1. Introduction
Dry, sensitive skin is a common condition that is associated with lack of water in the stratum corneum (SC). The SC of dry skin sufferers displays an altered lipid organization/composition and lipid content, thereby markedly contributing to the development of impaired skin barrier function with increased transepidermal water loss (TEWL) and consequently reduced skin hydration [
1]. Often, dry skin involves the hands. Dry hands can be a condition in itself, but in most instances, it is triggered by environmental factors, such as frequent washing, cold weather, or exposure to chemicals/detergents [
2,
3]. The hands feel dry and rough; they are tense, reddened, and painful cracks appear. People with dry hands have an increased risk of eczema formation [
3].
Regular use of a moisturizing hand cream can help to restore hydration and barrier function of the skin as illustrated by a study in healthy subjects showing that skin dryness and roughness caused by frequent hand washing can be alleviated by applying a moisturizing hand cream after each hand wash [
4]. However, to be accepted by users, a hand cream must have special features in addition to its moisturizing effect. It must be absorbed quickly so that the hands are immediately ready for use again. The hand cream should be non-greasy and non-sticky and instantly relieve the feeling of tightness and roughness of dry hands.
In this context, a new dexpanthenol-containing hand cream (ND-HC, Bepanthen
® Derma REPAIRING Hand Cream) was developed for people with dry, rough, sensitive, and/or environmentally stressed hands. The most important ingredients of ND-HC comprise argan oil, shea butter, squalane, isopropyl isostearate, niacinamide, glycerin, and dexpanthenol. ND-HC is an oil-in-water emulsion formulation and contains no preservatives, fragrance, or silicone. The composition of ND-HC is in line with recent recommendations for skin care products to be used for dry skin management [
1].
Our randomized controlled study in healthy subjects with sensitive and very dry skin on the hands had the objective to explore the performance, acceptability, satisfaction, and local tolerability of ND-HC when used several times a day. Established noninvasive methods were applied to measure the effects of ND-HC on SC hydration and skin barrier function over the 4-week study period. Cutaneous tolerability and product acceptability/satisfaction were determined by dermatological evaluations and a subject questionnaire, respectively.
2. Methods
Our trial in healthy adult volunteers was conducted from October to November 2021 at proDERM GmbH, Schenefeld/Hamburg, Germany, under surveillance of a dermatologist. The study followed the principles outlined in the Declaration of Helsinki with all its revisions, and the independent Ethics Committee of proDERM reviewed the study protocol for ethical approval (approval number: 2021/033). Each subject provided written informed consent prior to enrollment in the study. Bayer Consumer Care AG (Basel, Switzerland) supplied the new hand cream (Bepanthen® Derma REPAIRING Hand Cream, Bayer Consumer Care AG, Basel, Switzerland) used in the trial.
As this was an exploratory study, no formal sample size calculation was performed, and no primary and secondary variables were specified. Based on prior experience with similarly designed investigations, it was assumed that informative results could be obtained with the chosen sample size [
5,
6,
7].
2.1. Study Design
This was an exploratory, monocentric, open, randomized, intraindividual comparison study in healthy adult subjects with sensitive and very dry skin on the hands. Visits at the study site took place on study day 1 (baseline) and days 2, 3, and 29. On these days, the study participants came to the study center without having applied ND-HC before.
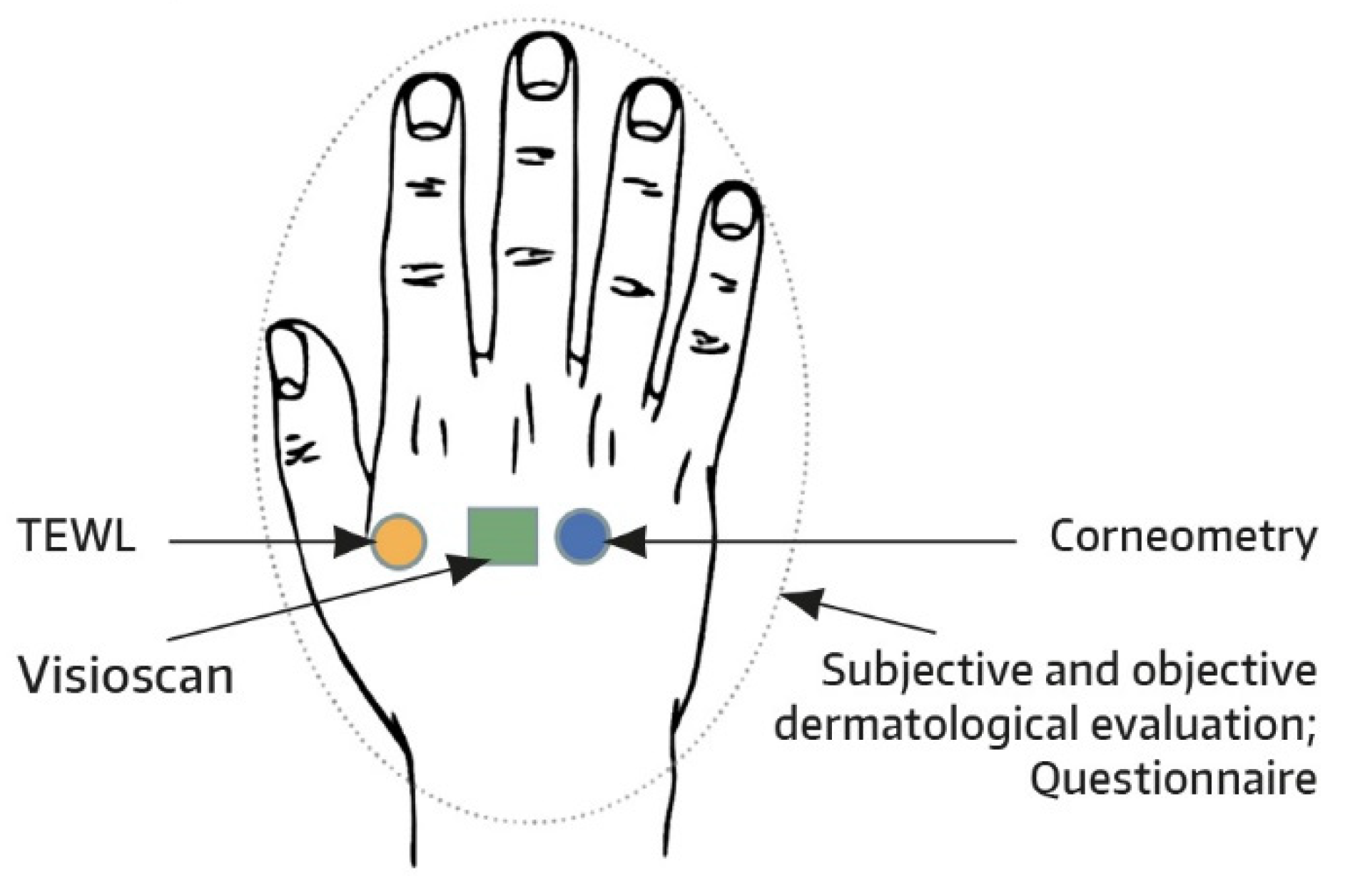
In accordance with previous studies examining a hand cream [
8], prespecified test areas were marked on the dorsal skin of both hands, with one hand serving as a control and remaining untreated (
Figure 1). For each subject, the treatment side was randomly chosen and balanced between both hands. One fingertip unit (approximately 0.5 g of ND-HC) was to be applied to the entire hand at least four times daily every 2-3 h using a glove. Hand washing right after ND-HC application was to be avoided to allow complete absorption of the test product. The first and second administrations of ND-HC were performed by the subjects under the guidance of a technician at the study site. The other applications were performed by the subjects at home. As soon as baseline assessments had been completed on day 1, ND-HC treatment commenced, with the caveat that the first and second ND-HC administrations were separated by a treatment-free period of 48 h. By weighing each tube with ND-HC (50 mL) before and after the study, compliance was checked and ensured.
2.2. Subjects
Healthy male and nonpregnant female subjects, aged 18–70 years, and having a body mass index of <30 were enrolled. For study participation, subjects had to have sensitive and very dry skin in the test areas. Very dry skin was defined as a corneometer value of ≤30 arbitrary units (a.u.), while the presence of sensitive skin was verified based on criteria established by the Autorité de Régulation Professionnelle de la Publicité [
9]. Women of childbearing potential had to use reliable contraceptive methods during the entire course of the trial.
Subjects could not enter the study if they suffered from any skin condition at the test areas that might influence the interpretation of study data; a condition requiring the use of drugs interfering with study results within 1 week (immunosuppressive drugs, antihistamines, any topical medication at the test areas) or within 3 days (anti-inflammatory agents, analgesics with the exception of acetylsalicylic acid and paracetamol) before or during the study; or had allergies to cosmetic products or ingredients. Subjects were further excluded if they were addicted to alcohol/drugs or had insulin-dependent diabetes, human immunodeficiency virus infection, infectious hepatitis, illnesses associated with reduced physical fitness (e.g., asthma, cardiovascular diseases), or received cancer treatment within the past 2 years.
Subjects were not permitted to have intensive exposure of the test areas to sun, UV-therapy and/or artificial tanning within 1 month before and during the study, or to apply any leave-on cosmetics on the hands from 1 week before until termination of the study. Furthermore, study participants were not allowed to change their lifestyle customs over the study course. On days of study site visits, subjects were requested to avoid the use of detergents and disinfection products on the test areas in the morning and not to consume any caffeinated beverages or to smoke within 2 h before instrumental measurements. For the same time interval, any contact of the test areas with water was discouraged.
2.3. Assessments
Measurements of SC hydration were performed by corneometry (Corneometer
® CM825, Courage & Khazaka, Cologne, Germany). This technique determines the electrical capacitance of the skin surface, which correlates with the amount of water in the SC (i.e., the higher the skin capacitance, the better the skin hydration) [
10,
11]. Electrical capacitance was quantified on one dorsal skin test area of both hands (treated and untreated) at baseline and on day 2 (24 h) and day 3 (48 h) after initial and single application of ND-HC. Another measurement was performed after 4 weeks of ND-HC use on day 29. Five readings were taken per test area and assessment time. The highest and lowest were removed, and the mean of the remaining three readings was used as an actual measurement value for further analysis, as reported before [
12].
TEWL as a measure of skin barrier function was determined on one dorsal skin test area of both hands (treated and untreated) using the Tewameter
® TM 300 (Courage & Khazaka, Cologne, Germany). Measurements were conducted at baseline and on day 29. For each assessment, the probe was held in place for 30 s. The average of the last 10 s (= 10 values) represented the actual measurement value [
12,
13]. One TEWL measurement was performed per test area and time point. An improvement in skin barrier function is reflected by a decrease in TEWL values [
14].
To illustrate the effects of ND-HC on treated versus untreated skin, images from predefined skin areas of the dorsa of hands were taken in a subset of study participants at baseline and on day 29. Specifically, in the first 10 subjects entering the study, skin images were made using Visioscan
® VC 20plus (Courage & Khazaka, Cologne, Germany). This is a noninvasive method that uses UVA light to image the skin surface in vivo [
15]. The images show the skin structure and the level of dryness with a high resolution. Because dry skin has not differentiated properly, the skin scaliness is seen as very bright pixels, whereas wrinkles and lines appear dark.
The instrumental measurements (corneometry, TEWL) as well as Visioscan® imaging took place in an air-conditioned room (22 ± 2 °C, 50 ± 7.5% relative humidity). Before assessments commenced, the subjects stayed in the climatized room for ≥ 30 min.
Acceptability of ND-HC was determined by means of a validated questionnaire consisting of 26 favorable statements related to trait and features of the product. The subjects had to fill in the questionnaire at the study site on day 29. All questions had predefined identical options to be ticked: −3 = strongly disagree, −2 = moderately disagree, −1 = slightly disagree, 0 = neither agree nor disagree, 1 = slightly agree, 2 = moderately agree, 3 = strongly agree. Furthermore, the tolerability of ND-HC was judged based on objective and subjective dermatological evaluations at baseline and after 4 weeks of ND-HC application. Adverse events (AEs) were recorded throughout the study using a diary.
2.4. Statistics
The computation of statistical data was carried out with SAS® 9.4 for Windows (IT@Cornell, Ithaca, NY, USA). For instrumental measurements, mean changes from baseline were calculated. Bilateral differences (ND-HC-treated vs. untreated sites) in the mean change from baseline at each post-application assessment time were analyzed statistically using the paired t-test. For each site separately (ND-HC-treated and untreated), it was also evaluated whether mean absolute values for skin surface capacitance and TEWL statistically differed between baseline and post-application assessment times using the paired t-test. A significance level of 0.05 was chosen. Due to the explorative nature of the study, no adjustment for multiplicity was made. Results from the questionnaire and dermatological evaluations as well as AE frequencies were evaluated descriptively.
3. Results
Our study enrolled 40 healthy subjects (37 Whites, 2 Asians, 1 Black) of whom 34 were females and six were males. The average age was 49.4 years (range: 20–70 years). No subject discontinued the trial prematurely.
3.1. Corneometry—SC Hydration
At baseline, the skin test areas allocated to be treated or untreated had comparable skin surface capacitance values corresponding to the definition of very dry skin (
Table 1).
In the treated skin area, the single and 4 weeks’ use of ND-HC produced an increase in SC hydration as seen by increased skin surface capacitance values in comparison to baseline (
Table 1). Skin hydration was significantly improved at 24 h after the first application of ND-HC and remained elevated until 48 h after the first application, although for the latter time point, statistical significance was missed. On day 29, skin hydration was improved by 72.8% compared to baseline (12.41 a.u.;
p < 0.001). In the untreated control area, there was also a significant increase in skin hydration at day 29; however, the increase in mean electrical skin surface capacitance from baseline was much less pronounced compared to the treated site. In fact, the mean change in skin capacitance from baseline over the 4-week treatment period was significantly greater when ND-HC was applied to the test area compared with the untreated control area on the contralateral hand (12.41 vs. 4.46 a.u.;
p < 0.001;
Table 2), thereby emphasizing the skin-moisturizing effect of ND-HC. The bilateral differences in the mean change of skin surface capacitance from baseline did not reach statistical significance after a single application of ND-HC, although they were numerically in favor of ND-HC (
Table 2).
3.2. Transepidermal Water Loss
Before initiation of product application, barrier function was similar at the skin areas designated to be treated or untreated, as reflected by comparable mean values for TEWL at baseline (
Table 3). Upon use of ND-HC over 4 weeks, mean TEWL decreased significantly, thereby indicating an improvement in SC barrier function. On day 29, a decrease by 7.9% from baseline was observed (−1.8 g/m
2/h;
p = 0.026), whereas in the untreated control area, the mean TEWL even slightly increased. Likewise, the bilateral difference (treated vs. untreated hand) in the mean change of TEWL from baseline reached statistical significance after 4 weeks’ use of ND-HC (−1.8 vs. 1.0 g/m
2/h;
p = 0.003;
Table 3).
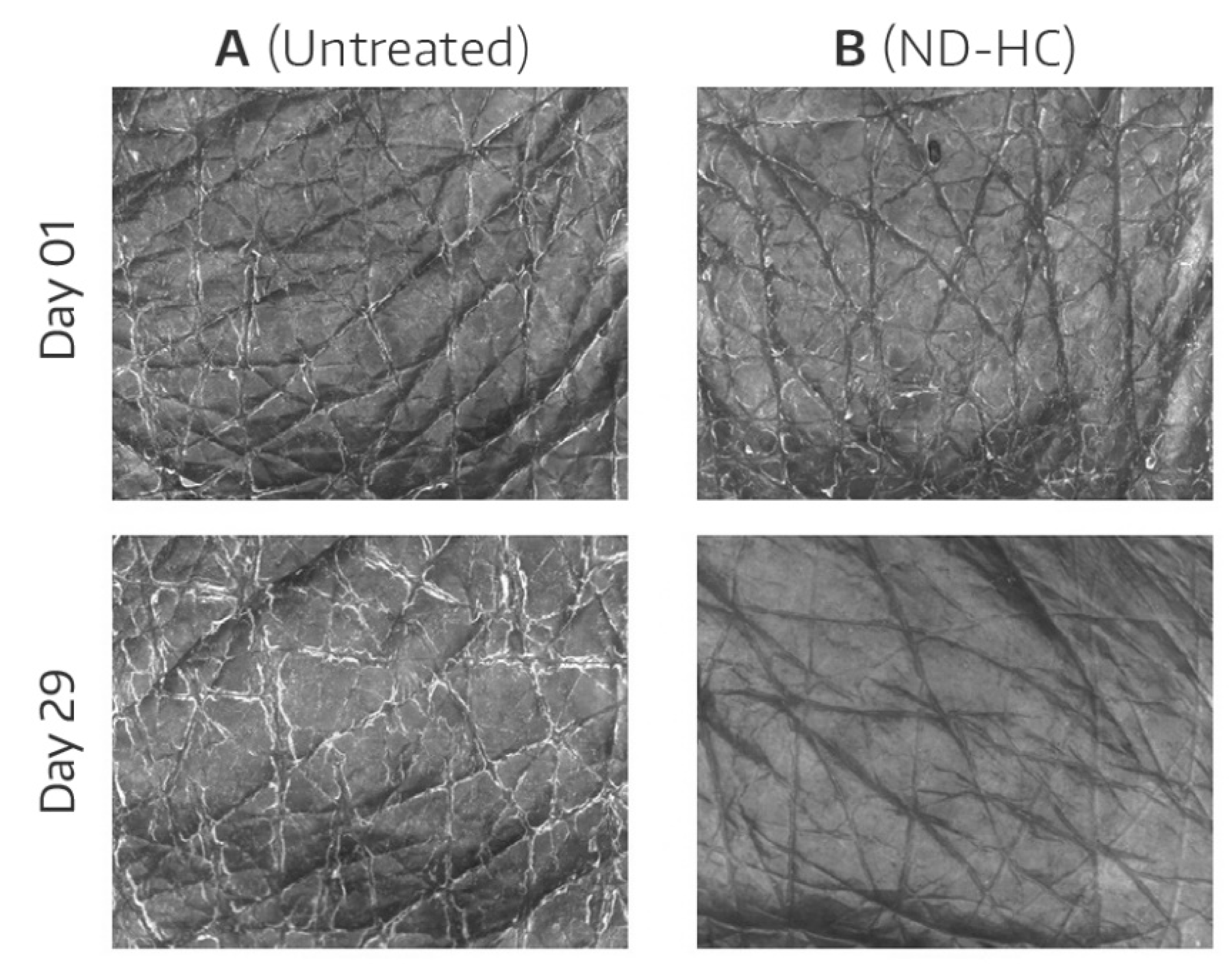
3.3. Visioscan® Images
The improved hydration of the ND-HC-treated skin was visually reflected in the Visioscan
® images as exemplified by
Figure 2, which shows treated and untreated skin areas of a study participant at baseline and at the end of the study. Dry corneocytes fluoresce when UVA hits the skin surface (i.e., visible light is emitted) [
15]. As a result of improved SC hydration, the brightness of the skin image is reduced.
3.4. Acceptability and Tolerability
The acceptability of ND-HC was highly scored by study participants. For 25 of 26 statements about the favorable traits and features of ND-HC, the rating was 3 (strongly agree), 2 (moderately agree), or 1 (slightly agree) by ≥80% of subjects after 4 weeks of ND-HC use. For example, 100%, 100%, 85%, and 88% of trial participants provided a rating of 1–3 for the claims that ND-HC “leaves hands feeling moisturized immediately after you apply, “immediately relieves the tightness of dry hands”, “provides a dry-touch (absorbs fast for a clean, non-greasy and non-sticky finish)”, and “totally satisfies me”, respectively.
Regarding safety, the 4 weeks’ use of ND-HC was well tolerated. No local AEs were recorded. The objective and subjective dermatological evaluations paralleled results from instrumental measurements and observations from the Visioscan® images. The presence of symptoms associated with dry hands (e.g., erythema, dryness, scaling, fissures)—as assessed by a dermatologist—markedly decreased in the ND-HC-treated skin area over the course of the study. Specifically, the proportion of subjects with some degree of dryness decreased from 100% at baseline to 15% after 4 weeks of ND-HC-treatment; no study participant revealed strong dryness any longer. In the untreated skin area, the proportion remained essentially unchanged during the study. Similar observations were made for subjective dermatological evaluations. For instance, the proportions of subjects with some degree of tightness and redness in the test area decreased from 75% and 50% at baseline to 18% and 13%, respectively, after 4 weeks of ND-HC application. No such pattern was observed for the untreated control skin area.
4. Discussion
A new dexpanthenol-containing hand cream (ND-HC) for multiple daily applications was developed for people with dry, rough, sensitive, and/or environmentally stressed hands. The aim of our study was to explore the performance, acceptability, satisfaction, and local tolerability of ND-HC in healthy subjects with sensitive and very dry skin on the hands. The effects on SC hydration, TEWL, and Visioscan® skin images were evaluated after single and/or 4 weeks’ use of ND-HC; local tolerability and acceptability/satisfaction were assessed by dermatological evaluations and a questionnaire, respectively.
Based on comparisons with baseline assessments and/or untreated skin areas on the contralateral hand, and considering the results from the questionnaire, the findings of our study can be summarized as follows: (1) after a single ND-HC application to very dry skin of the hand, skin surface capacitance improved significantly for up to 24 h, suggesting an immediate and long-lasting moisturizing effect; (2) after 4 weeks’ use of ND-HC (at least four times daily), a significantly increased skin surface capacitance was recorded, indicating that ND-HC provides long-term moisturization; (3) multiple daily applications of ND-HC over 4 weeks reduced TEWL significantly, corresponding to an improved skin barrier function; (4) the improved moisturization status of ND-HC-treated hands was confirmed by the results of Visioscan® images and dermatological examinations showing a reduction in signs and symptoms of dry hands over the course of the study; (5) the 4 weeks’ use of ND-HC was well tolerated and achieved a high level of acceptance and product satisfaction.
In our study, the newly developed hand cream (ND-HC) revealed a good performance in subjects with sensitive and very dry hands. In particular, ND-HC was an effective skin moisturizer and improved the skin barrier function. The high level of product acceptability/satisfaction among the overwhelming majority of study participants confirmed that ND-HC provides the required traits and attributes of a modern hand cream. The skin-hydrating effect of ND-HC, which we observed in our trial, was in accordance with earlier studies using topical skin care products (body lotions, face creams) with comparable principal components [
5,
7]. In these 4-week studies, daily use of the topical skin care products triggered a significant increase in hydration of the volar forearms and face, respectively, in dry skin suffers. Based on new insights into the management of dry skin [
1], it appears justified to infer that the beneficial effects of ND-HC on dry and sensitive hands is mediated by an additive or synergistic action of its key ingredients.
In line with recent recommendations for skin care products to be used for dry skin management, ND-HC contains a humectant (glycerin), a physiological lipid (isopropyl isostearate), non-physiological lipids (shea butter, squalane, argan oil), an antipruritic/soothing agent (niacinamide), and a multifunctional ingredient (dexpanthenol) [
1]. The non-physiological lipids act as a substitute for lost natural skin lipids in the outer layers of the SC and reduce TEWL by creating a hydrophobic barrier on the skin surface (occlusive effect) [
1]. Physiological lipids penetrate the SC and support the normalization of the affected lipid composition/organization [
1,
16]. Glycerin and niacinamide (nicotinamide) act as humectant and antipruritic/soothing agent, respectively [
17,
18,
19]. Dexpanthenol stimulates protein/lipid synthesis and supports epidermal regeneration by enhancing epidermal differentiation. In addition, it compensates for reduced hydration by retaining/increasing molecular mobility/fluidity of the SC lipid lamellae and proteins [
1,
20,
21].
An intriguing finding of our study was the significant increase in skin hydration in the untreated control area, albeit much less pronounced compared to the treated site. This observation is considered a spontaneous and random change in SC hydration during the 4-week study period.
Although we enrolled subjects with sensitive skin in our study, ND-HC was well tolerated and achieved a high level of product acceptance/satisfaction. This concurs with previous studies using topical skin care products with comparable principal components [
5,
7].
A limitation of our study is that only the dorsal skin of the hand was subjected to instrumental measurements. However, the palmar skin is several times thicker than that of the dorsum of the hands and thus a problematic test area with the risk of high variability in study results [
8]. The effects of ND-HC on the hand as a whole was assessed by acceptability scorings and evaluations by a dermatologist. ND-HC was not compared to an active comparator, which is another limitation. Therefore, no superiority claims over other hand creams can be made.