Three New Dexpanthenol-Containing Face Creams: Performance and Acceptability after Single and Repeated Applications in Subjects of Different Ethnicity with Dry and Sensitive Skin
Abstract
:1. Introduction
2. Methods
2.1. Study 1: DFC—Day Face Cream
2.1.1. Study Design
2.1.2. Subjects
2.1.3. Assessments
2.1.4. Statistical Evaluation
2.2. Studies 2 and 3: DFC-SPF—Day Face Cream with SPF25 and NFC—Night Face Cream
3. Results
3.1. Study 1: DFC—Day Face Cream
3.1.1. SC Hydration (Corneometry)
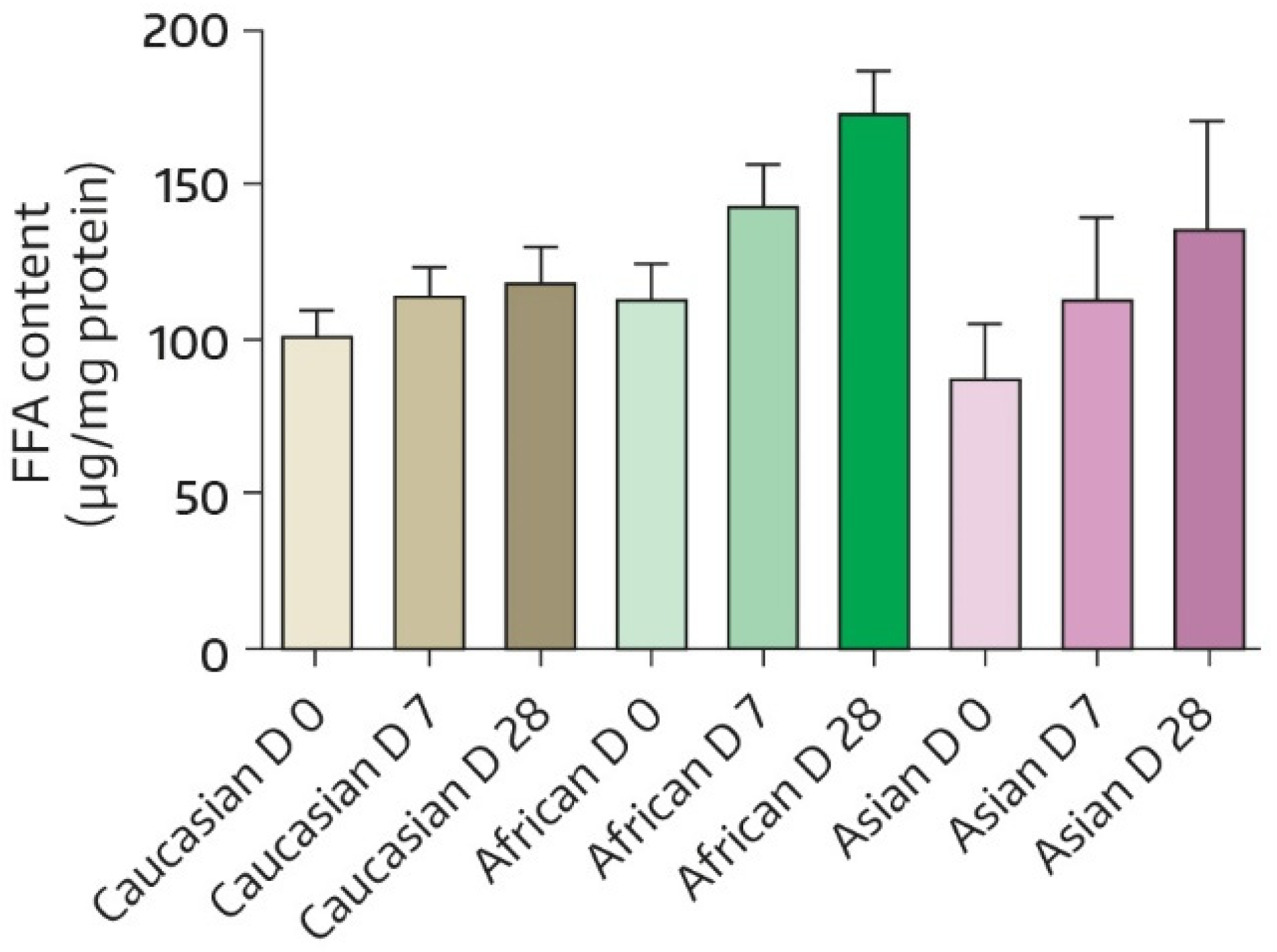
3.1.2. SC Lipid Content
3.1.3. Skin Elasticity Parameters
3.1.4. Self-Assessment Questionnaire, Acceptability, and Tolerability
3.2. Study 2: DFC-SPF—Day Face Cream with SPF25
3.2.1. SC Hydration (Corneometry)
3.2.2. SC Lipid Content
3.2.3. Skin Elasticity Parameters
3.2.4. Self-Assessment Questionnaire, Acceptability, and Tolerability
3.3. Study 3: NFC—Night Face Cream
3.3.1. SC Hydration (Corneometry)
3.3.2. SC Lipid Content
3.3.3. Skin Elasticity Parameters
3.3.4. Self-Assessment Questionnaire, Acceptability, and Tolerability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Proksch, E.; Berardesca, E.; Misery, L.; Engblom, J.; Bouwstra, J. Dry skin management: Practical approach in light of latest research on skin structure and function. J. Dermatol. Treat. 2020, 31, 716–722. [Google Scholar] [CrossRef]
- Sethi, A.; Kaur, T.; Malhotra, S.K.; Gambhir, M.L. Moisturizers: The slippery road. Indian J. Dermatol. 2016, 61, 279–287. [Google Scholar] [CrossRef]
- Voegeli, R.; Gierschendorf, J.; Summers, B.; Rawlings, A.V. Facial skin mapping: From single point bio-instrumental evaluation to continuous visualization of skin hydration, barrier function, skin surface pH, and sebum in different ethnic skin types. Int. J. Cosmet. Sci. 2019, 41, 411–424. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, K.; Kobayashi, H.; Hirao, T.; Ito, A.; Takahashi, H.; Tagami, H. Improvement of mild inflammatory changes of the facial skin induced by winter environment with daily applications of a moisturizing cream. A half-side test of biophysical skin parameters, cytokine expression pattern and the formation of cornified envelope. Dermatology 2003, 207, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Kobayashi, H.; Le Fur, I.; Tschachler, E.; Tagami, H. Winter season affects more severely the facial skin than the forearm skin: Comparative biophysical studies conducted in the same Japanese females in later summer and winter. Exog. Dermatol. 2002, 1, 32–38. [Google Scholar] [CrossRef]
- Hon, K.L.; Tsang, Y.C.; Pong, N.H.; Lee, V.W.; Luk, N.M.; Chow, C.M.; Leung, T.F. Patient acceptability, efficacy, and skin biophysiology of a cream and cleanser containing lipid complex with shea butter extract versus a ceramide product for eczema. Hong Kong Med. J. 2015, 21, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Lichterfeld, A.; Hauss, A.; Surber, C.; Peters, T.; Blume-Peytavi, U.; Kottner, J. Evidence-based skin care: A systematic literature review and the development of a basic skin care algorithm. J. Wound Ostomy Continence Nurs. 2015, 42, 501–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stettler, H.; Crowther, J.M.; Brandt, M.; Boxshall, A.; Lu, B.; de Salvo, R.; Laing, S.; Hennighausen, N.; Bielfeldt, S.; Blenkiron, P. Multi parametric biophysical assessment of treatment effects on xerotic skin. Skin Health Dis. 2021, 2, 1–10. [Google Scholar]
- Clarys, P.; Clijsen, R.; Taeymans, J.; Barel, A.O. Hydration measurements of the stratum corneum: Comparison between the capacitance method (digital version of the Corneometer CM 825®) and the impedance method (Skicon-200EX®). Skin Res. Technol. 2012, 18, 316–323. [Google Scholar] [CrossRef]
- Voegeli, R.; Rawlings, A.V.; Seroul, P.; Summers, B. A novel continuous colour mapping approach for visualization of facial skin hydration and transepidermal water loss for four ethnic groups. Int. J. Cosmet. Sci. 2015, 37, 595–605. [Google Scholar] [CrossRef]
- Fluhr, J.; Darlenski, R.; Surber, C. Glycerol and the skin: Holistic approach to its origin and functions. Br. J. Dermatol. 2008, 159, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Otte, N.; Borelli, C.; Korting, H.C. Nicotinamide—Biologic actions of an emerging cosmetic ingredient. Int. J. Cosmet. Sci. 2005, 27, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Wohlrab, J.; Kreft, D. Niacinamide—mechanisms of action and its topical use in dermatology. Skin Pharmacol. Physiol. 2014, 27, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; De Bony, R.; Trapp, S.; Boudon, S. Topical use of dexpanthenol: A 70th anniversary article. J. Dermatol. Treat. 2017, 28, 766–773. [Google Scholar] [CrossRef]
- Gehring, W.; Gloor, M. Effect of topically applied dexpanthenol on epidermal barrier function and stratum corneum hydration. Results of a human in vivo study. Arzneimittelforschung 2000, 50, 659–663. [Google Scholar] [CrossRef]
- Stettler, H.; Kurka, P.; Lunau, N.; Manger, C.; Böhling, A.; Bielfeldt, S.; Wilhelm, K.-P.; Dähnhardt-Pfeiffer, S.; Dähnhardt, D.; Brill, F.H.; et al. A new topical panthenol-containing emollient: Results from two randomized controlled studies assessing its skin moisturization and barrier restoration potential, and the effect on skin microflora. J. Dermatol. Treat. 2017, 28, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Barrionuevo-Gonzalez, A.; Trapp, S.; de Salvo, R.; Olsavszky, R.; Nanu, E.A.; Dopcea, I.; Dumitru, V.; Stettler, H. Two new dexpanthenol-containing wash gels: Skin Hydration, barrier function and cosmetic performance upon single and repeated usage in subjects with dry skin. Cosmetics 2021, 8, 44. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B. The validity and practicality of sun-reactive skin types I through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef]
- Autorité de Régulation Professionnelle de la Publicité. Cosmetic Products Code V8 (Effective on 1 July 2019). Available online: https://www.arpp.org/nous-consulter/regles/regles-de-deontologie/cosmetic-products-code-v8/#toc_0_20 (accessed on 8 July 2021).
- Heinrich, U.; Koop, U.; Leneveu-Duchemin, M.-C.; Osterrieder, K.; Bielfeldt, S.; Chkarnat, C.; Degwert, J.; Hantschel, D.; Jaspers, S.; Nissen, H.-P.; et al. Multicentre comparison of skin hydration in terms of physical-, physiological- and product-dependent parameters by the capacitive method (Corneometer CM 825). Int. J. Cosmet. Sci. 2003, 25, 45–53. [Google Scholar] [CrossRef]
- Stettler, H.; de Salvo, R.; Olsavszky, R.; Nanu, E.A.; Dumitru, V.; Trapp, S. Performance and tolerability of a new topical dexpanthenol-containing emollient line in subjects with dry skin: Results from three randomized studies. Cosmetics 2021, 8, 18. [Google Scholar] [CrossRef]
- Combarros, D.; Castilla-Castaño, E.; Lecru, L.; Pressanti, C.; Amalric, N.; Cadiergues, M.C. A prospective, randomized, double blind, placebo-controlled evaluation of the effects of an n-3 essential fatty acids supplement (Agepi® ω3) on clinical signs, and fatty acid concentrations in the erythrocyte membrane, hair shafts and skin surface of dogs with poor quality coats. Prostaglandins Leukot. Essent. Fat. Acids 2020, 159. [Google Scholar] [CrossRef]
- Dobrev, H. Use of Cutometer to assess epidermal hydration. Skin Res. Technol. 2000, 6, 239–244. [Google Scholar] [CrossRef]
- Ryu, H.S.; Joo, Y.H.; Kim, S.O.; Park, K.C.; Youn, S.W. Influence of age and regional differences on skin elasticity as measured by the Cutometer. Skin Res. Technol. 2008, 14, 354–358. [Google Scholar] [CrossRef]
- Krueger, N.; Luebberding, S.; Oltmer, M.; Streker, M.; Kerscher, M. Age-related changes in skin mechanical properties: A quantitative evaluation of 120 female subjects. Skin Res. Technol. 2011, 17, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Luebberding, S.; Krueger, N.; Kerscher, M. Mechanical properties of human skin in vivo: A comparative evaluation in 300 men and women. Skin Res. Technol. 2014, 20, 127–135. [Google Scholar] [CrossRef] [PubMed]
- American Society for Testing and Materials E1958-16a. Standard Guide for Sensory Claim Substantiation; ASTM International: West Conshohocken, PA, USA, 2016.
- Orth, D.S.; Appa, Y. Glycerine: A natural ingredient for moisturizing skin. In Dry Skin and Moisturizers, 1st ed.; Loden, M., Maibach, H.I., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 213–228. [Google Scholar]
- Björklund, S.; Pham, Q.D.; Jensen, L.B.; Knudsen, N.Ø.; Nielsen, L.D.; Ekelund, K.; Ruzgas, T.; Engblom, J.; Sparr, E. The effects of polar excipients transcutol and dexpanthenol on molecular mobility, permeability, and electrical impedance of the skin barrier. J. Colloid Interface Sci. 2016, 479, 207–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caussin, J.; Gooris, G.S.; Bouwstra, J.A. FTIR studies show lipophilic moisturizers to interact with stratum corneum lipids, rendering the more densely packed. Biochim. Biophys. Acta 2008, 1778, 1517–1524. [Google Scholar] [CrossRef] [Green Version]
- Overgaard Olsen, L.; Jemec, G.B. The influence of water, glycerin, paraffin oil and ethanol on skin mechanics. Acta Derm. Venereol. 1993, 73, 404–406. [Google Scholar] [CrossRef]
- Pierre, J.; Francois, G.; Benize, A.M.; Rubert, V.; Coutet, J.; Flament, F. Mapping, in vivo, the uniformity of two skin properties alongside the human face by a 3D virtual approach. Int. J. Cosmet. Sci. 2018, 40, 482–487. [Google Scholar] [CrossRef]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- van Smeden, J.; Bouwstra, J.A. Stratum corneum lipids: Their role for the skin barrier function in healthy subjects and atopic dermatitis patients. Curr. Probl. Dermatol. 2016, 49, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Tanno, O.; Ota, Y.; Kitamura, N.; Katsube, T.; Inoue, S. Nicotinamide increases biosynthesis of ceramides as well as other stratum corneum lipids to improve the epidermal permeability barrier. Br. J. Dermatol. 2000, 143, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Saya, Y.; Morita, E.; Matsunaka, H. Novel petrolatum-based ointment that is highly moisturizing and has superior usability with increased adherence in patients with facial dry skin. J. Cosmet. Dermatol. 2020, 19, 2650–2655. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. Thematic review series: Skin lipids. The role of epidermal lipids in cutaneous permeability barrier homeostasis. J. Lipid Res. 2007, 48, 2531–2546. [Google Scholar] [CrossRef] [Green Version]
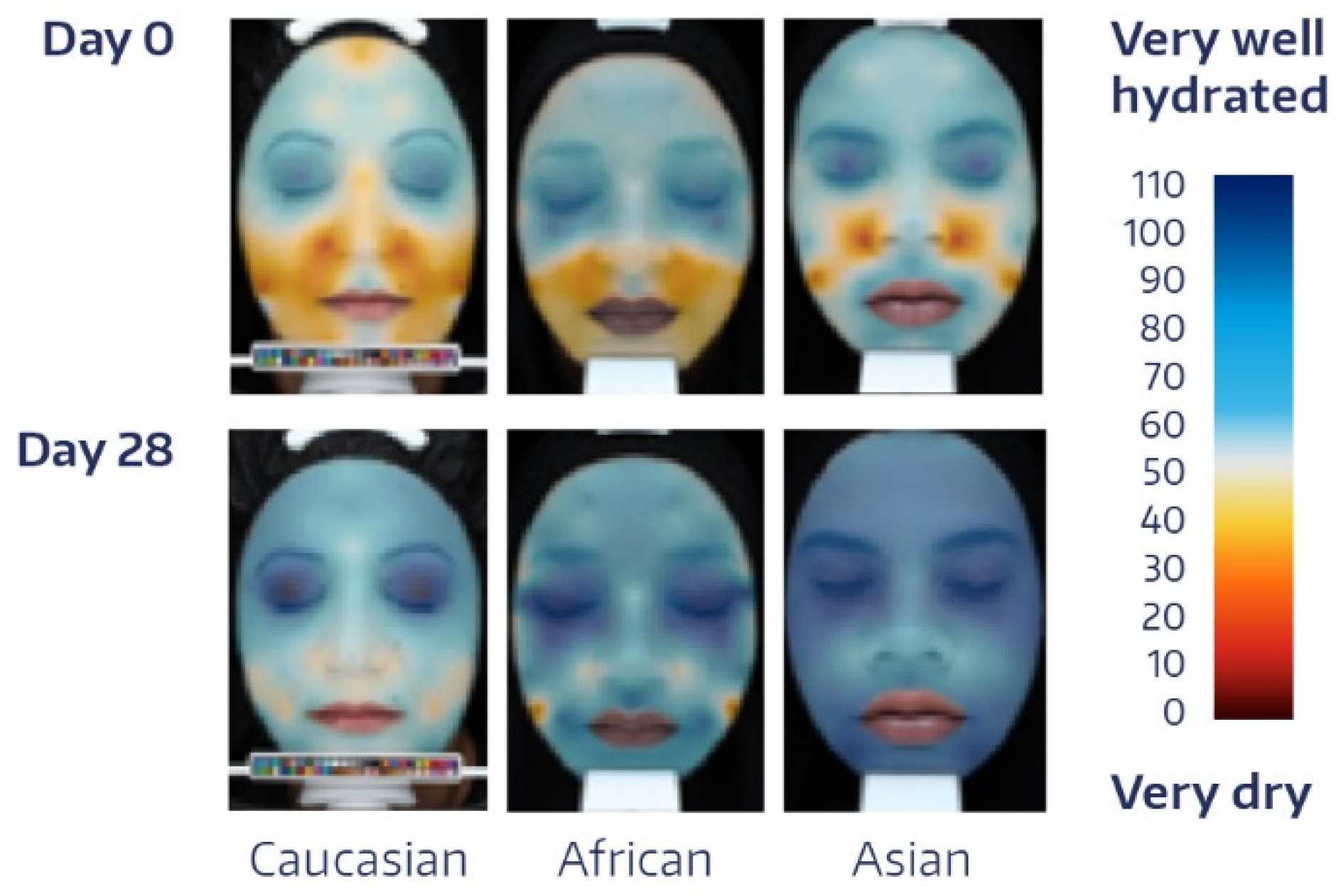
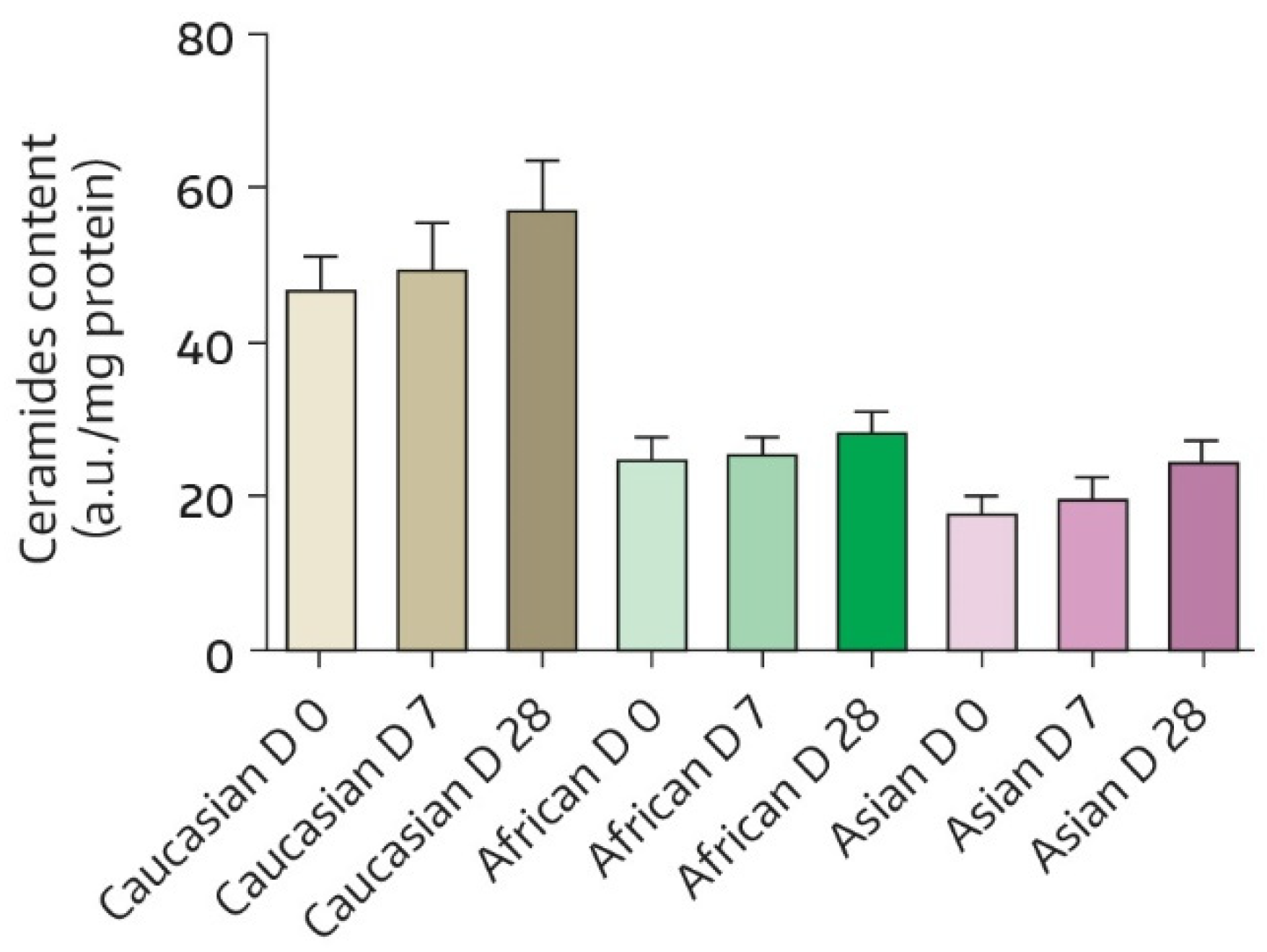

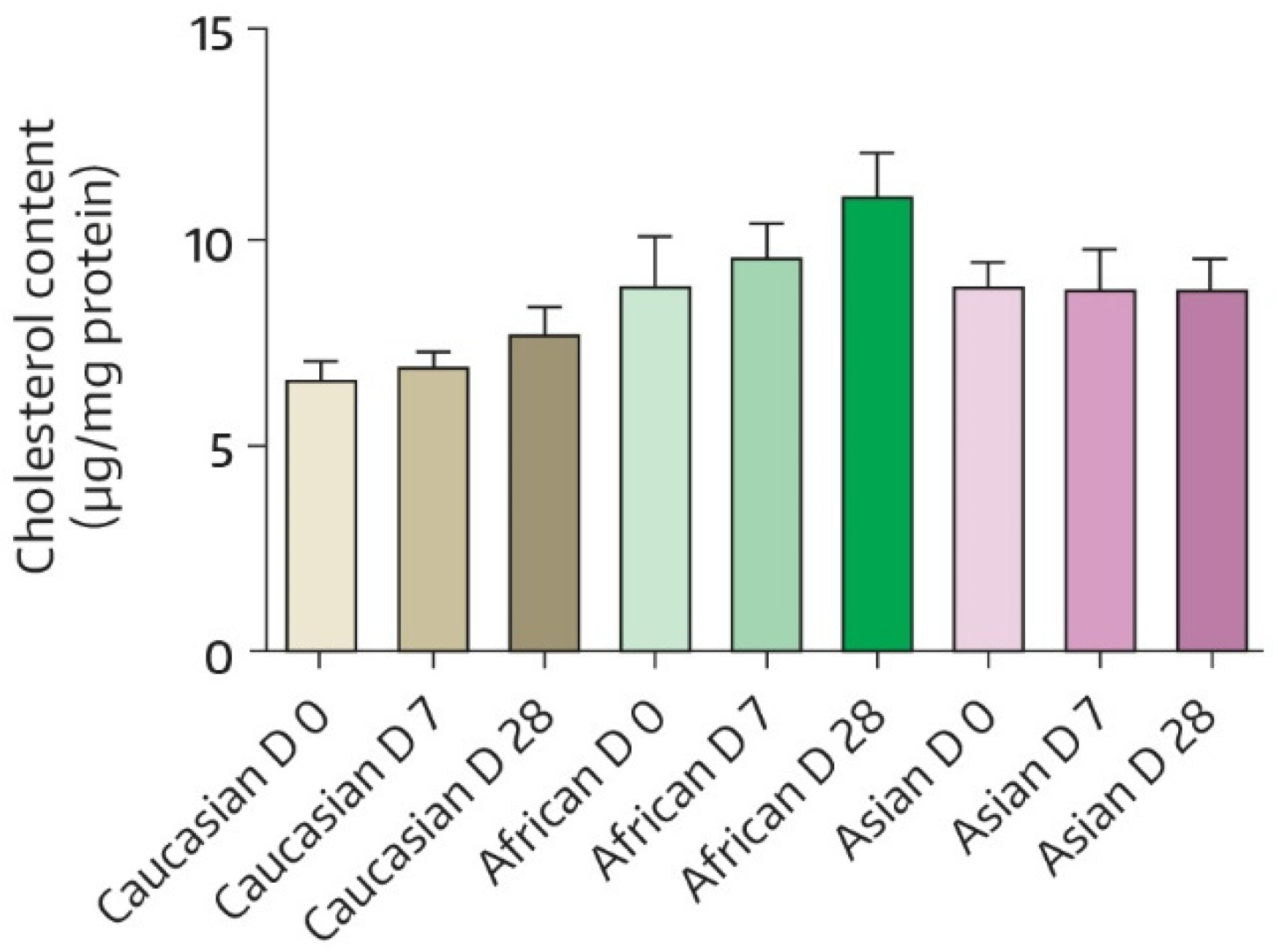
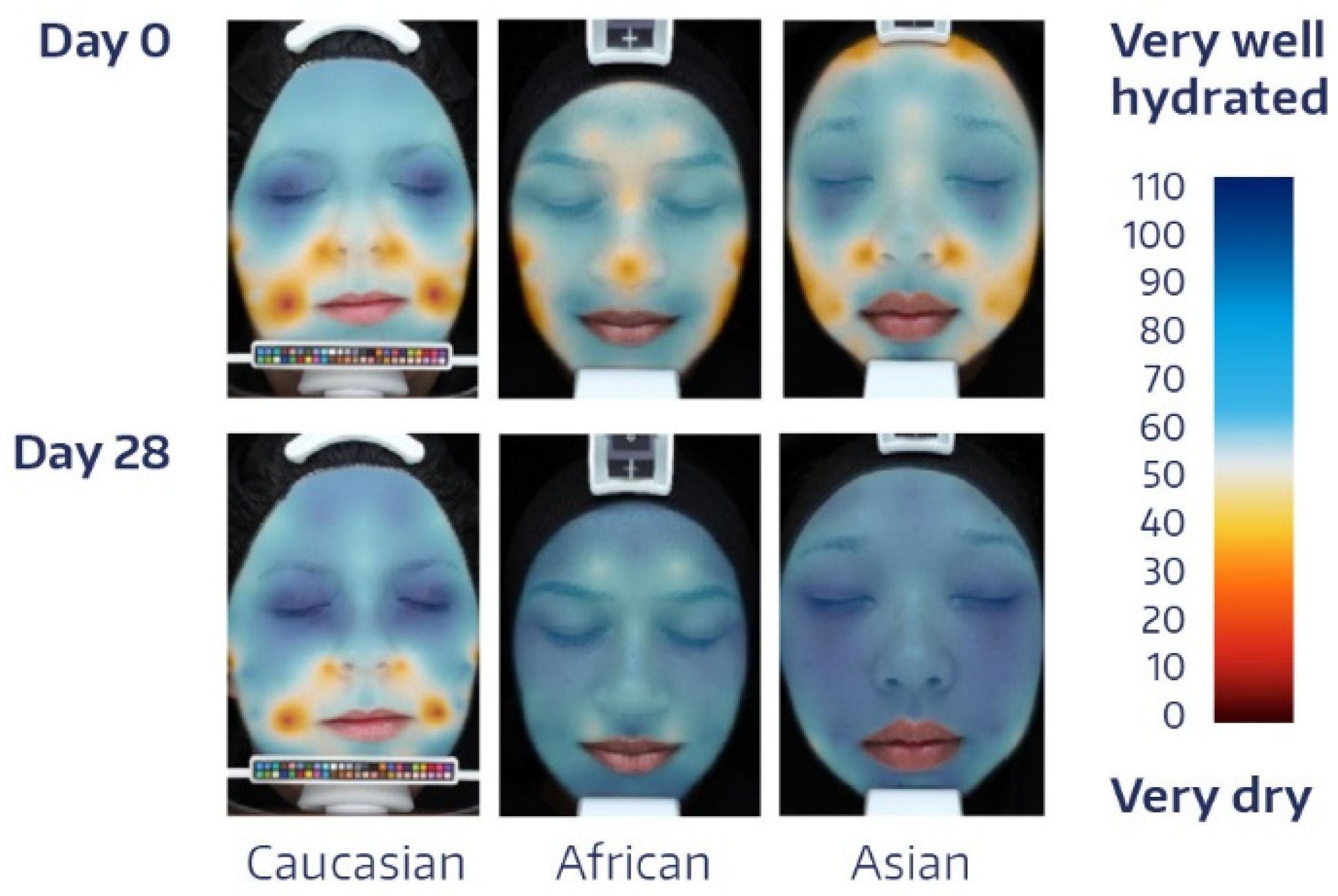
| Time | Absolute Value | Change from BL * | p-Value # |
|---|---|---|---|
| BL | 45.50 ± 7.41 | - | - |
| 24 h | 50.24 ± 10.66 | 4.85 [2.58, 7.13] | 0.0001 |
| 48 h | 48.44 ± 9.48 | 2.74 [0.56, 4.93] | 0.0151 |
| Day 7 | 51.83 ± 10.89 | 6.35 [3.53, 9.17] | <0.0001 |
| Day 14 | 52.85 ± 10.56 | 7.38 [4.90, 9.86] | <0.0001 |
| Day 21 | 56.14 ± 11.50 | 10.66 [7.93, 13.40] | <0.0001 |
| Day 28 | 57.61 ± 12.70 | 12.13 [8.90, 15.36] | <0.0001 |
| Time | Absolute Value | Change from BL * | p-Value # |
|---|---|---|---|
| BL | 47.77 ± 7.54 | - | - |
| 24 h | 51.20 ± 10.94 | 3.42 [1.41, 5.43] | 0.0014 |
| 48 h | 51.89 ± 11.09 | 4.12 [2.01, 6.22] | 0.0003 |
| Day 7 | 56.00 ± 9.61 | 8.22 [6.28, 10.17] | <0.0001 |
| Day 14 | 56.36 ± 10.33 | 8.59 [6.49, 10.69] | <0.0001 |
| Day 21 | 58.71 ± 9.77 | 10.70 [8.69, 12.71] | <0.0001 |
| Day 28 | 60.34 ± 8.49 | 12.57 [10.86, 14.28] | <0.0001 |
| Time | Absolute Value | Change from BL * | p-Value # |
|---|---|---|---|
| BL | 47.64 ± 5.67 | - | - |
| 24 h | 53.33 ± 7.03 | 5.69 [3.46, 7.92] | <0.0001 |
| 48 h | 53.95 ± 8.11 | 6.31 [3.97, 8.66] | <0.0001 |
| Day 7 | 58.38 ± 7.40 | 10.74 [8.67, 12.81] | <0.0001 |
| Day 14 | 58.00 ± 7.32 | 10.10 [7.47, 12.73] | <0.0001 |
| Day 21 | 61.29 ± 6.93 | 13.65 [11.54, 15.75] | <0.0001 |
| Day 28 | 60.30 ± 9.25 | 12.66 [9.92, 15.40] | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrionuevo-Gonzalez, A.; Trapp, S.; de Salvo, R.; Reitmann, M.; Cassar, E.; Rharbaoui, S.; Reber, F.; Stettler, H. Three New Dexpanthenol-Containing Face Creams: Performance and Acceptability after Single and Repeated Applications in Subjects of Different Ethnicity with Dry and Sensitive Skin. Cosmetics 2021, 8, 93. https://doi.org/10.3390/cosmetics8040093
Barrionuevo-Gonzalez A, Trapp S, de Salvo R, Reitmann M, Cassar E, Rharbaoui S, Reber F, Stettler H. Three New Dexpanthenol-Containing Face Creams: Performance and Acceptability after Single and Repeated Applications in Subjects of Different Ethnicity with Dry and Sensitive Skin. Cosmetics. 2021; 8(4):93. https://doi.org/10.3390/cosmetics8040093
Chicago/Turabian StyleBarrionuevo-Gonzalez, Ana, Sonja Trapp, Raffaella de Salvo, Marina Reitmann, Eva Cassar, Siham Rharbaoui, Florence Reber, and Hans Stettler. 2021. "Three New Dexpanthenol-Containing Face Creams: Performance and Acceptability after Single and Repeated Applications in Subjects of Different Ethnicity with Dry and Sensitive Skin" Cosmetics 8, no. 4: 93. https://doi.org/10.3390/cosmetics8040093
APA StyleBarrionuevo-Gonzalez, A., Trapp, S., de Salvo, R., Reitmann, M., Cassar, E., Rharbaoui, S., Reber, F., & Stettler, H. (2021). Three New Dexpanthenol-Containing Face Creams: Performance and Acceptability after Single and Repeated Applications in Subjects of Different Ethnicity with Dry and Sensitive Skin. Cosmetics, 8(4), 93. https://doi.org/10.3390/cosmetics8040093







