True Grit: A Story of Perseverance Making Two Out of Three the First Non-Animal Testing Strategy (Adopted as OECD Guideline No. 497)
Abstract
:1. Introduction
2. Setting the Scene
3. Embarking on the Journey
4. The Concomitantly Changing Regulatory Landscape
5. Heading down to the Finish Line: OECD GL 497
6. Looking Forward
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- SCCS. SCCS Notes of Guidance for Testing of Cosmetic Ingredients and Their Safety Evaluation. Available online: https://ec.europa.eu/health/sites/default/files/scientific_committees/consumer_safety/docs/sccs_o_250.pdf (accessed on 15 October 2021).
- EU. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02009R1223-20210823 (accessed on 15 October 2021).
- Rollin, B.E. The regulation of animal research and the emergence of animal ethics: A conceptual history. Theor. Med. Bioeth. 2006, 27, 285–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EU. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency, Amending Directive 1999/45/EC and Repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02006R1907-20211001 (accessed on 15 October 2021).
- EU. Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02008R1272-20211001 (accessed on 15 October 2021).
- ECHA. Interface between REACH and Cosmetics Regulations. Available online: https://echa.europa.eu/documents/10162/13628/reach_cosmetics_factsheet_en.pdf/2fbcf6bf-cc78-4a2c-83fa-43ca87cfb314 (accessed on 15 October 2021).
- Knight, J.; Rovida, C.; Kreiling, R.; Zhu, C.; Knudsen, M.; Hartung, T. Continuing Animal Tests on Cosmetic Ingredients for REACH in the EU. ALTEX 2021, 38, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Sauer, U.G.; Hill, E.H.; Curren, R.D.; Raabe, H.A.; Kolle, S.N.; Teubner, W.; Mehling, A.; Landsiedel, R. Local tolerance testing under REACH: Accepted non-animal methods are not on equal footing with animal tests. Altern. Lab. Anim. 2016, 44, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Fentem, J.; Malcomber, I.; Maxwell, G.; Westmoreland, C. Upholding the EU’s Commitment to ‘Animal Testing as a Last Resort’ Under REACH Requires a Paradigm Shift in How We Assess Chemical Safety to Close the Gap Between Regulatory Testing and Modern Safety Science. Altern. Lab. Anim. 2021, 49, 122–132. [Google Scholar] [CrossRef]
- Culliney, K. ‘A Big Step Forward’: BASF and Givaudan Receive OECD Approval on Animal-Free Skin Sensitisation and Allergen Potency Testing Strategy. Available online: HTTPS://WWW.COSMETICSDESIGN-EUROPE.COM/ARTICLE/2021/06/30/ANIMAL-FREE-TESTING-FOR-SKIN-SENSITISATION-ALLERGEN-POTENCY-FROM-BASF-AND-GIVAUDAN-RECEIVES-OECD-APPROVAL (accessed on 15 October 2021).
- OECD. Guideline No. 497: Defined Approaches on Skin Sensitisation. Available online: https://www.oecd-ilibrary.org/content/publication/b92879a4-en (accessed on 15 October 2021).
- Natsch, A.; Landsiedel, R.; Kolle, S.N. A triangular approach for the validation of new approach methods for skin sensitization. ALTEX 2021, 38, 669–677. [Google Scholar] [CrossRef]
- Urbisch, D.; Mehling, A.; Guth, K.; Ramirez, T.; Honarvar, N.; Kolle, S.; Landsiedel, R.; Jaworska, J.; Kern, P.S.; Gerberick, F.; et al. Assessing skin sensitization hazard in mice and men using non-animal test methods. Regul. Toxicol. Pharmacol. 2015, 71, 337–351. [Google Scholar] [CrossRef] [Green Version]
- Gell, P.G.H.C.R.R.A. Clinical Aspects of Immunology; Blackwell: Oxford, UK, 1963. [Google Scholar]
- Matzinger, P. The danger model: A renewed sense of self. Science 2002, 296, 301–305. [Google Scholar] [CrossRef] [Green Version]
- OECD. The Adverse Outcome Pathway for Skin Sensitisation Initiated by Covalent Binding to Proteins. Available online: https://www.oecd-ilibrary.org/content/publication/9789264221444-en (accessed on 15 October 2021).
- OECD. Test No. 406: Skin Sensitisation. Available online: https://www.oecd-ilibrary.org/content/publication/9789264070660-en (accessed on 15 October 2021).
- OECD. Test No. 429: Skin Sensitisation. Available online: https://www.oecd-ilibrary.org/content/publication/9789264071100-en (accessed on 15 October 2021).
- Taylor, K. Ten years of REACH—An animal protection perspective. Altern. Lab. Anim. 2018, 46, 347–373. [Google Scholar] [CrossRef]
- OECD. Test No. 442D: In Vitro Skin Sensitisation. Available online: https://www.oecd-ilibrary.org/content/publication/9789264229822-en (accessed on 15 October 2021).
- OECD. Test No. 442E: In Vitro Skin Sensitisation. Available online: https://www.oecd-ilibrary.org/content/publication/9789264264359-en (accessed on 15 October 2021).
- OECD. Test No. 442C: In Chemico Skin Sensitisation. Available online: http://www.oecd.org/env/ehs/testing/draft-test-guideline-442C-in-chemico-skin-sensitisation.pdf (accessed on 15 October 2021).
- Mehling, A.; Eriksson, T.; Eltze, T.; Kolle, S.; Ramirez, T.; Teubner, W.; van Ravenzwaay, B.; Landsiedel, R. Non-animal test methods for predicting skin sensitization potentials. Arch. Toxicol. 2012, 86, 1273–1295. [Google Scholar] [CrossRef]
- Teubner, W.; Mehling, A.; Schuster, P.X.; Guth, K.; Worth, A.; Burton, J.; van Ravenzwaay, B.; Landsiedel, R. Computer models versus reality: How well do in silico models currently predict the sensitization potential of a substance. Regul. Toxicol. Pharmacol. 2013, 67, 468–485. [Google Scholar] [CrossRef]
- Bauch, C.; Kolle, S.N.; Fabian, E.; Pachel, C.; Ramirez, T.; Wiench, B.; Wruck, C.J.; van Ravenzwaay, B.; Landsiedel, R. Intralaboratory validation of four in vitro assays for the prediction of the skin sensitizing potential of chemicals. Toxicology 2011, 25, 1162–1168. [Google Scholar] [CrossRef]
- Gerberick, G.F.; Vassallo, J.D.; Bailey, R.E.; Chaney, J.G.; Morrall, S.W.; Lepoittevin, J.P. Development of a peptide reactivity assay for screening contact allergens. Toxicol. Sci. 2004, 81, 332–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emter, R.; Ellis, G.; Natsch, A. Performance of a novel keratinocyte-based reporter cell line to screen skin sensitizers in vitro. Toxicol. Appl. Pharmacol. 2010, 245, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Ashikaga, T.; Yoshida, Y.; Hirota, M.; Yoneyama, K.; Itagaki, H.; Sakaguchi, H.; Miyazawa, M.; Ito, Y.; Suzuki, H.; Toyoda, H. Development of an in vitro skin sensitization test using human cell lines: The human Cell Line Activation Test (h-CLAT). I. Optimization of the h-CLAT protocol. Toxicology 2006, 20, 767–773. [Google Scholar] [CrossRef]
- Python, F.; Goebel, C.; Aeby, P. Assessment of the U937 cell line for the detection of contact allergens. Toxicol. Appl. Pharmacol. 2007, 220, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, H.; Ashikaga, T.; Miyazawa, M.; Yoshida, Y.; Ito, Y.; Yoneyama, K.; Hirota, M.; Itagaki, H.; Toyoda, H.; Suzuki, H. Development of an in vitro skin sensitization test using human cell lines; human Cell Line Activation Test (h-CLAT). II. An inter-laboratory study of the h-CLAT. Toxicology 2006, 20, 774–784. [Google Scholar] [CrossRef]
- Bauch, C.; Kolle, S.N.; Ramirez, T.; Eltze, T.; Fabian, E.; Mehling, A.; Teubner, W.; van Ravenzwaay, B.; Landsiedel, R. Putting the parts together: Combining in vitro methods to test for skin sensitizing potentials. Regul. Toxicol. Pharmacol. 2012, 63, 489–504. [Google Scholar] [CrossRef]
- OECD. Guidance Document on the Reporting of Defined Approaches and Individual Information Sources to be Used within Integrated Approaches to Testing and Assessment (IATA) for Skin Sensitisation. Available online: https://www.oecd-ilibrary.org/content/publication/9789264279285-en (accessed on 15 October 2021).
- Kolle, S.N.; Teubner, W.; Landsiedel, R. Modern Skin Toxicity Testing Strategies. In Environment and Skin; Krutmann, J., Merk, H.F., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 27–40. [Google Scholar]
- Urbisch, D.; Becker, M.; Honarvar, N.; Kolle, S.N.; Mehling, A.; Teubner, W.; Wareing, B.; Landsiedel, R. Assessment of Pre- and Pro-haptens Using Nonanimal Test Methods for Skin Sensitization. Chem. Res. Toxicol. 2016, 29, 901–913. [Google Scholar] [CrossRef] [Green Version]
- Patlewicz, G.; Casati, S.; Basketter, D.A.; Asturiol, D.; Roberts, D.W.; Lepoittevin, J.P.; Worth, A.P.; Aschberger, K. Can currently available non-animal methods detect pre and pro-haptens relevant for skin sensitization? Regul. Toxicol. Pharmacol. 2016, 82, 147–155. [Google Scholar] [CrossRef]
- Gabbert, S.; Mathea, M.; Kolle, S.N.; Landsiedel, R. Accounting for Precision Uncertainty of Toxicity Testing: Methods to Define Borderline Ranges and Implications for Hazard Assessment of Chemicals. Risk Anal. 2020. [Google Scholar] [CrossRef]
- Kolle, S.N.; Mathea, M.; Natsch, A.; Landsiedel, R. Assessing Experimental Uncertainty in Defined Approaches: Borderline Ranges for In Chemico and In Vitro Skin Sensitization Methods Determined from Ring Trial Data. Applied Toxicol. 2021, 7, 102–111. [Google Scholar] [CrossRef]
- Kleinstreuer, N.C.; Hoffmann, S.; Alepee, N.; Allen, D.; Ashikaga, T.; Casey, W.; Clouet, E.; Cluzel, M.; Desprez, B.; Gellatly, N.; et al. Non-animal methods to predict skin sensitization (II): An assessment of defined approaches (*). Crit. Rev. Toxicol. 2018, 48, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Kolle, S.N.; Natsch, A.; Gerberick, G.F.; Landsiedel, R. A review of substances found positive in 1 of 3 in vitro tests for skin sensitization. Regul. Toxicol. Pharmacol. 2019, 106, 352–368. [Google Scholar] [CrossRef] [PubMed]
- EU. EU Reference Laboratory for Alternatives to Animal Testing. Available online: https://eurl-ecvam.jrc.ec.europa.eu/validation-regulatory-acceptance/eurl-ecvams-validation-process/eurl-ecvams-validation-process#figure-1-the-eurl (accessed on 15 October 2021).
- EU. Validation and Submission Process. Available online: https://ec.europa.eu/jrc/en/eurl/ecvam/alternative-methods-toxicity-testing/validation (accessed on 15 October 2021).
- OECD. Annex I: Case Studies to the Guidance Document on the Reporting of Defined Approaches and Individual Information Sources to be Used within Integrated Approaches to Testing and Assessment (IATA) for Skin Sensitisation. Available online: https://one.oecd.org/document/ENV/JM/WRPR(2016)62/ANN1/en/pdf (accessed on 15 October 2021).
- OECD. Annex II: Information Sources Used within the Case Studies to ThevGuidance Document on the Reporting of Defined Approaches and Individual Information Sources to be Used within Integrated Approaches to Testing and Assessment (IATA) for Skin Sensitisation. Available online: https://one.oecd.org/document/ENV/JM/WRPR(2016)62/ANN2/en/pdf (accessed on 15 October 2021).
- Strickland, J.; Daniel, A.B.; Allen, D.; Aguila, C.; Ahir, S.; Bancos, S.; Craig, E.; Germolec, D.; Ghosh, C.; Hudson, N.L.; et al. Skin sensitization testing needs and data uses by US regulatory and research agencies. Arch. Toxicol. 2019, 93, 273–291. [Google Scholar] [CrossRef]
- Kolle, S.N.; Landsiedel, R.; Natsch, A. Replacing the refinement for skin sensitization testing: Considerations to the implementation of adverse outcome pathway (AOP)-based defined approaches (DA) in OECD guidelines. Regul. Toxicol. Pharmacol. 2020, 115, 104713. [Google Scholar] [CrossRef]
- Hoffmann, S.; Kleinstreuer, N.; Alepee, N.; Allen, D.; Api, A.M.; Ashikaga, T.; Clouet, E.; Cluzel, M.; Desprez, B.; Gellatly, N.; et al. Non-animal methods to predict skin sensitization (I): The Cosmetics Europe database. Crit. Rev. Toxicol. 2018, 48, 344–358. [Google Scholar] [CrossRef] [Green Version]
- Basketter, D.A.; Alepee, N.; Ashikaga, T.; Barroso, J.; Gilmour, N.; Goebel, C.; Hibatallah, J.; Hoffmann, S.; Kern, P.; Martinozzi-Teissier, S.; et al. Categorization of chemicals according to their relative human skin sensitizing potency. Dermatitis 2014, 25, 11–21. [Google Scholar] [CrossRef]
- EPA. Interim Science Policy: Use of Alternative Approaches for Skin Sensitization as a Replacement for Laboratory Animal Testing. Available online: https://www.regulations.gov/document?D=EPA-HQ-OPP-2016-0093-0090 (accessed on 15 October 2021).
- Bergal, M.; Puginier, M.; Gerbeix, C.; Groux, H.; Roso, A.; Cottrez, F.; Milius, A. In vitro testing strategy for assessing the skin sensitizing potential of “difficult to test” cosmetic ingredients. Toxicology 2020, 65, 104781. [Google Scholar] [CrossRef]
- Cottrez, F.; Boitel, E.; Ourlin, J.C.; Peiffer, J.L.; Fabre, I.; Henaoui, I.S.; Mari, B.; Vallauri, A.; Paquet, A.; Barbry, P.; et al. SENS-IS, a 3D reconstituted epidermis based model for quantifying chemical sensitization potency: Reproducibility and predictivity results from an inter-laboratory study. Toxicology 2016, 32, 248–260. [Google Scholar] [CrossRef]
- Forreryd, A.; Zeller, K.S.; Lindberg, T.; Johansson, H.; Lindstedt, M. From genome-wide arrays to tailor-made biomarker readout—Progress towards routine analysis of skin sensitizing chemicals with GARD. Toxicology 2016, 37, 178–188. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.W. Is a combination of assays really needed for non-animal prediction of skin sensitization potential? Performance of the GARD (Genomic Allergen Rapid Detection) assay in comparison with OECD guideline assays alone and in combination. Regul. Toxicol. Pharmacol. 2018, 98, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Natsch, A.; Haupt, T.; Wareing, B.; Landsiedel, R.; Kolle, S.N. Predictivity of the kinetic direct peptide reactivity assay (kDPRA) for sensitizer potency assessment and subclassification. ALTEX 2020, 37, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Wareing, B.; Kolle, S.N.; Birk, B.; Alepee, N.; Haupt, T.; Kathawala, R.; Kern, P.S.; Nardelli, L.; Raabe, H.; Rucki, M.; et al. The kinetic Direct Peptide Reactivity Assay (kDPRA): Intra- and inter-laboratory reproducibility in a seven-laboratory ring trial. ALTEX 2020, 37, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Barthe, M.; Bavoux, C.; Finot, F.; Mouche, I.; Cuceu-Petrenci, C.; Forreryd, A.; Chérouvrier Hansson, A.; Johansson, H.; Lemkine, G.F.; Thénot, J.-P.; et al. Safety Testing of Cosmetic Products: Overview of Established Methods and New Approach Methodologies (NAMs). Cosmetics 2021, 8, 50. [Google Scholar] [CrossRef]
- Teubner, W.; Landsiedel, R. Read-across for hazard assessment: The ugly duckling is growing up. Altern. Lab. Anim. 2015, 43, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Janer, G.; Ag-Seleci, D.; Sergent, J.A.; Landsiedel, R.; Wohlleben, W. Creating sets of similar nanoforms with the ECETOC NanoApp: Real-life case studies. Nanotoxicology 2021, 15, 1016–1034. [Google Scholar] [CrossRef]
- Luechtefeld, T.; Rowlands, C.; Hartung, T. Big-data and machine learning to revamp computational toxicology and its use in risk assessment. Toxicol. Res. 2018, 7, 732–744. [Google Scholar] [CrossRef] [Green Version]
- Garcia de Lomana, M.; Morger, A.; Norinder, U.; Buesen, R.; Landsiedel, R.; Volkamer, A.; Kirchmair, J.; Mathea, M. ChemBioSim: Enhancing Conformal Prediction of In Vivo Toxicity by Use of Predicted Bioactivities. J. Chem. Inf. Model 2021, 61, 3255–3272. [Google Scholar] [CrossRef]
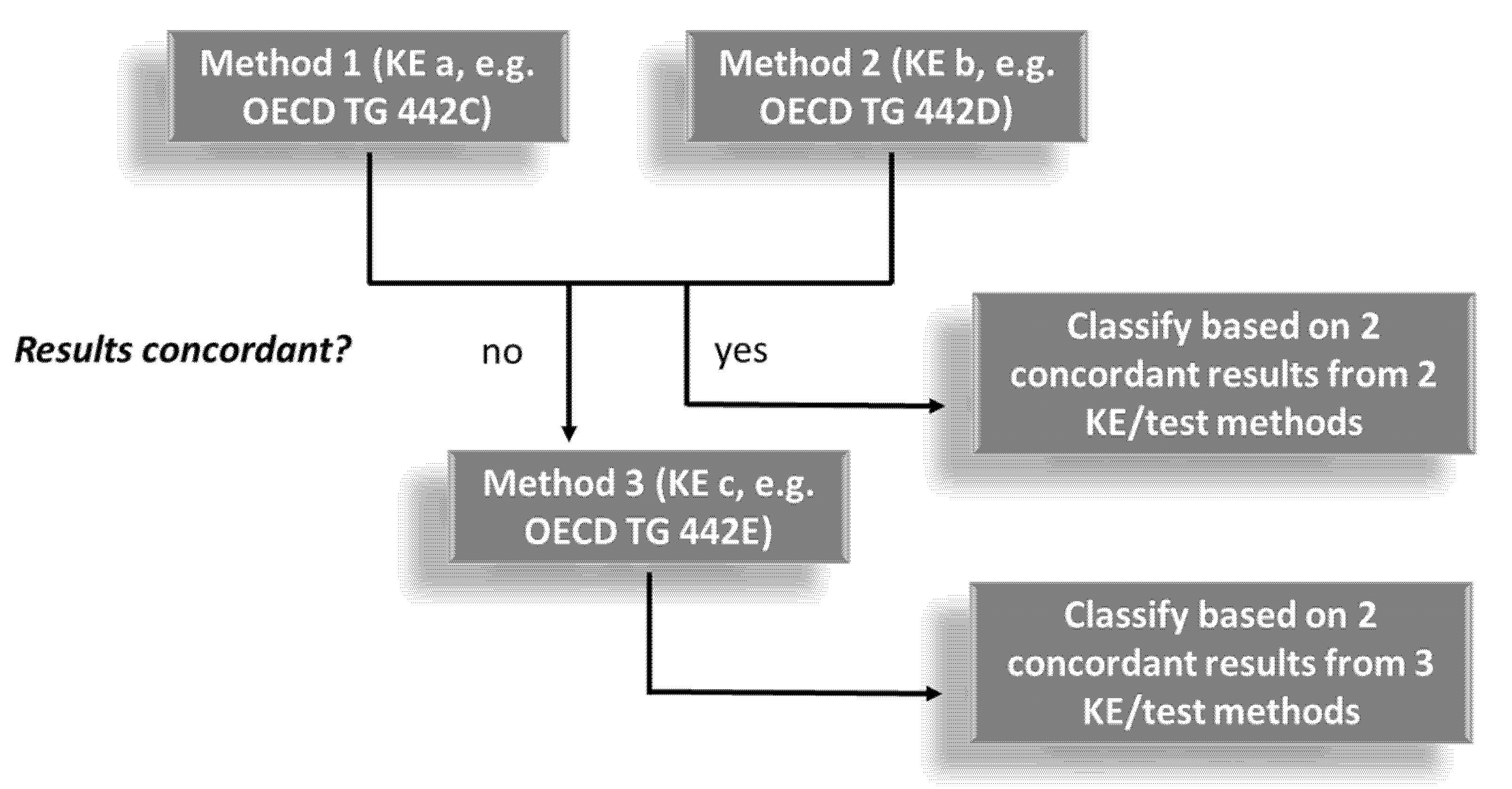

| Chemical Set and Reference Data | Subset B | |||||||
|---|---|---|---|---|---|---|---|---|
| Human Data a | LLNA Data | |||||||
| Cooper Statistics | Se b [%] | Sp b [%] | Acc b [%] | n | Se b [%] | Sp b [%] | Acc b [%] | n |
| “2 out of 3” WoE approach | 90 | 90 | 90 | 101 | 81 | 83 | 82 | 103 |
| DPRA | 84 | 84 | 84 | 102 | 77 | 85 | 79 | 105 |
| KeratinoSensTM | 82 | 84 | 82 | 102 | 74 | 73 | 74 | 103 |
| h-CLAT | 89 | 64 | 82 | 98 | 86 | 68 | 81 | 101 |
| LuSens | 78 | 79 | 79 | 60 | 73 | 70 | 71 | 62 |
| (m)MUSST | 74 | 88 | 78 | 85 | 71 | 83 | 75 | 87 |
| LLNA | 91 | 64 | 82 | 111 | - | - | - | - |
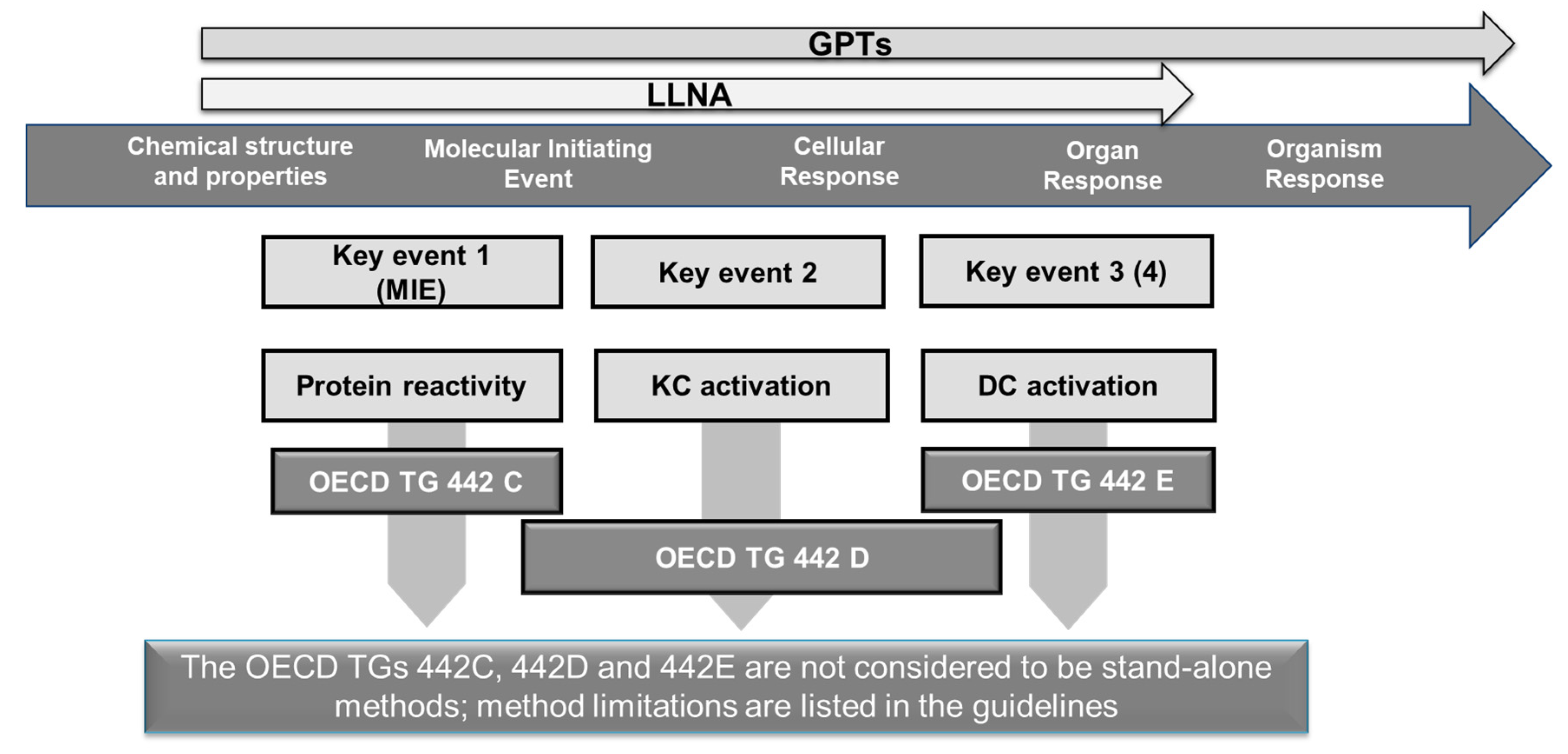
| OECD (Test) Guideline Key Event Addressed | Last Updated | AOP-Based Test Method or DA | Outcome |
|---|---|---|---|
| OECD TG 442C Key Event 1 (peptide/protein binding) | 2021 | DPRA | yes/no information on KE1 as part of an integrated assessment |
| ADRA | yes/no information on KE1 as part of an integrated assessment | ||
| kDPRA | quantitative potency information as stand-alone or part of an integrated assessment: Cat a 1A or Cat 1B/NS | ||
| OECD TG 442D Key Event 2 (Keratinocyte responses) | 2018 | Keratinos ensTM | yes/no information on KE2 as part of an integrated assessment |
| LuSens | yes/no information on KE2 as part of an integrated assessment | ||
| OECD TG 442E Key Event 3 (monocyte/dendritic cell responses) | 2018 | h-CLAT | yes/no information on KE3 as part of an integrated assessment |
| U-SENSTM | yes/no information on KE3 as part of an integrated assessment | ||
| IL-8 Luc | yes/no information on KE3 as part of an integrated assessment | ||
| OECD GL 497 Combining Key Events 1, 3 and/or 2 | 2021 | 2 out of 3 | non-sensitizer or non-sensitizer |
| ITS v1 and v2 | non-sensitizer or sensitizer (Cat 1A or 1B) |
| Sensitivity [%] | Specificity [%] | Balanced Accuracy [%] | n | |
|---|---|---|---|---|
| Bauch et al., 2012 [31] | 96 | 81 | 88 | 50 |
| Urbisch et al., 2015 [13] | 91 | 64 | 77.5 | 111 |
| Hoffmann et al., 2018 and Kleinstreuer et al., 2018 [38,46] | 85.2 | 50.0 | 67.6 | 128 |
| OECD LLNA database [11] vs. human data [47] | 99 | 39 | 69 | 96 |
| OECD database [11] | 94 | 22 | 58 | 56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehling, A.; Kolle, S.N.; Wareing, B.; Landsiedel, R. True Grit: A Story of Perseverance Making Two Out of Three the First Non-Animal Testing Strategy (Adopted as OECD Guideline No. 497). Cosmetics 2022, 9, 22. https://doi.org/10.3390/cosmetics9010022
Mehling A, Kolle SN, Wareing B, Landsiedel R. True Grit: A Story of Perseverance Making Two Out of Three the First Non-Animal Testing Strategy (Adopted as OECD Guideline No. 497). Cosmetics. 2022; 9(1):22. https://doi.org/10.3390/cosmetics9010022
Chicago/Turabian StyleMehling, Annette, Susanne N. Kolle, Britta Wareing, and Robert Landsiedel. 2022. "True Grit: A Story of Perseverance Making Two Out of Three the First Non-Animal Testing Strategy (Adopted as OECD Guideline No. 497)" Cosmetics 9, no. 1: 22. https://doi.org/10.3390/cosmetics9010022
APA StyleMehling, A., Kolle, S. N., Wareing, B., & Landsiedel, R. (2022). True Grit: A Story of Perseverance Making Two Out of Three the First Non-Animal Testing Strategy (Adopted as OECD Guideline No. 497). Cosmetics, 9(1), 22. https://doi.org/10.3390/cosmetics9010022





