Molecular Docking, Tyrosinase, Collagenase, and Elastase Inhibition Activities of Argan By-Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Extraction Methods
2.3.1. Supercritical Fluid Extraction
2.3.2. Ultrasound-Assisted Extraction
2.3.3. Determination of the Extraction Yields
2.4. Dermocosmetic Activities
2.4.1. Tyrosinase Inhibition Activity
2.4.2. Collagenase Inhibition Activity
2.4.3. Elastase Inhibition Activity
2.5. Determination of Total Phenolic Content
2.6. Molecular Docking
2.7. Statistical Analysis
3. Results and Discussion
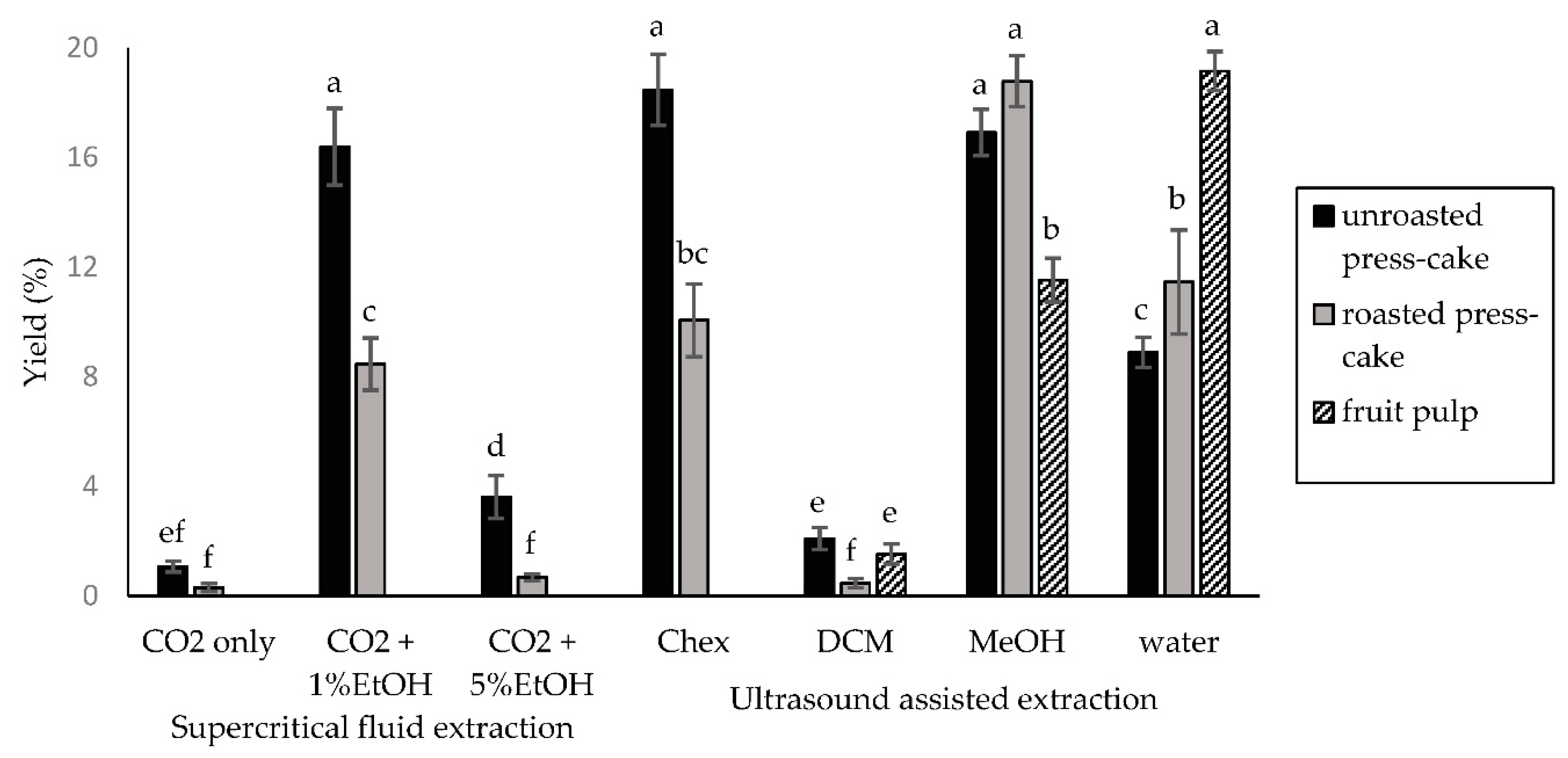
3.1. Extraction Yields
3.2. Enzyme Inhibition Assays
3.3. Determination of the Total Phenolic Content
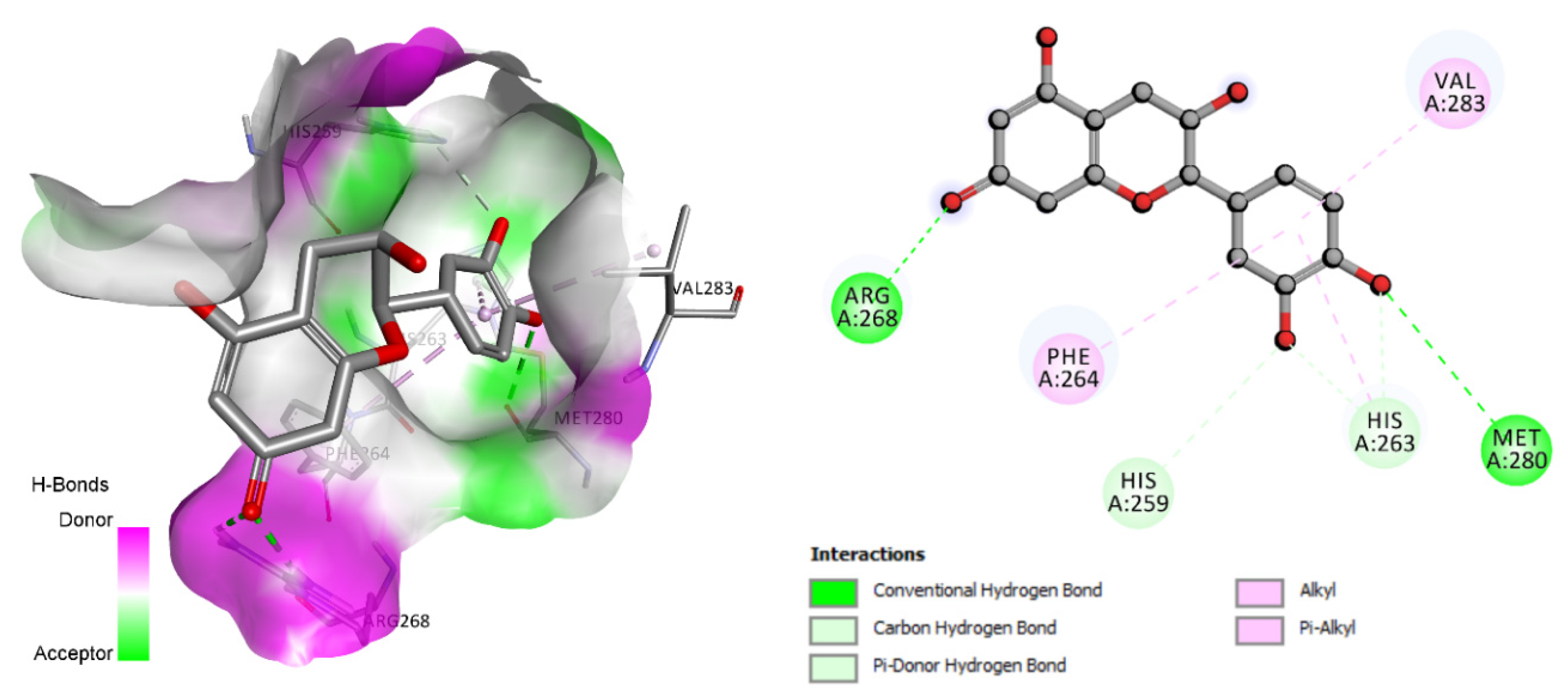
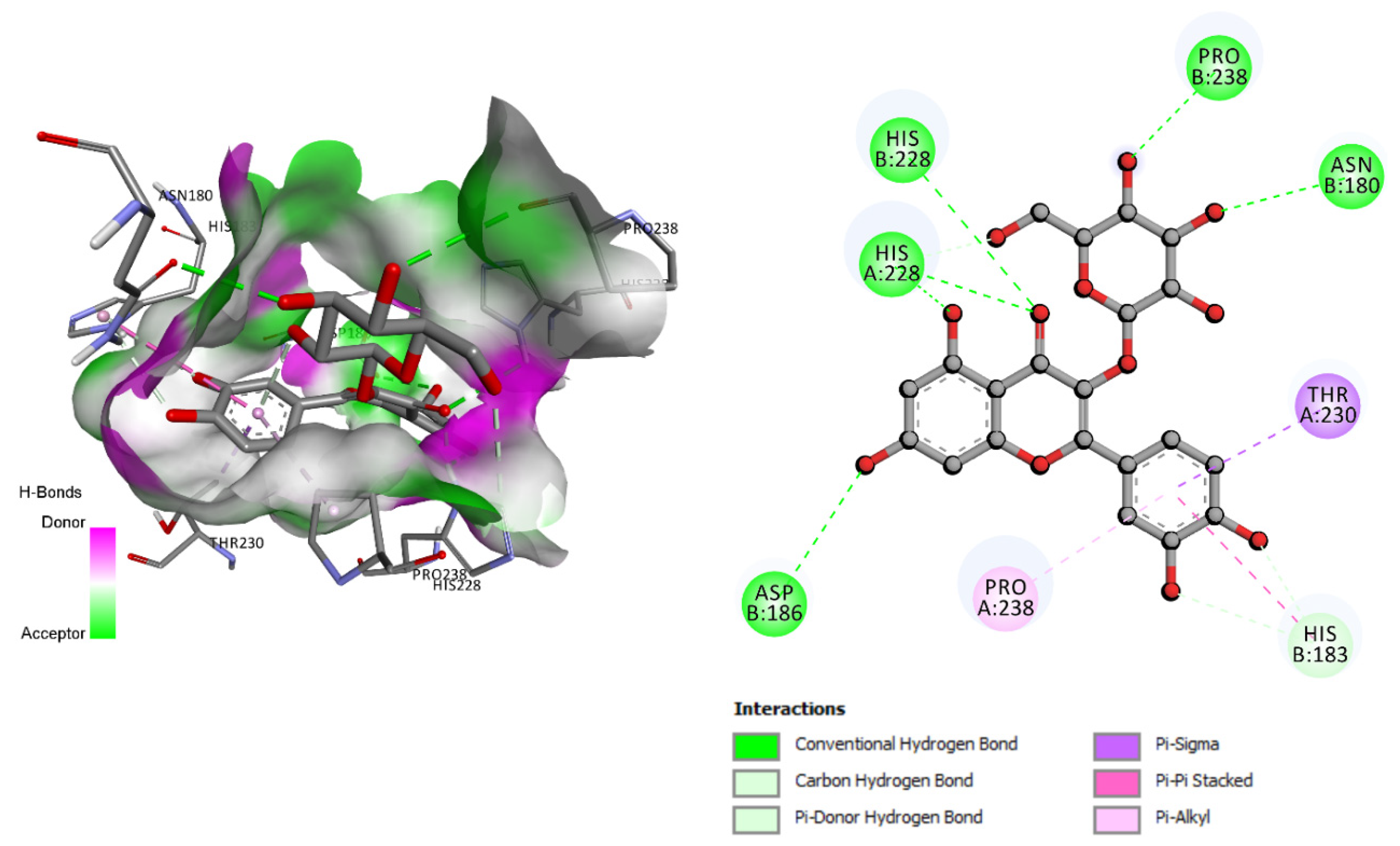
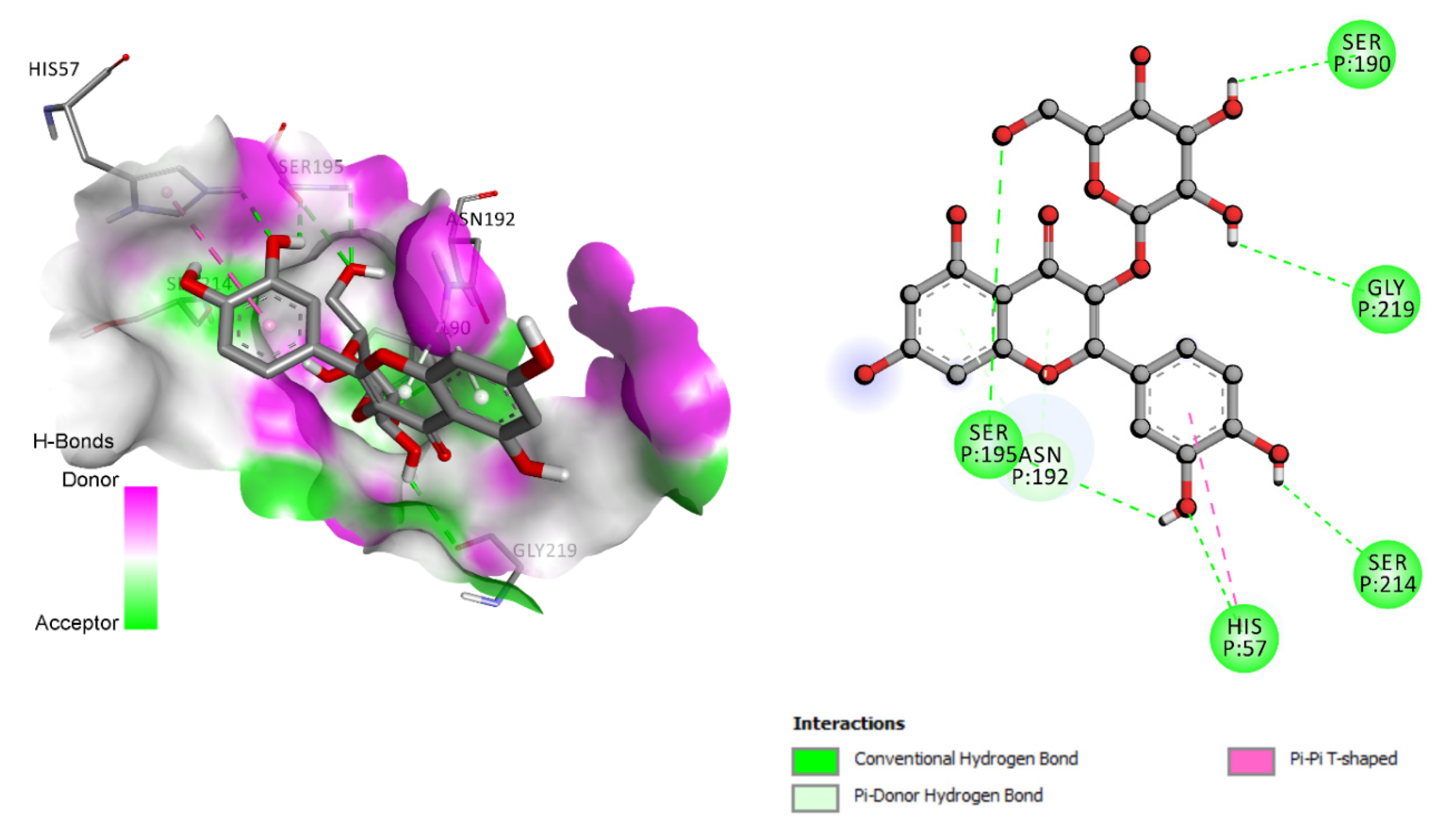
3.4. Molecular Docking Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Venus, M.; Waterman, J.; McNab, I. Basic physiology of the skin. Surgery (Oxford) 2010, 28, 469–472. [Google Scholar] [CrossRef]
- Escoffier, C.; de Rigal, J.; Rochefort, A.; Vasselet, R.; Lévêque, J.-L.; Agache, P.G. Age-related mechanical properties of human skin: An in vivo study. J. Investig. Dermatol. 1989, 93, 353–357. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Structural Characteristics of the Aging Skin: A Review. Cutan. Ocul. Toxicol. 2007, 26, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Buffoli, B.; Rinaldi, F.; Labanca, M.; Sorbellini, E.; Trink, A.; Guanziroli, E.; Rezzani, R.; Rodella, L.F. The human hair: From anatomy to physiology. Int. J. Dermatol. 2013, 53, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Ebling, F.J.G. The Biology of Hair. Dermatol. Clin. 1987, 5, 467–481. [Google Scholar] [CrossRef]
- Sjerobabski-Masnec, I.; Šitum, M. Skin aging. Acta Clin. Croat. 2010, 49, 515–518. [Google Scholar] [PubMed]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Tobin, D.J. Biochemistry of human skin—Our brain on the outside. Chem. Soc. Rev. 2006, 35, 52–67. [Google Scholar] [CrossRef]
- Boucetta, K.Q.; Charrouf, Z.; Derouiche, A.; Rahali, Y.; Bensouda, Y. Skin hydration in postmenopausal women: Argan oil benefit with oral and/or topical use. Menopausal Rev. 2014, 5, 280–288. [Google Scholar] [CrossRef] [Green Version]
- El Hafian, M.; Benlandini, N.; Elyacoubi, H.; Zidane, L.; Rochdi, A. Étude floristique et ethnobotanique des plantes médicinales utilisées au niveau de la préfecture d’Agadir-Ida-Outanane (Maroc). J. Appl. Biosci. 2014, 81, 7198. [Google Scholar] [CrossRef] [Green Version]
- Moukal, A. L’arganier, Argania spinosa L. (skeels), usage thérapeutique, cosmétique et alimentaire. Phytothérapie 2004, 2, 135–141. [Google Scholar] [CrossRef]
- Ouhaddou, H.; Boubaker, H.; Msanda, F.; El Mousadik, A. An ethnobotanical study of medicinal plants of the Agadir Ida Ou Tanane province (southwest Morocco). J. Appl. Biosci. 2015, 84, 7707. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.R.; Grosso, C.; Delerue-Matos, C.; Rocha, J.M. Comprehensive review on the interaction between natural compounds and brain receptors: Benefits and toxicity. Eur. J. Med. Chem. 2019, 174, 87–115. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Odaka, Y.; Ogawa, N.; Nakamoto, K.; Kuninaga, H. Identification of Geranic Acid, a Tyrosinase Inhibitor in Lemongrass (Cymbopogon citratus). J. Agric. Food Chem. 2007, 56, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, D.; Angelis, A.; Aligiannis, N.; Mitakou, S.; Skaltsounis, L. Recovery of Sesamin, Sesamolin, and Minor Lignans From Sesame Oil Using Solid Support-Free Liquid–Liquid Extraction and Chromatography Techniques and Evaluation of Their Enzymatic Inhibition Properties. Front. Pharmacol. 2019, 10, 723. [Google Scholar] [CrossRef] [PubMed]
- Gendron, R.; Grenier, D.; Sorsa, T.; Mayrand, D. Inhibition of the Activities of Matrix Metalloproteinases 2, 8, and 9 by Chlorhexidine. Clin. Diagn. Lab. Immunol. 1999, 6, 437–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelis, A.; Hubert, J.; Aligiannis, N.; Michalea, R.; Abedini, A.; Nuzillard, J.-M.; Gangloff, S.C.; Skaltsounis, A.-L.; Renault, J.-H. Bio-Guided Isolation of Methanol-Soluble Metabolites of Common Spruce (Picea abies) Bark by-Products and Investigation of Their Dermo-Cosmetic Properties. Molecules 2016, 21, 1586. [Google Scholar] [CrossRef] [Green Version]
- Vermerris, W.; Nicholson, R. Biosynthesis of Phenolic Compounds; Springer Science and Business Media LLC: Dordrecht, The Netherlands, 2008; pp. 63–149. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Fan, L.; Duan, Z. Five individual polyphenols as tyrosinase inhibitors: Inhibitory activity, synergistic effect, action mechanism, and molecular docking. Food Chem. 2019, 297, 124910. [Google Scholar] [CrossRef]
- Osorio, E.; Bravo, K.; Cardona, W.; Yepes, A.; Coa, J.C. Antiaging activity, molecular docking, and prediction of percutaneous absorption parameters of quinoline–hydrazone hybrids. Med. Chem. Res. 2019, 28, 1959–1973. [Google Scholar] [CrossRef]
- Karatoprak, G.Ş.; Göger, F.; Çelik, I.; Budak, Ü.; Akkol, E.K.; Aschner, M. Phytochemical profile, antioxidant, antiproliferative, and enzyme inhibition-docking analyses of Salvia ekimiana Celep & Doğan. S. Afr. J. Bot. 2021, 146, 36–47. [Google Scholar] [CrossRef]
- Gharby, S.; Harhar, H.; Guillaume, D.; Haddad, A.; Charrouf, Z. The Origin of Virgin Argan Oil’s High Oxidative Stability Unraveled. Nat. Prod. Commun. 2012, 7, 621–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taribak, C.; Casas, L.; Mantell, C.; Elfadli, Z.; Metni, R.E.; de la Ossa, E.M. Quality of Cosmetic Argan Oil Extracted by Supercritical Fluid Extraction from Argania spinosa L. J. Chem. 2013, 2013, 408194. [Google Scholar] [CrossRef] [Green Version]
- Harhar, H.; Gharby, S.; Ghanmi, M.; EL Monfalouti, H.; Guillaume, D.; Charrouf, Z. Composition of the Essential Oil of Argania spinosa (Sapotaceae) Fruit Pulp. Nat. Prod. Commun. 2010, 5, 935–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harhar, H.; Gharby, S.; Kartah, B.; El Monfalouti, H.; Guillaume, D.; Charrouf, Z. Influence of Argan Kernel Roasting-time on Virgin Argan Oil Composition and Oxidative Stability. Mater. Veg. 2011, 66, 163–168. [Google Scholar] [CrossRef]
- El Monfalouti, H.; Charrouf, Z.; Belviso, S.; Ghirardello, D.; Scursatone, B.; Guillaume, D.; Denhez, C.; Zeppa, G. Analysis and antioxidant capacity of the phenolic compounds from argan fruit (Argania spinosa (L.) Skeels). Eur. J. Lipid Sci. Technol. 2012, 114, 446–452. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Quantitative Analysis of Eumelanin and Pheomelanin in Humans, Mice, and Other Animals: A Comparative Review. Pigment Cell Res. 2003, 16, 523–531. [Google Scholar] [CrossRef]
- Gillbro, J.M.; Olsson, M.J. The melanogenesis and mechanisms of skin-lightening agents—Existing and new approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef]
- Hearing, V.J.; Jiménez, M. Mammalian tyrosinase—The critical regulatory control point in melanocyte pigmentation. Int. J. Biochem. 1987, 19, 1141–1147. [Google Scholar] [CrossRef]
- Bourhim, T.; Villareal, M.O.; Gadhi, C.; Hafidi, A.; Isoda, H. Depigmenting effect of argan press-cake extract through the down-regulation of Mitf and melanogenic enzymes expression in B16 murine melanoma cells. Cytotechnology 2018, 70, 1389–1397. [Google Scholar] [CrossRef]
- Di Petrillo, A.; González-Paramás, A.M.; Era, B.; Medda, R.; Pintus, F.; Santos-Buelga, C.; Fais, A. Tyrosinase inhibition and antioxidant properties of Asphodelus microcarpus extracts. BMC Complement. Altern. Med. 2016, 16, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Curtis-Long, M.J.; Lee, B.W.; Yuk, H.J.; Kim, D.W.; Tan, X.F.; Park, K.H. Inhibition of tyrosinase activity by polyphenol compounds from Flemingia philippinensis roots. Bioorg. Med. Chem. 2014, 22, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Cui, F.; Li, H.; Huang, Q.; Li, Y. Understanding the inhibitory mechanism of tea polyphenols against tyrosinase using fluorescence spectroscopy, cyclic voltammetry, oximetry, and molecular simulations. RSC Adv. 2018, 8, 8310–8318. [Google Scholar] [CrossRef] [Green Version]
- Madani, W.; Kermasha, S.; Bisakowski, B. Inhibition of tyrosinase activity by a polyphenol esterase using selected phenolic substrates. Phytochemistry 1999, 52, 1001–1008. [Google Scholar] [CrossRef]
- Jeong, C.H.; Shim, K.H. Tyrosinase Inhibitor Isolated from the Leaves of Zanthoxylum piperitum. Biosci. Biotechnol. Biochem. 2004, 68, 1984–1987. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.-S. An Updated Review of Tyrosinase Inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [Green Version]
- Seo, S.-Y.; Sharma, V.K.; Sharma, N. Mushroom Tyrosinase: Recent Prospects. J. Agric. Food Chem. 2003, 51, 2837–2853. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Sari, S.; Barut, B.; Özel, A. Tyrosinase inhibition by some flavonoids: Inhibitory activity, mechanism by in vitro and in silico studies. Bioorg. Chem. 2018, 81, 168–174. [Google Scholar] [CrossRef]
- Chang, T.-S.; Ding, H.-Y.; Tai, S.S.-K.; Wu, C.-Y. Mushroom tyrosinase inhibitory effects of isoflavones isolated from soygerm koji fermented with Aspergillus oryzae. BCRC Food Chem. 2007, 105, 1430–1438. [Google Scholar] [CrossRef]
- Tortora, G.J.; Funke, B.R.; Case, C.L. Microbiology: An Introduction; Pearson Benjamin Cummings: San Francisco, CA, USA, 2007. [Google Scholar]
- Imokawa, G.; Ishida, K. Biological Mechanisms Underlying the Ultraviolet Radiation-Induced Formation of Skin Wrinkling and Sagging I: Reduced Skin Elasticity, Highly Associated with Enhanced Dermal Elastase Activity, Triggers Wrinkling and Sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.-H.; Kim, Y.-H.; Choo, S.-J.; Ryoo, I.-J.; Yoo, J.-K.; Ahn, J.-S.; Yoo, I.-D. Chemical constituents from the leaves of Ilex paraguariensis inhibit human neutrophil elastase. Arch. Pharmacal Res. 2009, 32, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Ghimeray, A.K.; Jung, U.S.; Lee, H.Y.; Kim, Y.H.; Ryu, E.K.; Chang, M.S. In vitro antioxidant, collagenase inhibition, and in vivo anti-wrinkle effects of combined formulation containing Punica granatum, Ginkgo biloba, Ficus carica, and Morus alba fruits extract. Clin. Cosmet. Investig. Dermatol. 2015, 8, 389–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhan, B.; Krishnamoorthy, G.; Rao, J.R.; Nair, B.U. Role of green tea polyphenols in the inhibition of collagenolytic activity by collagenase. Int. J. Biol. Macromol. 2007, 41, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef]
- Charrouf, Z.; Hilali, M.; Jauregui, O.; Soufiaoui, M.; Guillaume, D. Separation and characterization of phenolic compounds in argan fruit pulp using liquid chromatography–negative electrospray ionization tandem mass spectroscopy. Food Chem. 2007, 100, 1398–1401. [Google Scholar] [CrossRef]
- Rojas, L.B.; Quideau, S.; Pardon, A.P.; Charrouf, Z. Colorimetric Evaluation of Phenolic Content and GC-MS Characterization of Phenolic Composition of Alimentary and Cosmetic Argan Oil and Press Cake. J. Agric. Food Chem. 2005, 53, 9122–9127. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular Docking and Structure-Based Drug Design Strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef]
- Yuriev, E.; Holien, J.; Ramsland, P.A. Improvements, trends, and new ideas in molecular docking: 2012–2013 in review. J. Mol. Recognit. 2015, 28, 581–604. [Google Scholar] [CrossRef]
- Ashooriha, M.; Khoshneviszadeh, M.; Khoshneviszadeh, M.; Rafiei, A.; Kardan, M.; Yazdian-Robati, R.; Emami, S. Kojic acid–natural product conjugates as mushroom tyrosinase inhibitors. Eur. J. Med. Chem. 2020, 201, 112480. [Google Scholar] [CrossRef]
- Sin, B.Y.; Kim, H.P. Inhibition of collagenase by naturally-occurring flavonoids. Arch. Pharmacal Res. 2005, 28, 1152–1155. [Google Scholar] [CrossRef]
- Lim, H.; Kim, H.P. Inhibition of Mammalian Collagenase, Matrix Metalloproteinase-1, by Naturally-Occurring Flavonoids. Planta Med. 2007, 73, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.H.; Moon, Y.-H.; Ryu, Y.-B.; Kim, Y.-M.; Nam, S.-H.; Kim, M.-S.; Kimura, A.; Kim, D. The influence of flavonoid compounds on the in vitro inhibition study of a human fibroblast collagenase catalytic domain expressed in E. coli. Enzym. Microb. Technol. 2013, 52, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Priani, S.E.; Fakih, T.M. Insights into Molecular Interaction of Flavonoid Compounds in Citrus Peel Bound to Collagenase and Elastase Enzymes: A Computational Study. Pharm. Sci. Res. 2021, 8, 5. [Google Scholar]
- Taherkhani, A.; Moradkhani, S.; Orangi, A.; Jalalvand, A.; Khamverdi, Z. Molecular docking study of flavonoid compounds for possible matrix metalloproteinase-13 inhibition. J. Basic Clin. Physiol. Pharmacol. 2020, 32, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F. Potential Role of Natural Compounds Against Skin Aging. Curr. Med. Chem. 2015, 22, 1515–1538. [Google Scholar] [CrossRef] [PubMed]
- Bras, N.F.; Goncalves, R.; Mateus, N.; Fernandes, P.A.; Ramos, M.J.; de Freitas, V. Inhibition of pancreatic elastase by polyphenolic compounds. J. Agric. Food Chem. 2010, 58, 10668–10676. [Google Scholar] [CrossRef]


| Extraction Method | Extraction Solvent | Plant Material | Tyrosinase Inhibition (%) | Collagenase Inhibition (%) | Elastase Inhibition (%) |
|---|---|---|---|---|---|
| Supercritical fluid extraction(SFE) | CO2 only | Unroasted press-cake | 2.05 ± 1.21 | 1.85 ± 0.56 | 0 ± 0 |
| Roasted press-cake | 0.15 ± 0.21 | 2.18 ± 0.75 | 0 ± 0 | ||
| CO2 + 1% EtOH | Unroasted press-cake | 1.03 ± 0.27 | 2.7 ± 0.31 | 1.24 ± 0.2 | |
| Roasted press-cake | 1.23 ± 0.79 | 2.52 ± 0.94 | 1.38 ± 0.14 | ||
| CO2 + 5% EtOH | Unroasted press-cake | 1.94 ± 1.01 | 1.32 ± 0.39 | 0.1 ± 0.06 | |
| Roasted press-cake | 0.73 ± 0.19 | 2.4 ± 0.72 | 0.15 ± 0.1 | ||
| Ultrasound-assisted extraction (UAE) | Cyclohexane | Unroasted press-cake | 0.81 ± 0.21 | 0.98 ± 0.13 | 0 ± 0 |
| Roasted press-cake | 1.54 ± 0.36 | 1.63 ± 0.51 | 0 ± 0 | ||
| Dichloromethan | Unroasted press-cake | 1.52 ± 0.38 | 1.54 ± 0.95 | 3.77 ± 1.01 | |
| Roasted press cake | 1.11 ± 0.59 | 0.87 ± 0.68 | 3.28 ± 1.0 | ||
| Pulp | 11.39 ± 1.23 | 23.6 ± 2.5 | 4.39 ± 2.08 | ||
| Methanol | Unroasted press cake | 7.3 ± 1.63 | 15.09 ± 2.7 | 12.13 ± 1.95 | |
| Roasted press cake | 6.78 ± 1.47 | 19.22 ± 1.96 | 13.41 ± 2.1 | ||
| Pulp | 54.74 ± 3.7 | 52.29 ± 1.71 | 25.02 ± 1.69 | ||
| Water | Unroasted press cake | 5.75 ± 2.47 | 28.34 ± 2.62 | 19.63 ± 2.14 | |
| Roasted press cake | 3.49 ± 1.04 | 31.43 ± 3.06 | 19.98 ± 1.28 | ||
| Pulp | 23.12 ± 2.61 | 60.97 ± 2.36 | 38.41 ± 2.18 | ||
| Positive control | Kojic acid 95.18 ± 2.36 | chlorhexidine 98.24 ± 1.97 | Elastrinal 99.96 ± 0.02 | ||
| Sample | TPC (mgeqAG/gDM) |
|---|---|
| UAE Methanol—Unroasted press cake | 7.9 ± 0.7 |
| UAE Methanol—Roasted press cake | 3.1 ± 0.3 |
| UAE Methanol—Fruit pulp | 68.2 ± 1.5 |
| UAE water—Unroasted press cake | 6.5 ± 0.9 |
| UAE water—Roasted press cake | 10.3 ± 1.2 |
| UAE water—Fruit pulp | 56.8 ± 3.7 |
| Ligand | Score (Kcal/mol) | Active Site Residues Involved in H-Bond |
|---|---|---|
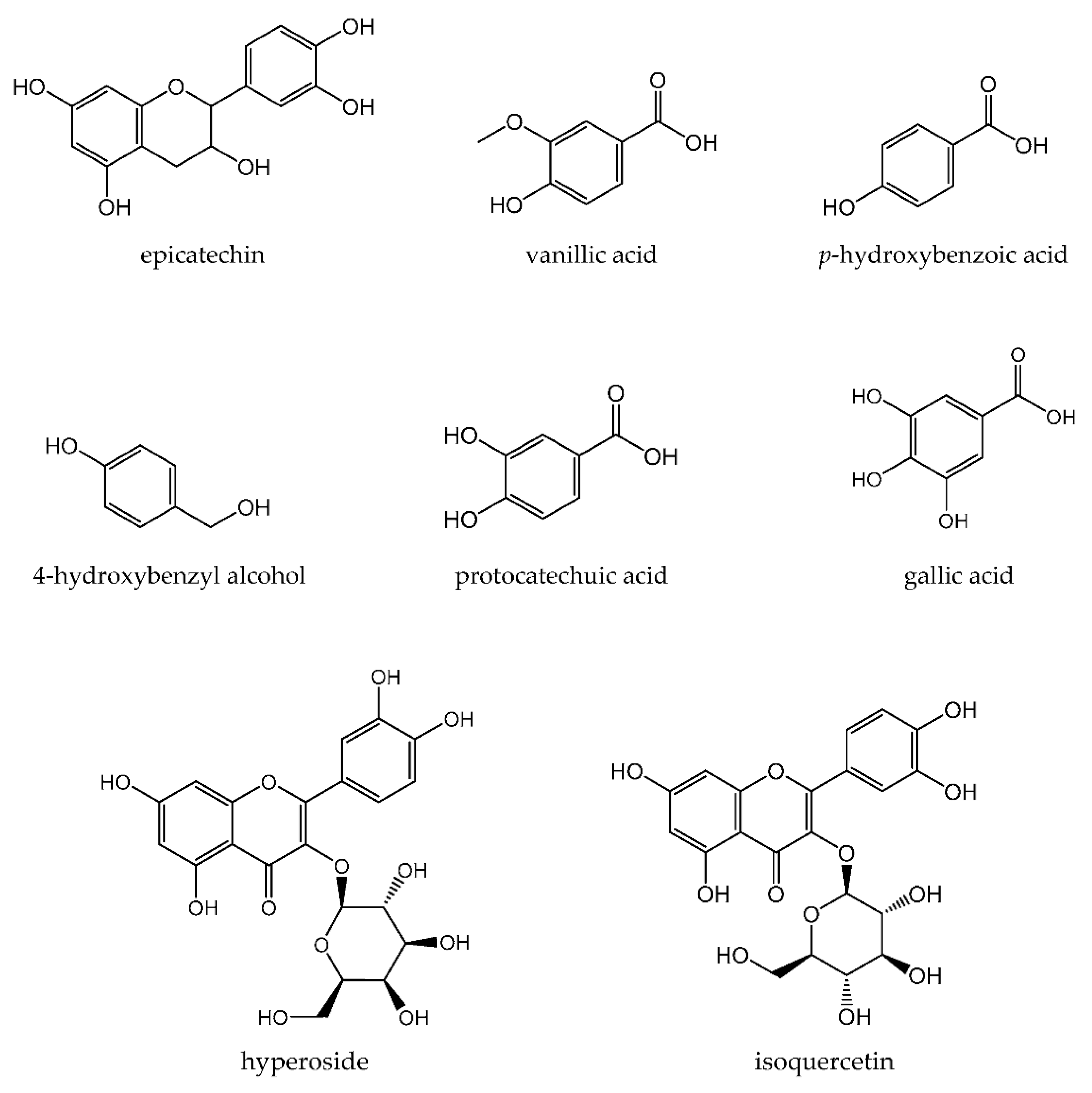
| Epicatechin | −6.7 | His259, His263, Arg268, Met280 |
| Vanillic acid | −5.8 | ND |
| p-hydroxybenzoic acid | −6.0 | ND |
| 4-hydroxybenzyl alcohol | −5.6 | His61, His85 |
| Hyperoside | −6.3 | His85, His263, Met280 |
| Isoquercetin | −6.3 | Met280, Gly281, Val283 |
| Protocatechic acid | −3.3 | His85, His259, Asn260, Phe264, His263 |
| Gallic acid | −5.9 | His85, Phe264 |
| Ligand | Score (Kcal/mol) | Active Site Residues Involved in H-Bond |
|---|---|---|
| Epicatechin | −8.5 | Asn180, Ala182, His218, Tyr237, Tyr240 |
| Vanillic acid | −6.5 | Val101, Leu226, His228 |
| p-hydroxybenzoic acid | −6.7 | Leu181, Ala182, Arg214, His218, Glu219, Ser239 |
| 4-hydroxybenzyl alcohol | −6.2 | His218 |
| Hyperoside | −9.2 | Ala182, Ala184, His222, His228 |
| Isoquercetin | −10.5 | Asn180, His183, Asp186, His228, Pro238 |
| Protocatechic acid | −6.4 | Leu181, Ala182, Arg214, Val215 |
| Gallic acid | −7.1 | Leu181, Ala182, Arg214, Glu219 |
| Ligand | Score (Kcal/mol) | Active Site Residues Involved in H-Bond |
|---|---|---|
| Epicatechin | −6.5 | Ser190, Asn192, Ser195, Cys220 |
| Vanillic acid | −5.2 | Asn192, Ser195 |
| p-hydroxybenzoic acid | −4.8 | Cys220 |
| 4-hydroxybenzyl alcohol | −4.9 | His57, Ser190, Ser195, Ser214 |
| Hyperoside | −7.7 | His57, Ser190, Asn192, Ser195, Ser214, Gly219 |
| Isoquercetin | −7.8 | Arg143, Asn147, Cys191, Ser195, Cys220 |
| Protocatechic acid | −5.3 | Asn192, Cly216 |
| Gallic acid | −5.7 | Asn192, Ser195 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mechqoq, H.; Hourfane, S.; El Yaagoubi, M.; El Hamdaoui, A.; da Silva Almeida, J.R.G.; Rocha, J.M.; El Aouad, N. Molecular Docking, Tyrosinase, Collagenase, and Elastase Inhibition Activities of Argan By-Products. Cosmetics 2022, 9, 24. https://doi.org/10.3390/cosmetics9010024
Mechqoq H, Hourfane S, El Yaagoubi M, El Hamdaoui A, da Silva Almeida JRG, Rocha JM, El Aouad N. Molecular Docking, Tyrosinase, Collagenase, and Elastase Inhibition Activities of Argan By-Products. Cosmetics. 2022; 9(1):24. https://doi.org/10.3390/cosmetics9010024
Chicago/Turabian StyleMechqoq, Hicham, Sohaib Hourfane, Mohamed El Yaagoubi, Abdallah El Hamdaoui, Jackson Roberto Guedes da Silva Almeida, Joao Miguel Rocha, and Noureddine El Aouad. 2022. "Molecular Docking, Tyrosinase, Collagenase, and Elastase Inhibition Activities of Argan By-Products" Cosmetics 9, no. 1: 24. https://doi.org/10.3390/cosmetics9010024
APA StyleMechqoq, H., Hourfane, S., El Yaagoubi, M., El Hamdaoui, A., da Silva Almeida, J. R. G., Rocha, J. M., & El Aouad, N. (2022). Molecular Docking, Tyrosinase, Collagenase, and Elastase Inhibition Activities of Argan By-Products. Cosmetics, 9(1), 24. https://doi.org/10.3390/cosmetics9010024











