Multi-Ingredient Supplement Supports Mitochondrial Health through Interleukin-15 Signaling in Older Adult Human Dermal Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. RNA Isolation and cDNA Synthesis
2.3. Quantitative PCR
2.4. Western Blotting
2.5. Cytotoxicity and Cellular Senescence
2.6. Statistics
3. Results
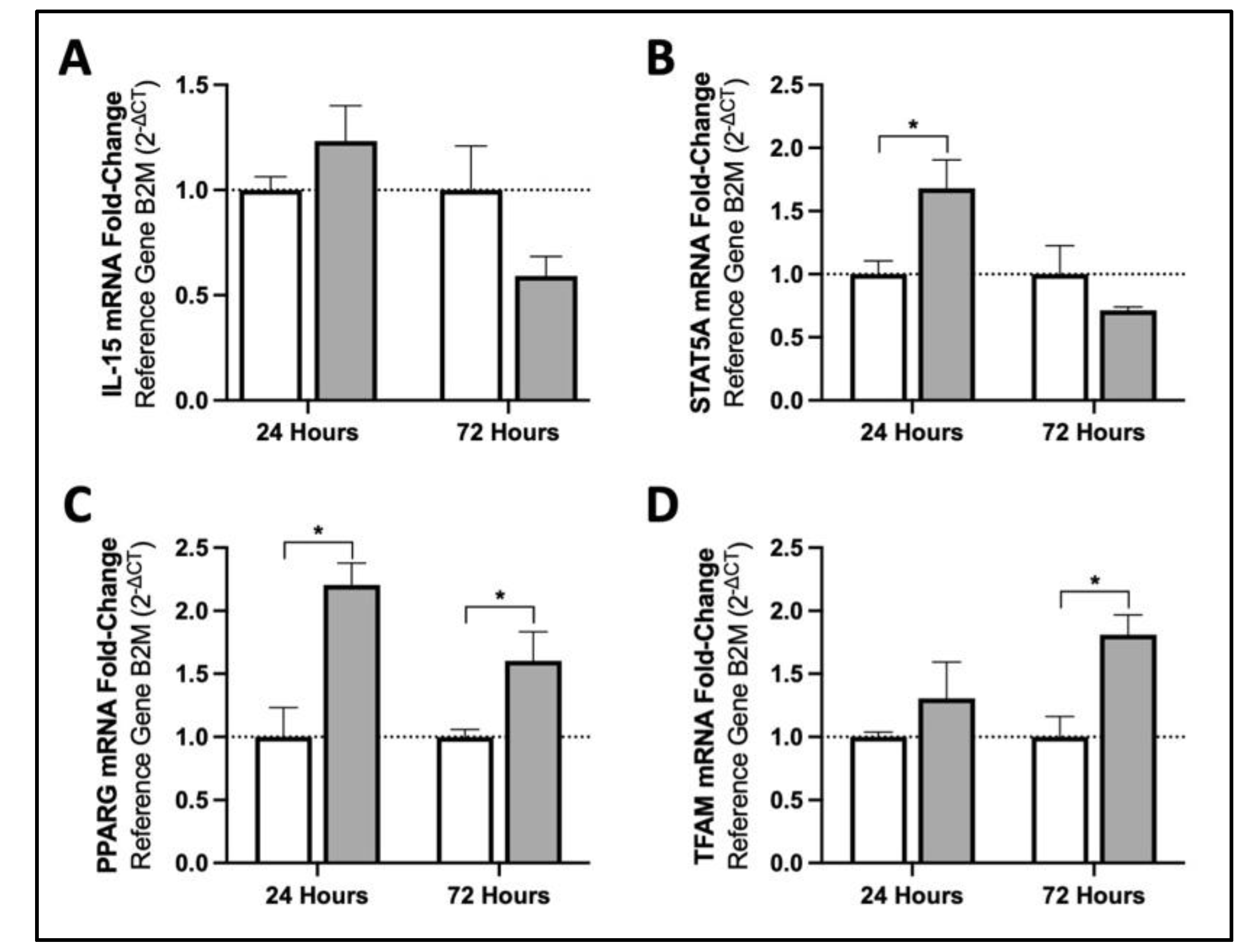
3.1. MIS Treatment Increases mRNA Content of Mitochondrial Biogenesis Activators in Human Fibroblasts via Downstream Targets of IL15
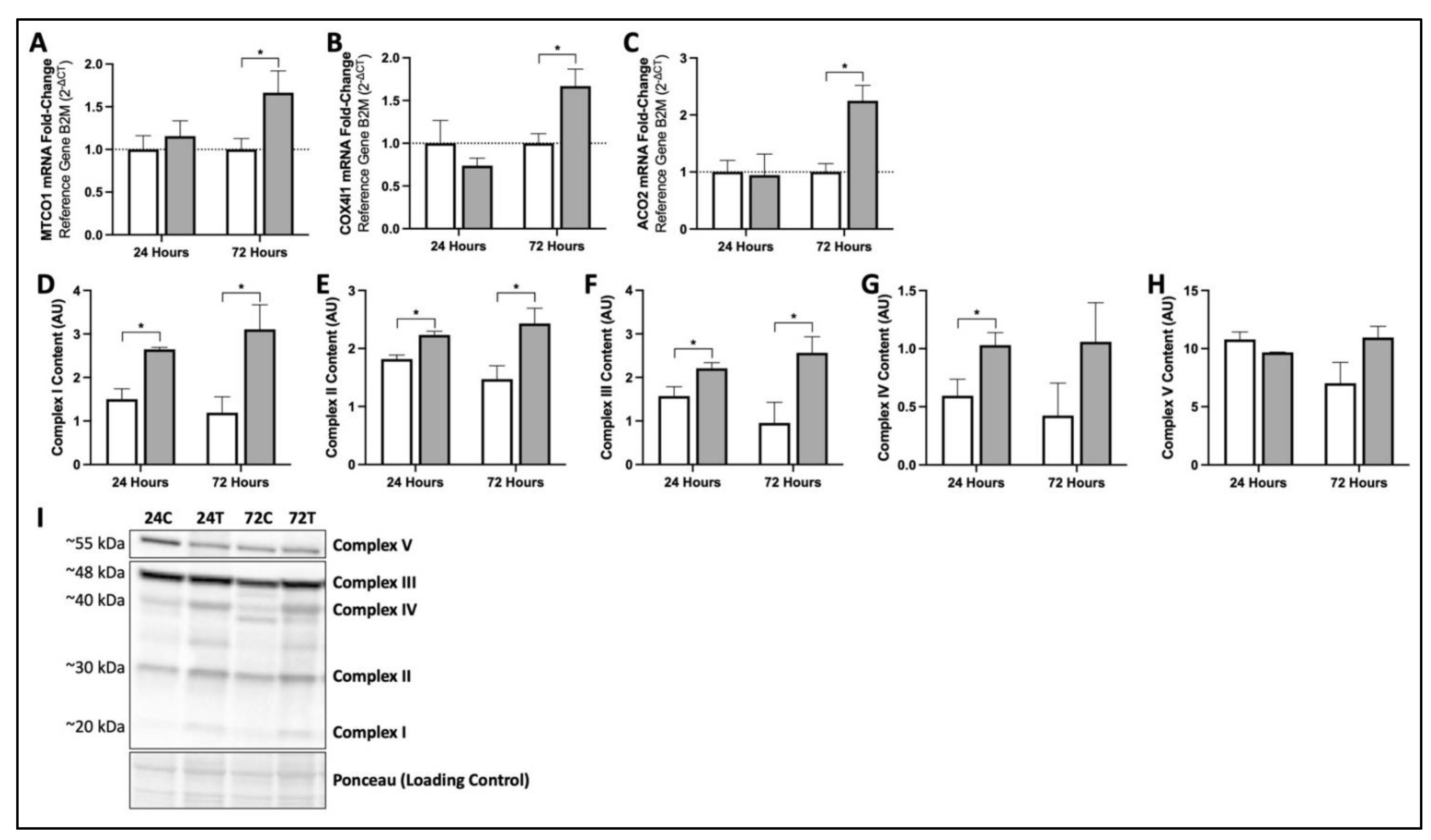
3.2. MIS Treatment Increases Production of Key mRNA and Proteins Responsible for Mitochondrial Function and Oxidative Phosphorylation in Human Fibroblasts
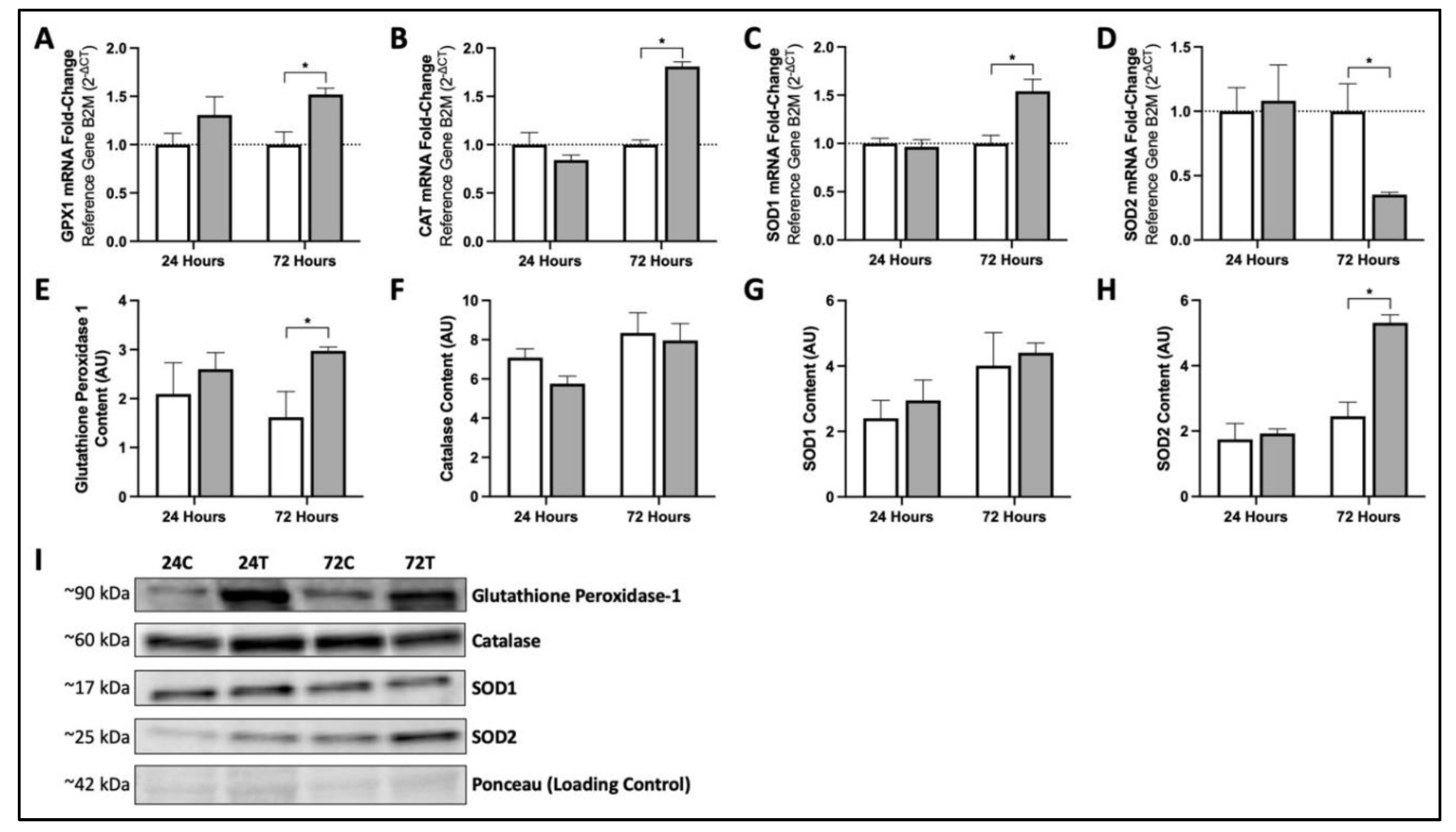
3.3. Seventy-Two Hours of MIS Supplementation Modifies mRNA and Protein Content of Key Cellular Antioxidants in Human Fibroblasts
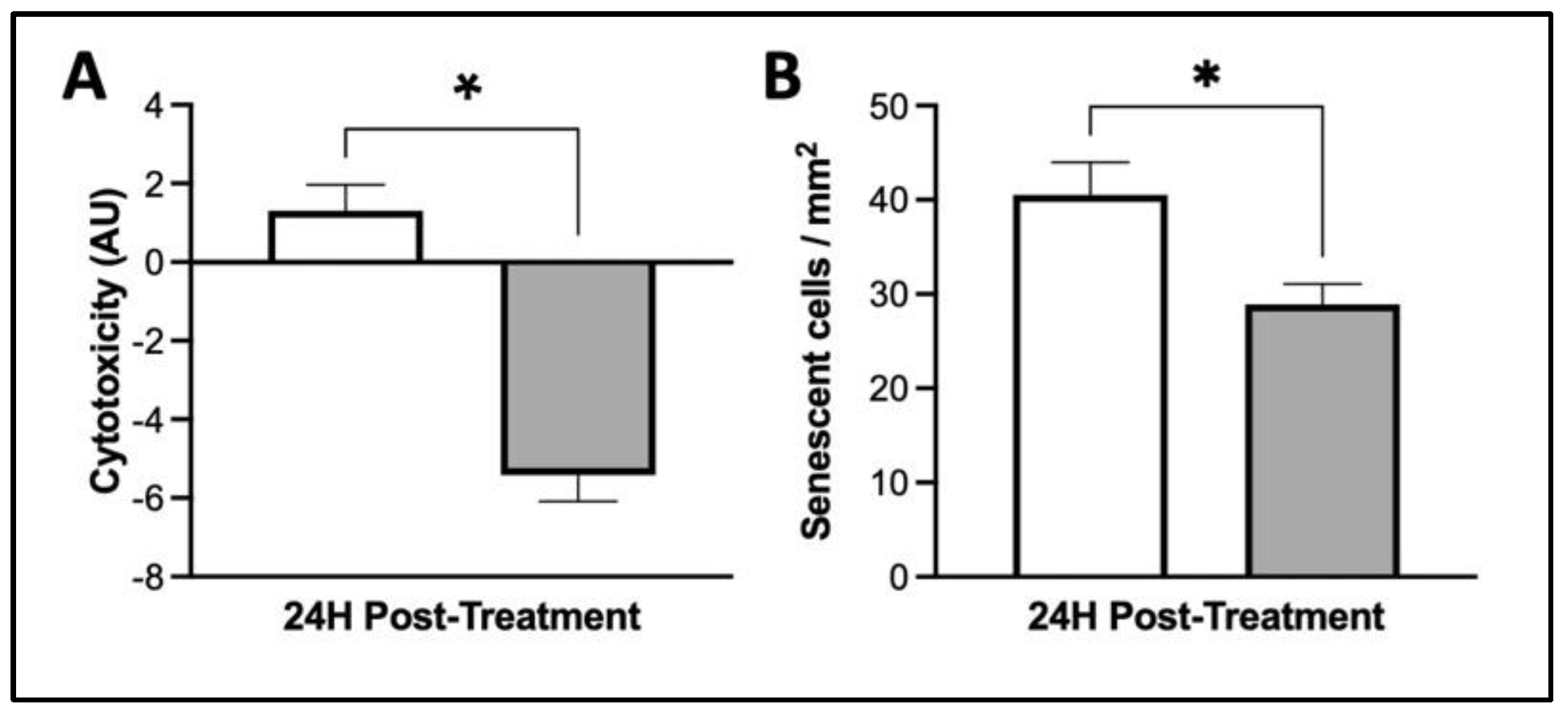
3.4. MIS Treatment Supports Whole-Cell Health in Human Dermal Fibroblasts
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194. [Google Scholar] [CrossRef] [PubMed]
- Menon, G.K.; Farra, C.D.; Botto, J.-M.; Domloge, N. Mitochondria: A new focus as an anti-aging target in skin care. J. Cosmet. Dermatol. 2010, 9, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, A.; Aguilera-Aguirre, L.; Singh, K.K. Mitochondria in skin health, aging, and disease. Cell Death Dis. 2020, 11, 444. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.D.; Macneil, L.G.; Lally, J.S.; Ford, R.J.; Bujak, A.L.; Brar, I.K.; Kemp, B.E.; Raha, S.; Steinberg, G.R.; Tarnopolsky, M.A. Exercise-stimulated interleukin-15 is controlled by AMPK and regulates skin metabolism and aging. Aging Cell 2015, 14, 625–634. [Google Scholar] [CrossRef]
- Stout, R.; Birch-Machin, M. Mitochondria’s Role in Skin Ageing. Biology 2019, 8, 29. [Google Scholar] [CrossRef]
- Ma, Y.S.; Wu, S.B.; Lee, W.Y.; Cheng, J.S.; Wei, Y.H. Response to the increase of oxidative stress and mutation of mitochondrial DNA in aging. Biochim. Biophys. Acta-Gen. Subj. 2009, 1790, 1021–1029. [Google Scholar] [CrossRef]
- Poljšak, B.; Dahmane, R.G.; Godic, A. Intrinsic skin aging: The role of oxidative stress. Acta Dermatovenerol Alp Pannonica Adriat. 2012, 21, 33–36. [Google Scholar] [CrossRef]
- Feichtinger, R.G.; Sperl, W.; Bauer, J.W.; Kofler, B. Mitochondrial dysfunction: A neglected component of skin diseases. Exp. Dermatol. 2014, 23, 607–614. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545. [Google Scholar] [CrossRef]
- Tamura, Y.; Watanabe, K.; Kantani, T.; Hayashi, J.; Ishida, N.; Kaneki, M. Upregulation of circulating IL-15 by treadmill running in healthy individuals: Is IL-15 an endocrine mediator of the beneficial effects of endurance exercise? Endocr. J. 2011, 58, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.C.; MacDonald, J.R.; Mahoney, D.J.; Parise, G.; Beal, M.F.; Tarnopolsky, M.A. Beneficial effects of creatine, CoQ10, and lipoic acid in mitochondrial disorders. Muscle Nerve 2007, 35, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Glover, E.I.; Martin, J.; Maher, A.; Thornhill, R.E.; Moran, G.R.; Tarnopolsky, M.A. A randomized trial of coenzyme Q10 in mitochondrial disorders. Muscle Nerve 2010, 42, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Abadi, A.; Crane, J.D.; Ogborn, D.; Hettinga, B.; Akhtar, M.; Stokl, A.; MacNeil, L.; Safdar, A.; Tarnopolsky, M. Supplementation with α-Lipoic Acid, CoQ10, and Vitamin E Augments Running Performance and Mitochondrial Function in Female Mice. PLoS ONE 2013, 8, e60722. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, R.; Fogelholm, M.; Himberg, J.J.; Laakso, J.; Salorinne, Y. Ubiquinone supplementation and exercise capacity in trained young and older men. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 72, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.A.; Costill, D.L.; Zachwieja, J.J.; Fink, W.J.; Wagner, E.; Folkers, K. The effect of oral coenzyme Q10 on the exercise tolerance of middle-aged, untrained men. Int. J. Sports Med. 1995, 16, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Gaeini, A.A.; Rahnama, N.; Hamedinia, M.R. Effects of vitamin E supplementation on oxidative stress at rest and after exercise to exhaustion in athletic students-ProQuest. J. Sports Med. Phys. Fit. 2006, 46, 458–461. [Google Scholar]
- Boyera, N.; Galey, I.; Bernard, B.A. Effect of vitamin C and its derivatives on collagen synthesis and cross-linking by normal human fibroblasts. Int. J. Cosmet. Sci. 1998, 20, 151–158. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- Offord, E.A.; Gautier, J.C.; Avanti, O.; Scaletta, C.; Runge, F.; Krämer, K.; Applegate, L.A. Photoprotective potential of lycopene, β-carotene, vitamin E, vitamin C and carnosic acid in UVA-irradiated human skin fibroblasts. Free Radic. Biol. Med. 2002, 32, 1293–1303. [Google Scholar] [CrossRef]
- Jurkiewicz, B.A.; Bissett, D.L.; Buettner, G.R. Effect of Topically Applied Tocopherol on Ultraviolet Radiation-Mediated Free Radical Damage in Skin. J. Investig. Dermatol. 1995, 104, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Petruk, G.; Del Giudice, R.; Rigano, M.M.; Monti, D.M. Antioxidants from plants protect against skin photoaging. Oxid. Med. Cell. Longev. 2018, 2018, 1454936. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Bertoglio, J.C.; Polimeni, A.; Scapagnini, G. Cytoprotective Polyphenols Against Chronological Skin Aging and Cutaneous Photodamage. Curr. Pharm. Des. 2018, 24, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Adhami, V.M.; Ahmad, N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol. Appl. Pharmacol. 2003, 186, 28–37. [Google Scholar] [CrossRef]
- Schagen, S.K.; Zampeli, V.A.; Makrantonaki, E.; Zouboulis, C.C. Discovering the link between nutrition and skin aging. Dermato-Endocrinol. 2012, 4, 298. [Google Scholar] [CrossRef]
- Giardina, D.; Michelotti, A.; Zavattini, G.; Finzi, S.; Ghisalberti, C.; Marzatico, F. Efficacy study in vitro: Assessment of the properties of resveratrol and resveratrol + N-acetyl-cysteine on proliferation and inhibition of collagen activity. Minerva Ginecol. 2010, 62, 195–201. [Google Scholar] [PubMed]
- Heng, M.C.Y. Curcumin targeted signaling pathways: Basis for anti-photoaging and anti-carcinogenic therapy. Int. J. Dermatol. 2010, 49, 608–622. [Google Scholar] [CrossRef]
- Lima, C.F.; Pereira-Wilson, C.; Rattan, S.I.S. Curcumin induces heme oxygenase-1 in normal human skin fibroblasts through redox signaling: Relevance for anti-aging intervention. Mol. Nutr. Food Res. 2011, 55, 430–442. [Google Scholar] [CrossRef]
- Vaughn, A.R.; Branum, A.; Sivamani, R.K. Effects of Turmeric (Curcuma longa) on Skin Health: A Systematic Review of the Clinical Evidence. Phytother. Res. 2016, 30, 1243–1264. [Google Scholar] [CrossRef]
- Laura, V.; Mattia, F.; Roberta, G.; Federico, I.; Emi, D.; Chiara, T.; Luca, B.; Elena, C. Potential of Curcumin in Skin Disorders. Nutrients 2019, 11, 2169. [Google Scholar] [CrossRef]
- McMahon, R.J. Biotin in metabolism and molecular biology. Annu. Rev. Nutr. 2003, 22, 221–239. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, D.L.; West, V.A.; Lephart, E.D. Enhancing Skin Health: By Oral Administration of Natural Compounds and Minerals with Implications to the Dermal Microbiome. Int. J. Mol. Sci. 2018, 19, 3059. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, Y.B.; Cohen, A.D.; Bibi Nitzan, Y.; Cohen, A.D. Zinc in skin pathology and care. J. Dermatol. Treat. 2009, 17, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.R.; Marsh, R.G.; Draelos, Z.D. Zinc and Skin Health: Overview of Physiology and Pharmacology. Dermatol. Surg. 2005, 31, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Rostan, E.F.; DeBuys, H.V.; Madey, D.L.; Pinnell, S.R. Evidence supporting zinc as an important antioxidant for skin. Int. J. Dermatol. 2002, 41, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Stahl, W.; Krutmann, J. Molecular evidence that oral supplementation with lycopene or lutein protects human skin against ultraviolet radiation: Results from a double-blinded, placebo-controlled, crossover study. Br. J. Dermatol. 2017, 176, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Faulhaber, D.; Hanson, K.M.; Ding, W.; Peters, S.; Kodali, S.; Granstein, R.D. Dietary Lutein Reduces Ultraviolet Radiation-Induced Inflammation and Immunosuppression. J. Investig. Dermatol. 2004, 122, 510–517. [Google Scholar] [CrossRef]
- Palombo, P.; Fabrizi, G.; Ruocco, V.; Ruocco, E.; Fluhr, J.; Roberts, R.; Morganti, P. Beneficial Long-Term Effects of Combined Oral/Topical Antioxidant Treatment with the Carotenoids Lutein and Zeaxanthin on Human Skin: A Double-Blind, Placebo-Controlled Study. Ski. Pharmacol. Physiol. 2007, 20, 199–210. [Google Scholar] [CrossRef]
- Komatsu, T.; Sasaki, S.; Manabe, Y.; Hirata, T.; Sugawara, T. Preventive effect of dietary astaxanthin on UVA-induced skin photoaging in hairless mice. PLoS ONE 2017, 12, e0171178. [Google Scholar] [CrossRef]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef]
- Lyons, N.M.; O’Brien, N.M. Modulatory effects of an algal extract containing astaxanthin on UVA-irradiated cells in culture. J. Dermatol. Sci. 2002, 30, 73–84. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.; Heilborn, J.D.; Jimenez, C.I.C.; Hammarsjö, A.; Törmä, H.; Ståhle, M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J. Investig. Dermatol. 2005, 124, 1080–1082. [Google Scholar] [CrossRef]
- Chang, A.L.S.; Fu, T.; Amir, O.; Tang, J.Y. Association of facial skin aging and vitamin D levels in middle-aged white women. Cancer Causes Control 2010, 21, 2315–2316. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bastianetto, S.; Dumont, Y.; Duranton, A.; Vercauteren, F.; Breton, L.; Quirion, R. Protective action of resveratrol in human skin: Possible involvement of specific receptor binding sites. PLoS ONE 2010, 5, e12935. [Google Scholar] [CrossRef]
- Schauber, J.; Gallo, R.L. The vitamin D pathway: A new target for control of the skin’s immune response? Exp. Dermatol. 2008, 17, 633–639. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J.; Lyakhovitsky, A.; Huszar, M. Improvement of facial skin characteristics using copper oxide containing pillowcases: A double-blind, placebo-controlled, parallel, randomized study. Int. J. Cosmet. Sci. 2009, 31, 437–443. [Google Scholar] [CrossRef]
- Borkow, G. Using Copper to Improve the Well-Being of the Skin. Curr. Chem. Biol. 2014, 8, 89. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Rebalka, I.A.; Monaco, C.M.F.; Varah, N.E.; Berger, T.; D’souza, D.M.; Zhou, S.; Mak, T.W.; Hawke, T.J. Loss of the adipokine lipocalin-2 impairs satellite cell activation and skeletal muscle regeneration. Am. J. Physiol.-Cell Physiol. 2018, 315, C714–C721. [Google Scholar] [CrossRef] [PubMed]
- Marçais, A.; Cherfils-Vicini, J.; Viant, C.; Degouve, S.; Viel, S.; Fenis, A.; Rabilloud, J.; Mayol, K.; Tavares, A.; Bienvenu, J.; et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat. Immunol. 2014, 15, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Legchenko, E.; Chouvarine, P.; Borchert, P.; Fernandez-Gonzalez, A.; Snay, E.; Meier, M.; Maegel, L.; Mitsialis, S.A.; Rog-Zielinska, E.A.; Kourembanas, S.; et al. PPARγ agonist pioglitazone reverses pulmonary hypertension and prevents right heart failure via fatty acid oxidation. Sci. Transl. Med. 2018, 10, eaao0303. [Google Scholar] [CrossRef]
- Yin, Y.; Wu, Y.; Zhang, X.; Zhu, Y.; Sun, Y.; Yu, J.; Gong, Y.; Sun, P.; Lin, H.; Han, X. PPA1 Regulates Systemic Insulin Sensitivity by Maintaining Adipocyte Mitochondria Function as a Novel PPARγ Target Gene. Diabetes 2021, 70, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, R.; Damirchi, A.; Babaei, P. Vitamin D increases PPARγ expression and promotes beneficial effects of physical activity in metabolic syndrome. Nutrition 2017, 36, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Aloysius Dhivya, M.; Aberami, S.; Nikhalashree, S.; Biswas, J.; Liu, W.; Irudayaraj, J.; Sulochana, K.N.; Coral, K.; Bharathi Devi, S.R. Copper mediates mitochondrial biogenesis in retinal pigment epithelial cells. Biochim. Biophys. Acta. Mol. Basis Dis. 2020, 1866, 165843. [Google Scholar] [CrossRef]
- Bernardo, A.; Plumitallo, C.; De Nuccio, C.; Visentin, S.; Minghetti, L. Curcumin promotes oligodendrocyte differentiation and their protection against TNF-α through the activation of the nuclear receptor PPAR-γ. Sci. Rep. 2021, 11, 4952. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.E.; Hu, L.; Zhao, L.; Huang, J. Carotenoids inhibit proliferation and regulate expression of peroxisome proliferators-activated receptor gamma (PPARγ) in K562 cancer cells. Arch. Biochem. Biophys. 2011, 512, 96–106. [Google Scholar] [CrossRef]
- Nanjaiah, H.; Vallikannan, B. Lutein upregulates the PGC-1α, NRF1, and TFAM expression by AMPK activation and downregulates ROS to maintain mtDNA integrity and mitochondrial biogenesis in hyperglycemic ARPE-19 cells and rat retina. Biotechnol. Appl. Biochem. 2019, 66, 999–1009. [Google Scholar] [CrossRef]
- Lee, D.; Kim, K.Y.; Shim, M.S.; Kim, S.Y.; Ellisman, M.H.; Weinreb, R.N.; Ju, W.K. Coenzyme Q10 ameliorates oxidative stress and prevents mitochondrial alteration in ischemic retinal injury. Apoptosis 2014, 19, 603–614. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Z.; Shen, R.; Zhong, W.; Zheng, H.; Chen, Z.; Tang, J.; Zhu, J. Resveratrol Improves Mitochondrial Biogenesis Function and Activates PGC-1α Pathway in a Preclinical Model of Early Brain Injury Following Subarachnoid Hemorrhage. Front. Mol. Biosci. 2021, 8, 223. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.; Zhang, B.; Deng, Z. The synergistic and antagonistic antioxidant interactions of dietary phytochemical combinations. Crit. Rev. Food Sci. Nutr. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.; Hughes, J.; Della Gatta, P.A.; Mason, S.; Lamon, S.; Russell, A.P.; Wadley, G.D. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic. Biol. Med. 2015, 89, 852–862. [Google Scholar] [CrossRef]
- Paulsen, G.; Cumming, K.T.; Holden, G.; Hallén, J.; Rønnestad, B.R.; Sveen, O.; Skaug, A.; Paur, I.; Bastani, N.E.; Østgaard, H.N.; et al. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: A double-blind, randomised, controlled trial. J. Physiol. 2014, 592, 1887–1901. [Google Scholar] [CrossRef] [PubMed]
- Yfanti, C.; Åkerström, T.; Nielsen, S.; Nielsen, A.R.; Mounier, R.; Mortensen, O.H.; Lykkesfeldt, J.; Rose, A.J.; Fischer, C.P.; Pedersen, B.K. Antioxidant supplementation does not alter endurance training adaptation. Med. Sci. Sports Exerc. 2010, 42, 1388–1395. [Google Scholar] [CrossRef]
- Higashida, K.; Kim, S.H.; Higuchi, M.; Holloszy, J.O.; Han, D.H. Normal adaptations to exercise despite protection against oxidative stress. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E779–E784. [Google Scholar] [CrossRef]
- Nederveen, J.P.; Manta, K.; Bujak, A.L.; Simone, A.C.; Fuda, M.R.; Nilsson, M.I.; Hettinga, B.P.; Hughes, M.C.; Perry, C.G.R.; Tarnopolsky, M.A. A Novel Multi-Ingredient Supplement Activates a Browning Program in White Adipose Tissue and Mitigates Weight Gain in High-Fat Diet-Fed Mice. Nutrients 2021, 13, 3726. [Google Scholar] [CrossRef]
- Yan, L.J.; Levine, R.L.; Sohal, R.S. Oxidative damage during aging targets mitochondrial aconitase. Proc. Natl. Acad. Sci. USA 1997, 94, 11168. [Google Scholar] [CrossRef]
- Krutmann, J.; Schroeder, P. Role of Mitochondria in Photoaging of Human Skin: The Defective Powerhouse Model. J. Investig. Dermatol. Symp. Proc. 2009, 14, 44–49. [Google Scholar] [CrossRef]
- Eshaghian, A.; Vleugels, R.A.; Canter, J.A.; McDonald, M.A.; Stasko, T.; Sligh, J.E. Mitochondrial DNA deletions serve as biomarkers of aging in the skin, but are typically absent in nonmelanoma skin cancers. J. Investig. Dermatol. 2006, 126, 336–344. [Google Scholar] [CrossRef][Green Version]
- Lee, H.C.; Wei, Y.H.; Yang, J.H. Photoageing-associated mitochondrial DNA length mutations in human skin. Arch. Dermatol. Res. 1995, 287, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Shadel, G.S. Mitochondrial DNA, aconitase “wraps” it up. Trends Biochem. Sci. 2005, 30, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Z.; Hu, C.W.; Gao, Z.M. Aconitase couples metabolic regulation to mitochondrial DNA maintenance. Science 2005, 307, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Wiley, C.D.; Velarde, M.C.; Lecot, P.; Liu, S.; Sarnoski, E.A.; Freund, A.; Shirakawa, K.; Lim, H.W.; Davis, S.S.; Ramanathan, A.; et al. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. 2016, 23, 303–314. [Google Scholar] [CrossRef]
- Lauri, A.; Pompilio, G.; Capogrossi, M.C. The mitochondrial genome in aging and senescence. Ageing Res. Rev. 2014, 18, 1–15. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 485. [Google Scholar] [CrossRef]
- Low, E.; Alimohammadiha, G.; Smith, L.A.; Costello, L.F.; Przyborski, S.A.; von Zglinicki, T.; Miwa, S. How good is the evidence that cellular senescence causes skin ageing? Ageing Res. Rev. 2021, 71, 101456. [Google Scholar] [CrossRef]
- Takenaka, Y.; Inoue, I.; Nakano, T.; Ikeda, M.; Kakinuma, Y. Prolonged disturbance of proteostasis induces cellular senescence via temporal mitochondrial dysfunction and subsequent mitochondrial accumulation in human fibroblasts. FEBS J. 2021, 289, 1650–1667. [Google Scholar] [CrossRef]
- Van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439. [Google Scholar] [CrossRef]
- Campisi, J.; D’Adda Di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Birar, V.C.; Sheerin, A.N.; Ostler, E.L.; Faragher, R.G.A. Novel resveratrol derivatives have diverse effects on the survival, proliferation and senescence of primary human fibroblasts. Biogerontology 2020, 21, 817–826. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebalka, I.A.; May, L.; Nederveen, J.P.; Tarnopolsky, M.A. Multi-Ingredient Supplement Supports Mitochondrial Health through Interleukin-15 Signaling in Older Adult Human Dermal Fibroblasts. Cosmetics 2022, 9, 47. https://doi.org/10.3390/cosmetics9030047
Rebalka IA, May L, Nederveen JP, Tarnopolsky MA. Multi-Ingredient Supplement Supports Mitochondrial Health through Interleukin-15 Signaling in Older Adult Human Dermal Fibroblasts. Cosmetics. 2022; 9(3):47. https://doi.org/10.3390/cosmetics9030047
Chicago/Turabian StyleRebalka, Irena Alexandra, Linda May, Joshua Peter Nederveen, and Mark Andrew Tarnopolsky. 2022. "Multi-Ingredient Supplement Supports Mitochondrial Health through Interleukin-15 Signaling in Older Adult Human Dermal Fibroblasts" Cosmetics 9, no. 3: 47. https://doi.org/10.3390/cosmetics9030047
APA StyleRebalka, I. A., May, L., Nederveen, J. P., & Tarnopolsky, M. A. (2022). Multi-Ingredient Supplement Supports Mitochondrial Health through Interleukin-15 Signaling in Older Adult Human Dermal Fibroblasts. Cosmetics, 9(3), 47. https://doi.org/10.3390/cosmetics9030047






