Anti-Skin-Aging Effect of a Treatment with a Cosmetic Product and a Food Supplement Based on a New Hyaluronan: A Randomized Clinical Study in Healthy Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Investigational Products
2.3. Study Design
2.4. Sample Size
2.5. Blinding and Randomization
2.6. Outcome Measures
2.7. Self-Assessment Questionnaire
2.8. Statistics
3. Results
3.1. Tolerability
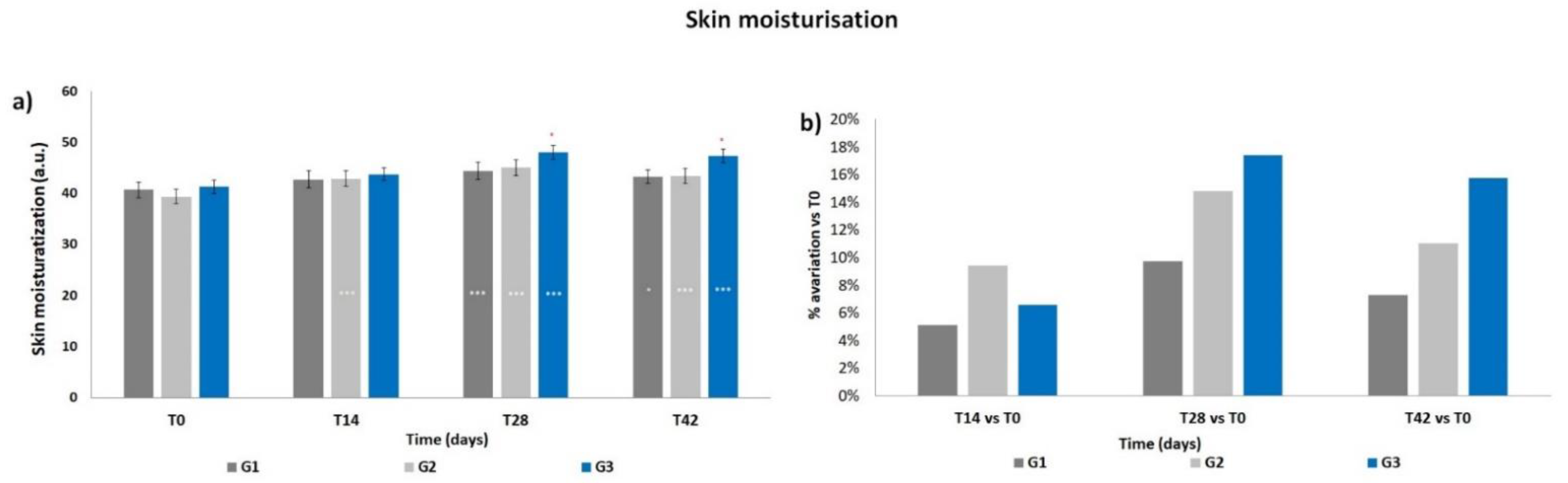
3.2. Skin Moisturization
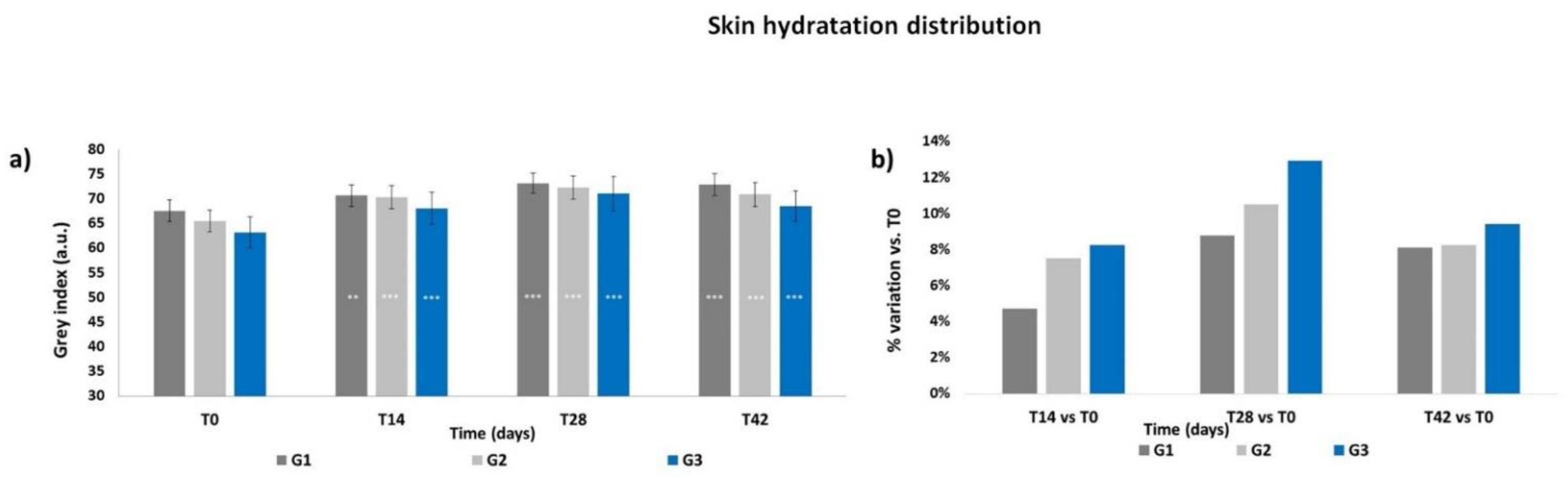
3.3. Skin Hydration Distribution
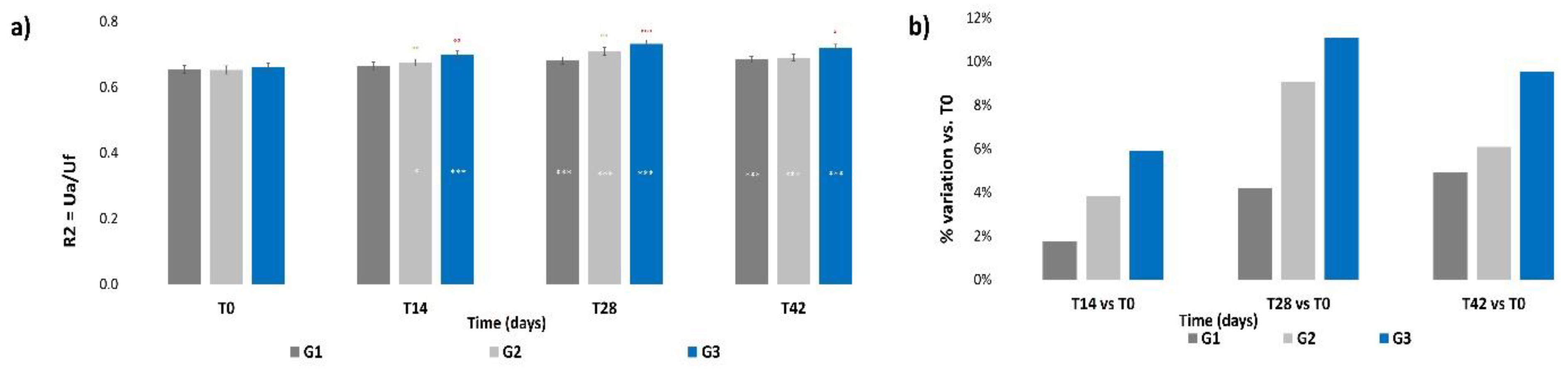
3.4. Skin Elasticity
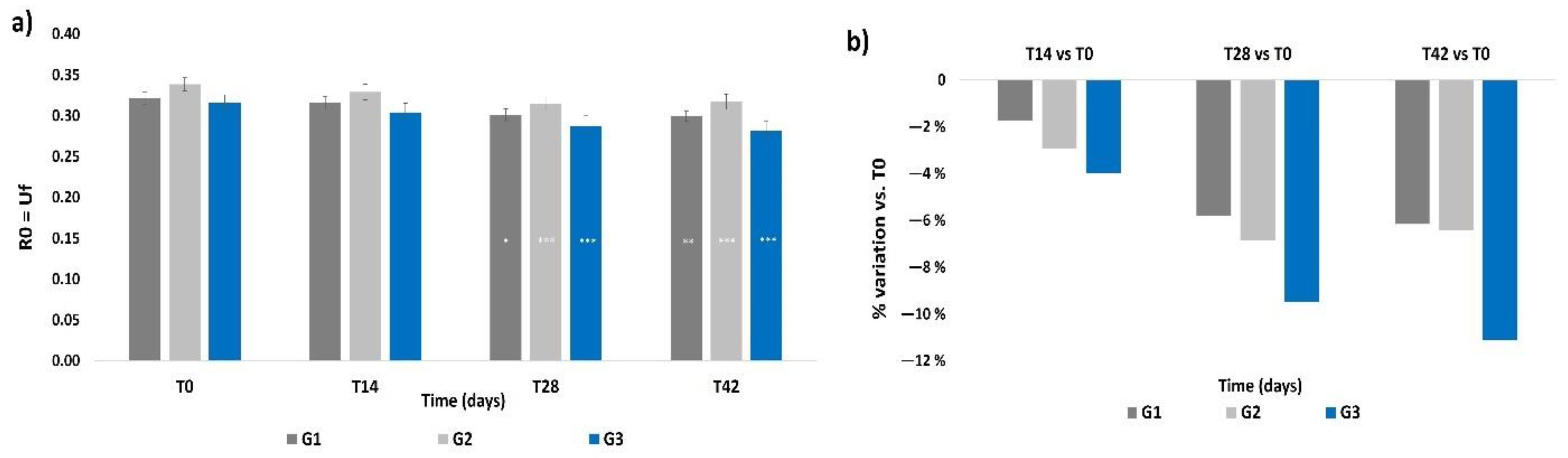
3.5. Skin Firmness
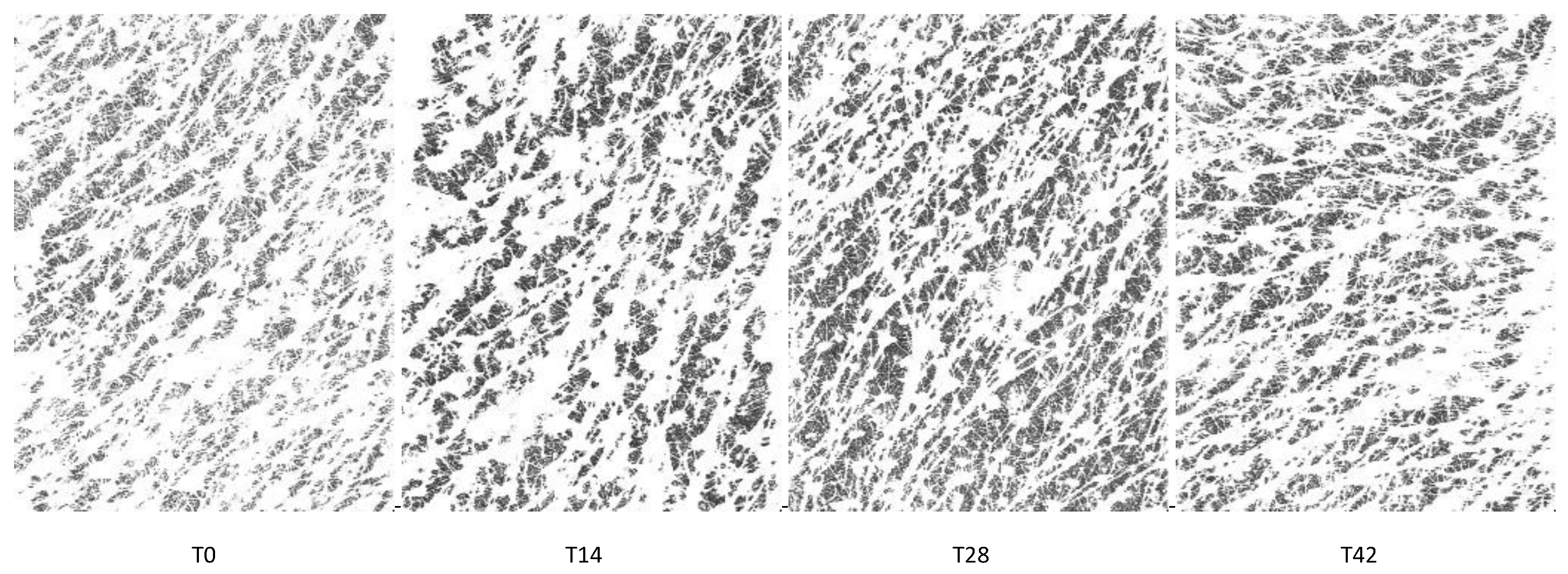
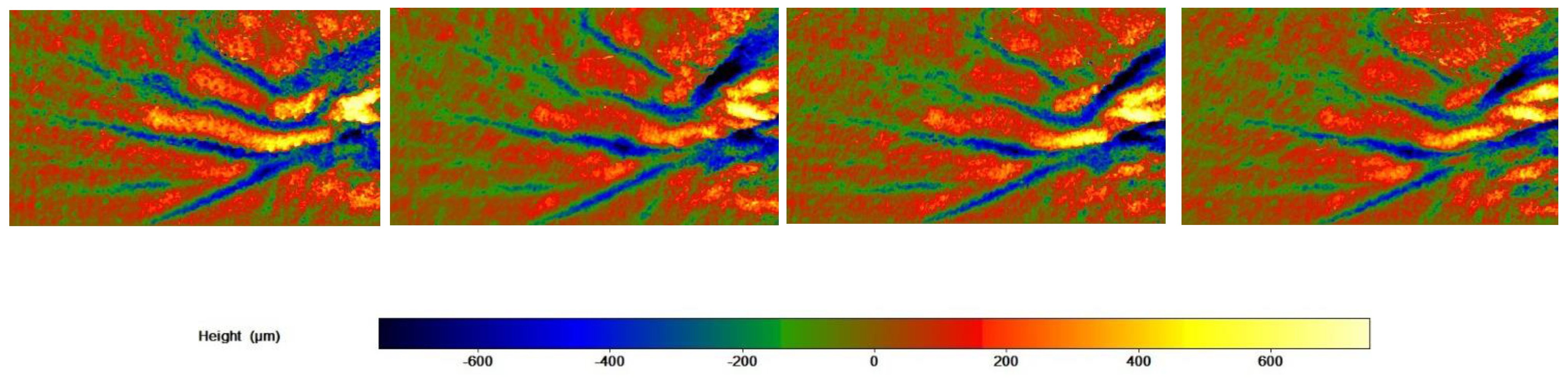
3.6. Skin Profilometry
3.7. Self-Assessment Questionnaire
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tobin, D.J. Introducing to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rittié, L.; Fisher, G.J. Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hong, Y.; Kim, M. Structural and Functional Changes and Possible Molecular Mechanisms in Aged Skin. Int. J. Mol. Sci. 2021, 22, 12489. [Google Scholar] [CrossRef] [PubMed]
- Genovese, L.; Corbo, A.; Sibilla, S. An insight into the changes in skin texture and properties following dietary intervention with a nutricosmeceutical containing a blend of collagen bioactive peptides and antioxidants. Ski. Pharmacol. Physiol. 2017, 30, 146–158. [Google Scholar] [CrossRef] [Green Version]
- Kawada, C.; Kimura, M.; Masuda, Y.; Nomura, Y. Orally administered hyaluronan affects skin dryness and epidermal thickening in photoaged hairless mice. Biosci. Biotechnol. Biochem. 2016, 80, 1192–1195. [Google Scholar] [CrossRef] [Green Version]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef]
- Tanaka, M.; Yamamoto, Y.; Misawa, E.; Nabeshima, K.; Saito, M.; Yamauchi, K.; Abe, F.; Furukawa, F. Effects of aloe sterol supplementation on skin elasticity, hydration, and collagen score: A 12-week double-blind, randomized, controlled trial. Ski. Pharmacol. Physiol. 2016, 29, 309–317. [Google Scholar] [CrossRef]
- Göllner, I.; Voss, W.; von Hehn, U.; Kammerer, S. Ingestion of an oral hyaluronan solution improves skin hydration, wrinkle reduction, elasticity, and skin roughness: Results of a clinical study. J. Evid.-Based Complementary Altern. Med. 2017, 22, 816–823. [Google Scholar] [CrossRef]
- Oe, M.; Sakai, S.; Yoshida, H.; Okado, N.; Kaneda, H.; Masuda, Y.; Urushibata, O. Oral hyaluronan relieves wrinkles: A double-blinded, placebo-controlled study over a 12-week period. Clin. Cosmet. Investig. Dermatol. 2017, 10, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Longas, M.O.; Russel, C.S.; He, X.Y. Evidence for structural changes in dermatan sulfate and hyaluronic acid with aging. Carbohydr. Res. 1987, 159, 127–136. [Google Scholar] [CrossRef]
- Stern, R.; Maibach, H.I. Hyaluronan in skin: Aspects of aging and its pharmacologic modulation. Clin Dermatol. 2008, 26, 106–122. [Google Scholar] [CrossRef]
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic Acid: A Key Ingredient in the Therapy of Inflammation. Biomolecules 2021, 11, 1518. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an Immune Regulator in Human Diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.; Jiang, D.; Noble, P.W. Hyaluronan as a therapeutic target in human diseases. Adv. Drug Deliv. Rev. 2016, 97, 186–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastow, E.R.; Byers, S.; Golub, S.B.; Clarkin, C.E.; Pitsillides, A.A.; Fosang, A.J. Hyaluronan synthesis and degradation in cartilage and bone. Cell Mol. Life Sci. 2008, 65, 395–413. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic acid in the third millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [Green Version]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds A Compend. Clin. Res. Pract. 2016, 28, 78–88. [Google Scholar]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermato-Endocrinology 2012, 4, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.J.; Alcorn, D.; Fraser, J.R.E. Absorption of hyaluronan applied to the surface of intact skin. J. Investig. Dermatol. 1999, 113, 740–746. [Google Scholar] [CrossRef] [Green Version]
- Essendoubi, M.; Gobinet, C.; Reynaud, R.; Angiboust, J.F.; Manfait, M.; Piot, O. Human skin penetration of hyaluronic acid of different molecular weights as probed by Raman spectroscopy. Ski. Res. Technol. 2016, 22, 55–62. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef] [PubMed]
- Sparavigna, A. Role of the extracellular matrix in skin aging and dedicated treatment—State of art. Plast. Aesthet. Res. 2020, 7, 14. [Google Scholar] [CrossRef]
- Kawada, C.; Yoshida, T.; Yoshida, H.; Matsuoka, R.; Sakamoto, W.; Odanaka, W.; Sato, T.; Yamasaki, T.; Kanemitsu, T.; Masuda, Y.; et al. Ingested hyaluronan moisturizes dry skin. Nutr. J. 2014, 13, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, T.F.; Su, Z.R.; Hsieh, Y.H.; Wang, M.F.; Oe, M.; Matsuoka, R.; Masuda, Y. Oral hyaluronan relieves wrinkles and improves dry skin: A 12-week double-blinded, placebo-controlled study. Nutrients 2021, 13, 2220. [Google Scholar] [CrossRef]
- Pavicic, T.; Gauglitz, G.G.; Lersch, P.; Schwach-Abdellaoui, K.; Malle, B.; Korting, H.C.; Farwick, M. Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. JDD 2011, 10, 990–1000. [Google Scholar]
- Michelotti, A.; Cestone, E.; De Ponti, I.; Pisati, M.; Tursi, F. Oral intake of a new Full Spectrum hyaluronan improves skin profilometry and ageing factors: A randomized, double-blind, placebo controlled clinical trial. Eur. J. Dermatol. 2021, 31, 798–805. [Google Scholar]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019, 286, 2883–2908. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Girolimetto, N.; Crescenzio Bentivenga, C.; Grandi, E.; Fogacci, F.; Borghi, C. Short-Term of a New Oral Sodium Hyaluronate Formulation on Knee Osteoarthritis: A Double-Blind, Randomized, Placebo-Controlled Clinical Trial. Diseases 2020, 8, 26. [Google Scholar] [CrossRef]
- Juncan, A.M.; Moisă, D.G.; Santini, A.; Morgovan, C.; Rus, L.L.; Vonica-Țincu, A.L.; Loghin, F. Advantages of hyaluronic acid and its combination with other bioactive ingredients in cosmeceuticals. Molecules 2021, 26, 4429. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic acid: Molecular mechanisms and therapeutic trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [Green Version]
- Cyphert, J.M.; Trempus, C.S.; Garantziotis, S. Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology. Int. J. Cell Biol. 2015, 2015, 563818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masi, S. The 2.0 Full Spectrum Hyaluronans Technology to improve bioavailability and efficacy performance. Agro. Food Ind. Hi-Tech 2020, 31, 21–25. [Google Scholar]

| Item | Question | G1 | G2 | G3 | |||
|---|---|---|---|---|---|---|---|
| T28 | T42 | T28 | T42 | T28 | T42 | ||
| Efficacy | Fine lines are reduced | 92% | 88% | 84% | 88% | 88% | 88% |
| Deep wrinkles are less visible | 80% | 72% | 76% | 76% | 72% | 74% | |
| The general appearance of the skin is improved | 92% | 96% | 92% | 88% | 92% | 84% | |
| The texture of the skin is redefined | 88% | 80% | 84% | 84% | 92% | 88% | |
| The skin is smoother | 92% | 96% | 92% | 88% | 96% | 92% | |
| The skin is deeply hydrated | 92% | 88% | 92% | 88% | 92% | 92% | |
| The skin is more elastic | 92% | 92% | 88% | 84% | 92% | 96% | |
| The skin is firmer and more compact | 92% | 88% | 84% | 80% | 92% | 88% | |
| The skin complexion is more even and the skin is brighter | 92% | 92% | 88% | 84% | 88% | 96% | |
| The skin looks like rejuvenated | 84% | 72% | 76% | 76% | 84% | 84% | |
| Characteristics | The food supplement is easy to take | 100% | - | 92% | - | 100% | - |
| The cream spreads easily | 96% | - | 96% | - | 96% | - | |
| The cream absorbs quickly and doesn’t leave any residues | 96% | - | 96% | - | 96% | - | |
| The cream has a good scent | 96% | - | 92% | - | 96% | - | |
| Tolerability | Was the food supplement well tolerated? | 96% | - | 100% | - | 100% | - |
| Was the cream well tolerated? | 100% | - | 100% | - | 100% | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlomagno, F.; Roveda, G.; Michelotti, A.; Ruggeri, F.; Tursi, F. Anti-Skin-Aging Effect of a Treatment with a Cosmetic Product and a Food Supplement Based on a New Hyaluronan: A Randomized Clinical Study in Healthy Women. Cosmetics 2022, 9, 54. https://doi.org/10.3390/cosmetics9030054
Carlomagno F, Roveda G, Michelotti A, Ruggeri F, Tursi F. Anti-Skin-Aging Effect of a Treatment with a Cosmetic Product and a Food Supplement Based on a New Hyaluronan: A Randomized Clinical Study in Healthy Women. Cosmetics. 2022; 9(3):54. https://doi.org/10.3390/cosmetics9030054
Chicago/Turabian StyleCarlomagno, Federica, Gloria Roveda, Angela Michelotti, Federica Ruggeri, and Francesco Tursi. 2022. "Anti-Skin-Aging Effect of a Treatment with a Cosmetic Product and a Food Supplement Based on a New Hyaluronan: A Randomized Clinical Study in Healthy Women" Cosmetics 9, no. 3: 54. https://doi.org/10.3390/cosmetics9030054
APA StyleCarlomagno, F., Roveda, G., Michelotti, A., Ruggeri, F., & Tursi, F. (2022). Anti-Skin-Aging Effect of a Treatment with a Cosmetic Product and a Food Supplement Based on a New Hyaluronan: A Randomized Clinical Study in Healthy Women. Cosmetics, 9(3), 54. https://doi.org/10.3390/cosmetics9030054






