholar]
- Philips, N.; Auler, S.; Hugo, R.; Gonzalez, S. Beneficial regulation of matrix metalloproteinases for skin health. Enzyme Res. 2011, 2011, 427285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demaria, M.; Desprez, P.Y.; Campisi, J.; Velarde, M.C. Cell autonomous and non-autonomous effects of senescent cells in the skin. J. Investig. Dermatol. 2015, 135, 1722–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittenauer, J.; Mackle, S.; Sussmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Bharadwaj, S.; Yadava, U.; Kang, G.S. Evaluation of caffeine as inhibitor against collagenase, elastase and tyrosinase using in silico and in vitro approach. J. Enzyme Inhib. Med. Chem. 2019, 34, 927–936. [Google Scholar]
- Madaan, A.; Verma, R.; Singh, A.T.; Jaggi, M. Review of hair follicle dermal papilla cells as in vitro screening model for hair growth. Int. J. Cosmet. Sci. 2018, 40, 429–450. [Google Scholar] [CrossRef] [Green Version]
- Luanpitpon, S.; Nimmannit, U.; Pongrakhan, V.; Chanvorach, P. Emblica (Phyllanthus emblica Linn.) Fruit extract promotes proliferation in dermal papilla cells of human hair follicle. Res. J. Med. Plant 2011, 5, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kim, S.N.; Jeong, G.; Hong, M.J.; Lee, Y.; Shin, S.H.; Park, H.; Jung, Y.C.; Kim, E.J.; Park, B.C.; et al. Efficacy of caffeine in promoting hair growth by enhancing intracellular activity of hair follicles. Korean J. Cosmet. Sci. 2019, 1, 11–18. [Google Scholar] [CrossRef]
- Anderson, K.M.; Liao, S. Selective retention of dihydrotestosterone by prostatic nuclei. Nature 1968, 219, 277–279. [Google Scholar] [CrossRef]
- Randall, V.A.; Hibberts, N.A.; Thornton, M.J.; Hamada, K.; Merrick, A.E.; Kato, S.; Jenner, T.J.; De Oliveira, I.; Messenger, A.G. The hair follicle: A paradoxical androgen target organ. Horm. Res. 2000, 54, 243–250. [Google Scholar] [CrossRef]
- Junlatat, J.; Sripanidkulchai, B. Hair growth-promoting effect of Carthamus tinctorius floret extract. Phytother. Res. 2014, 28, 1030–1036. [Google Scholar] [CrossRef]
- Fischer, T.W.; Hipler, U.C.; Elsner, P. Effect of caffeine and testosterone on the proliferation of human hair follicles in vitro. Int. J. Dermatol. 2007, 46, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Richter, H.; Schanzer, S.; Klenk, A.; Sterry, W.; Patzelt, A. Analysis of the penetration of a caffeine containing shampoo into the hair follicles by in vivo laser scanning microscopy. Laser Phys. 2009, 20, 551–556. [Google Scholar] [CrossRef]
- Teichmann, A.; Richter, H.; Knorr, F.; Antoniou, C.; Sterry, W.; Lademann, J. Investigation of the penetration and storage of a shampoo formulation containing caffeine into the hair follicles by in vivo laser scanning microscopy. Laser Phys. Lett. 2007, 4, 464–468. [Google Scholar] [CrossRef]
- Prinyarux, T.; Saewan, N. Anti-hairloss efficacy of coffee berry extract. Food Appl. Biosci. J. 2020, 8, 27–39. [Google Scholar]
- Paus, R.; Cotsarelis, G. The biology of hair follicles. N. Engl. J. Med. 1999, 341, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Itami, S.; Kurata, S.; Takayasu, S. Androgen induction of follicular epithelial cell growth is mediated via insulin-like growth factor-I from dermal papilla cells. Biochem. Biophys. Res. Commun. 1995, 212, 988–994. [Google Scholar] [CrossRef]
- Su, H.Y.; Hickford, J.G.; Bickerstaffe, R.; Palmer, B.R. Insulin-like growth factor 1 and hair growth. Dermatol. Online J. 1999, 5, 1. [Google Scholar] [CrossRef]
- Tang, L.; Bernardo, O.; Bolduc, C.; Lui, H.; Madani, S.; Shapiro, J. The expression of insulin-like growth factor 1 in follicular dermal papillae correlates with therapeutic efficacy of finasteride in androgenetic alopecia. J. Am. Acad. Dermatol. 2003, 49, 229–233. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Li, Z.; Gu, L.; Wang, Y.; Sung, C. Exogenous IGF-1 promotes hair growth by stimulating cell proliferation and down regulating TGF-β1 in C57BL/6 mice in vivo. Growth Horm. IGF Res. 2014, 24, 89–94. [Google Scholar] [CrossRef]
- Danilenko, D.M.; Ring, B.D.; Yanagihara, D.; Benson, W.; Wiemann, B.; Starnes, C.O.; Pierce, G.F. Keratinocyte growth factor is an important endogenous mediator of hair follicle growth, development, and differentiation. Normalization of the nu/nu follicular differentiation defect and amelioration of chemotherapy-induced alopecia. Am. J. Pathol. 1995, 147, 145–154. [Google Scholar]
- Braun, S.; Krampert, M.; Bodó, E.; Kümin, A.; Born-Berclaz, C.; Paus, R.; Werner, S. Keratinocyte growth factor protects epidermis and hair follicles from cell death induced by UV irradiation, chemotherapeutic or cytotoxic agents. J. Cell Sci. 2006, 119, 4841–4849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.R.; Yamazaki, M.; Mitsui, S.; Tsuboi, R.; Ogawa, H. Hepatocyte growth factor (HGF) activator expressed in hair follicles is involved in in vitro HGF-dependent hair follicle elongation. J. Dermatol. Sci. 2001, 25, 156–163. [Google Scholar] [CrossRef]
- Qi, Y.; Li, M.; Xu, L.; Chang, Z.; Shu, X.; Zhou, L. Therapeutic role of human hepatocyte growth factor (HGF) in treating hair loss. Peer J. 2016, 4, e2624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lachgar, S.; Charveron, M.; Sarraute, J.; Gall, Y.; Bonafe, J.L. Anti-androgens and estrogens: Modulators of VEGF expression in cultured hair dermal papilla cells. Exp. Dermatol. 1999, 8, 336–338. [Google Scholar] [PubMed]
- Yano, K.; Brown, L.F.; Detmar, M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J. Clin. Investig. 2001, 107, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Dvorak, H.F.; Brown, L.F.; Detmar, M.; Dvorak, A.M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Clin. Pathol. 1995, 146, 1029–1039. [Google Scholar]
- Rho, S.S.; Park, S.J.; Hwang, S.L.; Lee, M.H.; Kim, C.D.; Lee, I.H.; Chang, S.Y.; Rang, M.J. The hair growth promoting effect of Asiasari radix extract and its molecular regulation. J. Dermatol. Sci. 2005, 38, 89–97. [Google Scholar] [CrossRef]
- Fischer, T.W.; Herczeg-Lisztes, E.; Funk, W.; Zillikens, D.; Biro, T.; Paus, R. Differential effects of caffeine on hair shaft elongation, matrix and outer root sheath keratinocyte proliferation, and transforming growth factor-beta2/insulin-like growth factor-1-mediated regulation of the hair cycle in male and female human hair follicles in vitro. Br. J. Dermatol. 2014, 171, 1031–1043. [Google Scholar]
| Growth Factor | Primer Sequence | Expected Size (bp) | |
|---|---|---|---|
| IGF-1 | Forward (5′–3′) | TCAACAAGCCCACAGGGTAT | 307 |
| Reverse (5′–3′) | ACTCGTGCAGAGCAAAGGAT | ||
| HGF | Forward (5′–3′) | CGAGGCCATGGTGCTATACT | 297 |
| Reverse (5′–3′) | ACACCAGGGTGATTCAGACC | ||
| KGF | Forward (5′–3′) | GACATGGATCCTGCCAACTT | 304 |
| Reverse (5′–3′) | AATTCCAACTGCCACTGTCC | ||
| VEGF | Forward (5′–3′) | TCTTCAAGCCATCCTGTGTG | 297 |
| Reverse (5′–3′) | GCGAGTCTGTGTTTTTGCAG | ||
| Samples | HDF | HFDP | ||
|---|---|---|---|---|
| Maximum Non-Toxic Dose (mg/mL) | IC50 (mg/mL) | Maximum Non-Toxic Dose (mg/mL) | IC50 (mg/mL) | |
| CBE | ≤1.2 | 3.08 ± 0.04 a | ≤1.2 | 3.07 ± 0.09 a |
| Caffeine | ≤0.2 | 2.63 ± 0.14 b | ≤0.2 | 2.38 ± 0.12 b |
| Chlorogenic acid | ≤0.2 | 2.71 ± 0.03 b | ≤0.2 | 2.62 ± 0.04 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saewan, N. Effect of Coffee Berry Extract on Anti-Aging for Skin and Hair—In Vitro Approach. Cosmetics 2022, 9, 66. https://doi.org/10.3390/cosmetics9030066
Saewan N. Effect of Coffee Berry Extract on Anti-Aging for Skin and Hair—In Vitro Approach. Cosmetics. 2022; 9(3):66. https://doi.org/10.3390/cosmetics9030066
Chicago/Turabian StyleSaewan, Nisakorn. 2022. "Effect of Coffee Berry Extract on Anti-Aging for Skin and Hair—In Vitro Approach" Cosmetics 9, no. 3: 66. https://doi.org/10.3390/cosmetics9030066
APA StyleSaewan, N. (2022). Effect of Coffee Berry Extract on Anti-Aging for Skin and Hair—In Vitro Approach. Cosmetics, 9(3), 66. https://doi.org/10.3390/cosmetics9030066





