Deciphering the Phytochemical Profile of an Alpine Rose (Rhododendron ferrugineum L.) Leaf Extract for a Better Understanding of Its Senolytic and Skin-Rejuvenation Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Extract, Chemicals, and Solvents
2.2. Extract Fractionation by Centrifugal Partition Chromatography
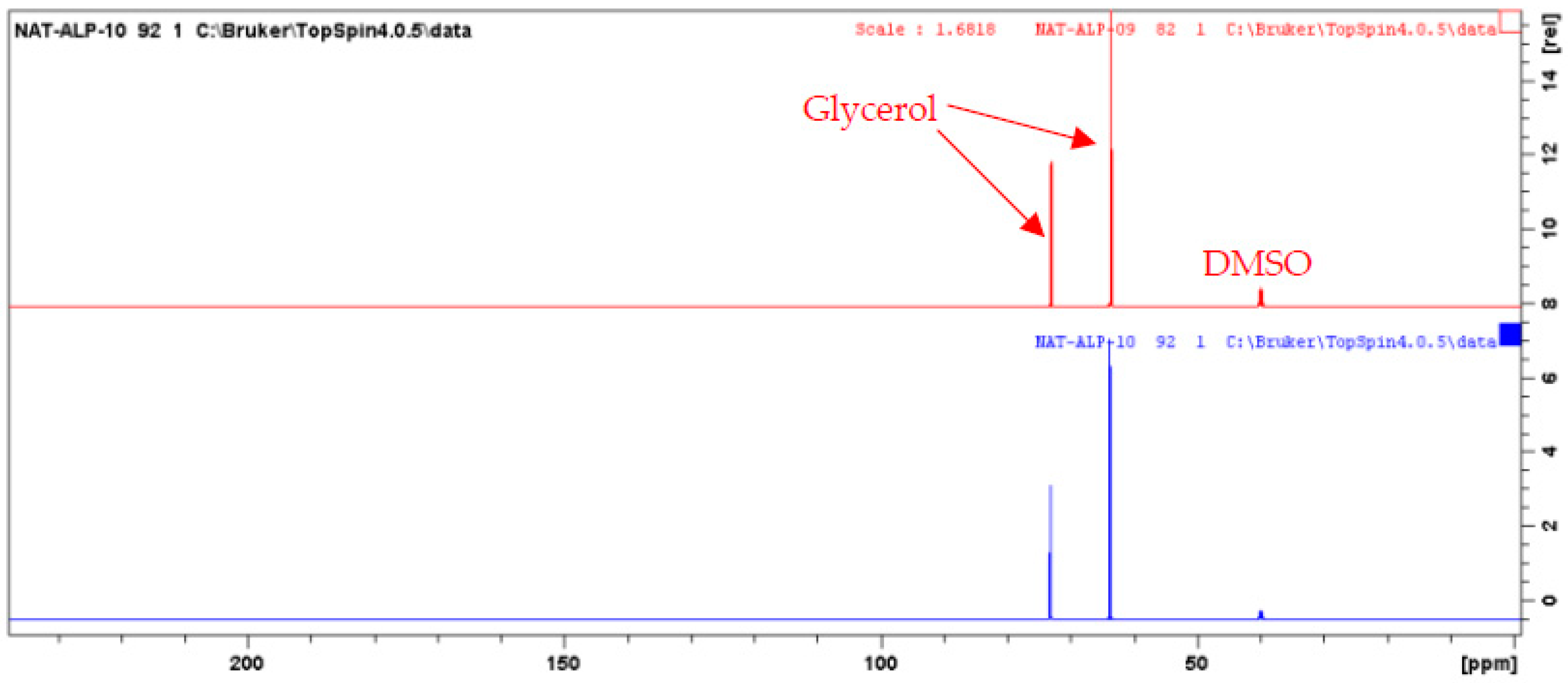
2.3. NMR Analyses and Identification of the Alpine Rose Extract Constituents by CARAMEL
2.4. LC/MS Analyses
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Francon, L.; Corona, C.; Till-Bottraud, I.; Choler, P.; Carlson, B.Z.; Charrier, G.; Améglio, T.; Morin, S.; Eckert, N.; Roussel, E.; et al. Assessing the effects of earlier snow melt-out on alpine shrub growth: The sooner the better? Ecol. Indic. 2020, 115, 106455. [Google Scholar]
- Francon, L.; Corona, C.; Roussel, E.; Lopez Saez, J.; Stoffel, M. Warm summers, and moderate winter precipitation boost Rhododendron ferrugineum L. growth in the Taillefer massif (French Alps). Sci. Total Environ. 2017, 586, 1020–1031. [Google Scholar] [PubMed]
- Wei, H.; Yang, Y.; Himmel, M.; Tucker, M.P.; Ding, S.Y.; Yang, S.; Arora, R. Identification and characterization of five cold stress-related rhododendron dehydrin genes: Spotlight on a FSK-Type dehydrin with multiple F-Segments. Front. Bioeng. Biotechnol. 2019, 7, 30. [Google Scholar] [PubMed] [Green Version]
- Gescher, K.; Kühn, J.; Hafezi, W.; Louis, A.; Derksen, A.; Deters, A.; Lorentzen, E.; Hensel, A. Inhibition of viral adsorption and penetration by an aqueous extract from Rhododendron ferrugineum L. as antiviral principle against herpes simplex virus type-1. Fitoterapia 2011, 82, 408–413. [Google Scholar] [PubMed]
- Löhr, G.; Beikler, T.; Hensel, A. Inhibition of in vitro adhesion and virulence of Porphyromonas gingivalis by aqueous extract and polysaccharides from Rhododendron ferrugineum L. A new way for prophylaxis of periodontitis? Fitoterapia 2015, 107, 105–113. [Google Scholar]
- Cosmetics Design Europe. Available online: https://www.cosmeticsdesign-europe.com/Product-innovations/Ecocert-Certified-Alpine-Rose-Active-Combats-Aging-by-Protecting-Skin-Proteins (accessed on 5 February 2022).
- Wandrey, F.; Schmid, D.; Zülli, F. Senolytics: Eliminating «zombie cells» in the skin—A novel anti-aging mechanism to combat senescent cells. Ski. Care HPC Today 2020, 15, 18–20. [Google Scholar]
- Chosson, E.; Chaboud, A.; Chulia, A.J.; Raynaud, J. Dihydroflavonol glycosides from Rhododendron ferrugineum. Phytochemistry 1998, 49, 1431–1433. [Google Scholar]
- Chosson, E.; Chaboud, A.; Chulia, A.J.; Raynaud, J. A phloracetophenone glucoside from Rhododendron ferrugineum. Phytochemistry 1998, 47, 87–88. [Google Scholar]
- Popescu, R.; Kopp, B. The genus Rhododendron: An ethnopharmacological and toxicological review. J. Ethnopharmacol. 2013, 147, 42–62. [Google Scholar]
- Seephonkai, P.; Popescu, R.; Zehl, M.; Krupitza, G.; Urban, E.; Kopp, B. Ferruginenes A–C from Rhododendron ferrugineum and their cytotoxic evaluation. J. Nat. Prod. 2011, 74, 712–717. [Google Scholar] [PubMed]
- Hubert, J.; Nuzillard, J.M.; Purson, S.; Hamzaoui, M.; Borie, N.; Reynaud, R.; Renault, J.H. Identification of natural metabolites in mixture: A pattern recognition strategy based on (13)C NMR. Anal. Chem. 2014, 86, 2955–2962. [Google Scholar] [PubMed]
- Abedini, A.; Colin, M.; Hubert, J.; Charpentier, E.; Angelis, A.; Bounasri, H.; Bertaux, B.; Kotland, A.; Reffuveille, F.; Nuzillard, J.M.; et al. Abundant Extractable Metabolites from Temperate Tree Barks: The Specific Antimicrobial Activity of Prunus Avium Extracts. Antibiotics 2020, 9, 111. [Google Scholar]
- Schmitt, M.; Alabdul Magid, A.; Hubert, J.; Etique, N.; Duca, L.; Voutquenne-Nazabadioko, L. Bio-guided isolation of new phenolic compounds from Hippocrepis emerus flowers and investigation of their antioxidant, tyrosinase and elastase inhibitory activities. Phytochem. Lett. 2020, 35, 28–36. [Google Scholar]
- Hammoud Mahdi, D.; Hubert, J.; Renault, J.H.; Martinez, A.; Schubert, A.; Engel, K.M.; Koudogbo, B.; Vissiennon, Z.; Ahyi, V.; Nieber, K.; et al. Chemical profile and antimicrobial activity of the fungus-growing termite strain Macrotermes bellicosus used in traditional medicine in the Republic of Benin. Molecules 2020, 25, 5015. [Google Scholar]
- Angelis, A.; Hubert, J.; Aligiannis, N.; Michalea, R.; Abedini, A.; Nuzillard, J.M.; Gangloff, S.C.; Skaltsounis, A.L.; Renault, J.H. Bio-guided isolation of methanol-soluble metabolites of common spruce (Picea abies) bark by-products and investigation of their dermo-cosmetic properties. Molecules 2016, 21, 1586. [Google Scholar]
- Hubert, J.; Chollet, S.; Purson, S.; Reynaud, R.; Harakat, D.; Martinez, A.; Nuzillard, J.M.; Renault, J.H. Exploiting the Complementarity between Dereplication and Computer-Assisted Structure Elucidation for the Chemical Profiling of Natural Cosmetic Ingredients: Tephrosia purpurea as a Case Study. J. Nat. Prod. 2015, 78, 1609–1617. [Google Scholar] [PubMed]
- Gu, Y.; Jérôme, F. Glycerol as a sustainable solvent for green chemistry. Green Chem. 2010, 12, 1127–1138. [Google Scholar]
- Canton, M.; Hubert, J.; Poigny, S.; Roe, R.; Brunel, Y.; Nuzillard, J.M.; Renault, J.H. Dereplication of natural extracts diluted in glycerin: Physical suppression of glycerin by Centrifugal Partition Chromatography combined with presaturation of solvent signals in 13C-Nuclear Magnetic Resonance spectroscopy. Molecules 2020, 25, 5061. [Google Scholar]
- Louis, A.; Petereit, F.; Lechtenberg, M.; Deters, A.; Hensel, A. Phytochemical characterization of Rhododendron ferrugineum and in vitro assessment of an aqueous extract on cell toxicity. Planta Med. 2010, 76, 1550–1557. [Google Scholar] [PubMed] [Green Version]
- Borghesan, M.; Hoogaars, W.M.H.; Varela-Eirin, M.; Talma, N.; Demaria, M. A Senescence-Centric View of Aging: Implications for Longevity and Disease. Trends Cell Biol. 2020, 30, 777–791. [Google Scholar]
- Ryu, S.J.; Oh, Y.S.; Park, S.C. Failure of stress-induced downregulation of Bcl-2 contributes to apoptosis resistance in senescent human diploid fibroblasts. Cell Death Differ. 2007, 14, 1020–1028. [Google Scholar]
- Qiu, J.; Zhang, T.; Zhu, X.; Yang, C.; Wang, Y.; Zhou, N.; Ju, B.; Zhou, T.; Deng, G.; Qiu, C. Hyperoside Induces Breast Cancer Cells Apoptosis via ROS-Mediated NF-κB Signaling Pathway. Int. J. Mol. Sci. 2019, 21, 131. [Google Scholar]
- Pirvu, L.; Stefaniu, A.; Neagu, G.; Albu, B.; Pintilie, L. In Vitro Cytotoxic and antiproliferative activity of Cydonia oblonga flower petals, leaf and fruit pellet ethanolic extracts. Docking simulation of the active flavonoids on anti-apoptotic protein Bcl-2. Open Chem. 2018, 16, 591–604. [Google Scholar]
- Rakhshan, R.; Atashi, H.A.; Hoseinian, M.; Jafari, A.; Haghighi, A.; Ziyadloo, F.; Razizadeh, N.; Ghasemian, H.; Nia, M.M.K.; Sefidi, A.B.; et al. The synergistic cytotoxic and apoptotic effect of resveratrol and naringenin on Y79 retinoblastoma cell line. Anti-Cancer Agents Med. Chem. 2021, 21, 2243–2249. [Google Scholar]
- Liu, E.; Liang, T.; Wang, X.; Ban, S.; Han, L.; Li, Q. Apoptosis induced by farrerol in human gastric cancer SGC-7901 cells through the mitochondrial-mediated pathway. Eur. J. Cancer Prev. 2015, 24, 365–372. [Google Scholar]
- Razak, S.; Afsar, T.; Ullah, A.; Almajwal, A.; Alkholief, M.; Alshamsan, A.; Jahan, S. Taxifolin, a natural flavonoid interacts with cell cycle regulators causes cell cycle arrest and causes tumor regression by activating Wnt/β-catenin signaling pathway. BMC Cancer 2018, 18, 1043. [Google Scholar]
- Franco, A.C.; Aveleira, C.; Cavadas, C. Skin senescence: Mechanisms and impact on whole-body aging. Trends Mol. Med. 2022, 28, 97–109. [Google Scholar]



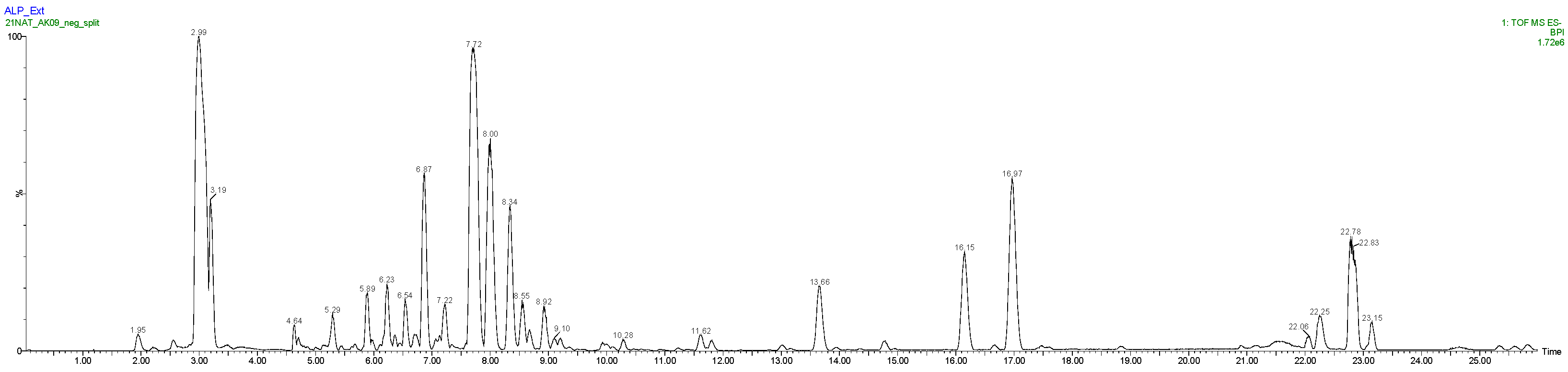
| LC Retention Time (min) | Observed m/z | Elemental Composition | Δppm | Tentative Identification |
|---|---|---|---|---|
| 2.92 | 191.0555 [M-H]− | C7H11O6 | 0.0 | Quinic acid |
| 3.21 | 341.1086 [M-H]− | C12H21O11 | 0.6 | Saccharose |
| 4.63 | 337.0769 407.1183 | C12H17O11 C16H23O12 | −0.6 −1.7 | Not assigned |
| 4.69 | 191.0191 [M-H]− 111.0082 | C6H7O7 C5H3O3 | −0.5 0.0 | Citric acid |
| 5.29 | 435.1143 | C17H23O13 | 0.9 | Not assigned |
| 5.44 | 329.0873 301.0559 | C14H17O9 C12H13O9 | 0.0 −0.3 | Not assigned |
| 5.88 | 417.1030 | C17H21O12 | −0.7 | Kaempferol pentoside |
| 6.22 | 329.0872 | C14H17O9 | −0.3 | Vanillic hexoside |
| 6.36 | 227.0553 | C10H11O6 | −1.3 | Not assigned |
| 6.55 | 417.1033 | C17H21O12 | 0.0 | Kaempferol pentoside isomer |
| 6.72 | 353.0876 [M-H]− 191.0557 quinic acid | C16H17O9 C7H11O6 | 0.8 0.5 | Mono-caffeoylquinic acid |
| 6.86 | 289.0717 [M-H]− | C15H13O6 | 1.7 | (+)-catechin * |
| 7.22 | 289.0714 [M-H]− 327.1079 | C15H13O6 C15H19O8 | 0.7 −0.3 | (−)-Epicatechin Coumaric acid hexoside |
| 7.64 | 435.0934 [M-H]− 285.0401 | C20H19O11 C15H9O6 | 1.6 0.7 | Taxifolin 3-O-arabinoside isomer 1 * |
| 7.98 | 463.0882 [M-H]− | C21H19O12 | 1.1 | Hyperoside * |
| 8.39 | 435.0928 [M-H]− 285.0399 | C20H19O11 C15H9O6 | 0.2 0.0 | Taxifolin 3-O-arabinoside isomer 2 * |
| 8.53 | 433.0767 [M-H]− | C20H17O11 | −0.9 | Quercetin xyloside or arabinoside |
| 8.67 | 447.0924 [M-H]− | C21H19O11 | −0.7 | Quercetin rhamnoside |
| 8.92 | 505.0986 [M-H]− 301.0341 Quercetin | C23H21O13 | 0.8 | Quercetin acetyl-hexoside |
| 9.10 | 303.0501 [M-H]− 285.0397 [M-H-H2O]- | C15H11O7 C15H9O6 | −1.3 −0.7 | Taxifolin |
| 9.20 | 149.0601 311.1129 [M-H]− | C9H9O2 C15H19O7 | −1.3 −0.6 | Cinnamic acid hexoside |
| 9.92 | 167.0345 [M-H]− | C8H7O4 | 0.6 | Isovanillic acid * |
| 10.27 | 455.1551 | C21H27O11 | −0.4 | Not assigned |
| 11.61 | 567.1141 | C21H27O18 | −9.9 | Not assigned |
| 13.65 | 271.0606 [M-H]− | C15H11O5 | 0.0 | Naringenin * |
| 14.76 | 285.0760 [M-H]− | C16H13O5 | −1.1 | Poriol * or isomer |
| 16.13 | 285.0763 [M-H]− | C16H13O5 | 0.0 | Poriol * or isomer |
| 16.96 | 299.0921 [M-H]− | C17H15O5 | 0.7 | Farrerol * |
| 16.63 | 297.0762 [M-H]− | C17H13O5 | −0.3 | Dihydrofarrerol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hubert, J.; Kotland, A.; Henes, B.; Poigny, S.; Wandrey, F. Deciphering the Phytochemical Profile of an Alpine Rose (Rhododendron ferrugineum L.) Leaf Extract for a Better Understanding of Its Senolytic and Skin-Rejuvenation Effects. Cosmetics 2022, 9, 37. https://doi.org/10.3390/cosmetics9020037
Hubert J, Kotland A, Henes B, Poigny S, Wandrey F. Deciphering the Phytochemical Profile of an Alpine Rose (Rhododendron ferrugineum L.) Leaf Extract for a Better Understanding of Its Senolytic and Skin-Rejuvenation Effects. Cosmetics. 2022; 9(2):37. https://doi.org/10.3390/cosmetics9020037
Chicago/Turabian StyleHubert, Jane, Alexis Kotland, Bernhard Henes, Stéphane Poigny, and Franziska Wandrey. 2022. "Deciphering the Phytochemical Profile of an Alpine Rose (Rhododendron ferrugineum L.) Leaf Extract for a Better Understanding of Its Senolytic and Skin-Rejuvenation Effects" Cosmetics 9, no. 2: 37. https://doi.org/10.3390/cosmetics9020037
APA StyleHubert, J., Kotland, A., Henes, B., Poigny, S., & Wandrey, F. (2022). Deciphering the Phytochemical Profile of an Alpine Rose (Rhododendron ferrugineum L.) Leaf Extract for a Better Understanding of Its Senolytic and Skin-Rejuvenation Effects. Cosmetics, 9(2), 37. https://doi.org/10.3390/cosmetics9020037







