N-Succinyl-S-Farnesyl-L-Cysteine (SFC): A Novel Isoprenylcysteine Analog with In Vitro Anti-Inflammatory Activity and Clinical Skin Protecting Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Treatments
2.3. Enzyme-Linked Immunosorbent Assays (ELISA)
2.4. Clinical Study
2.5. Statistical Analysis
3. Results
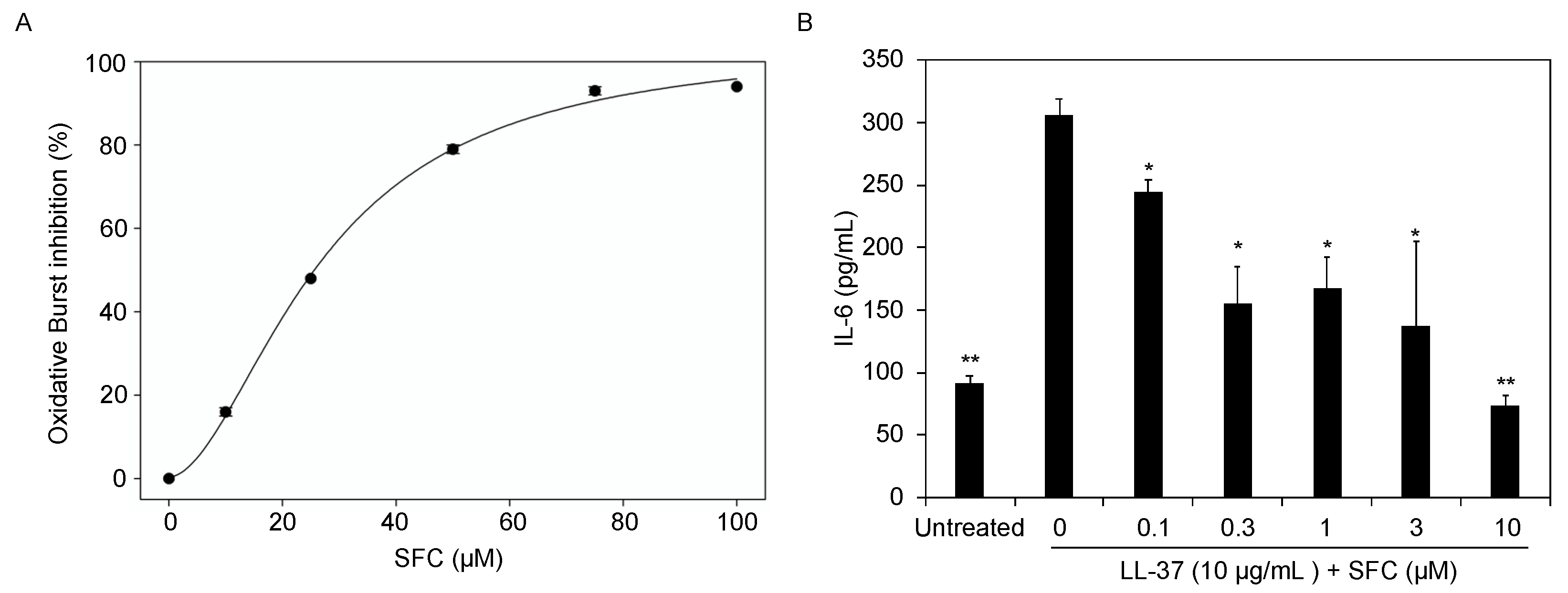
3.1. SFC Inhibits GPCR-Induced Oxidative Stress and Inflammation
3.2. SFC Protects against UVA and UVB-Induced Photoaging
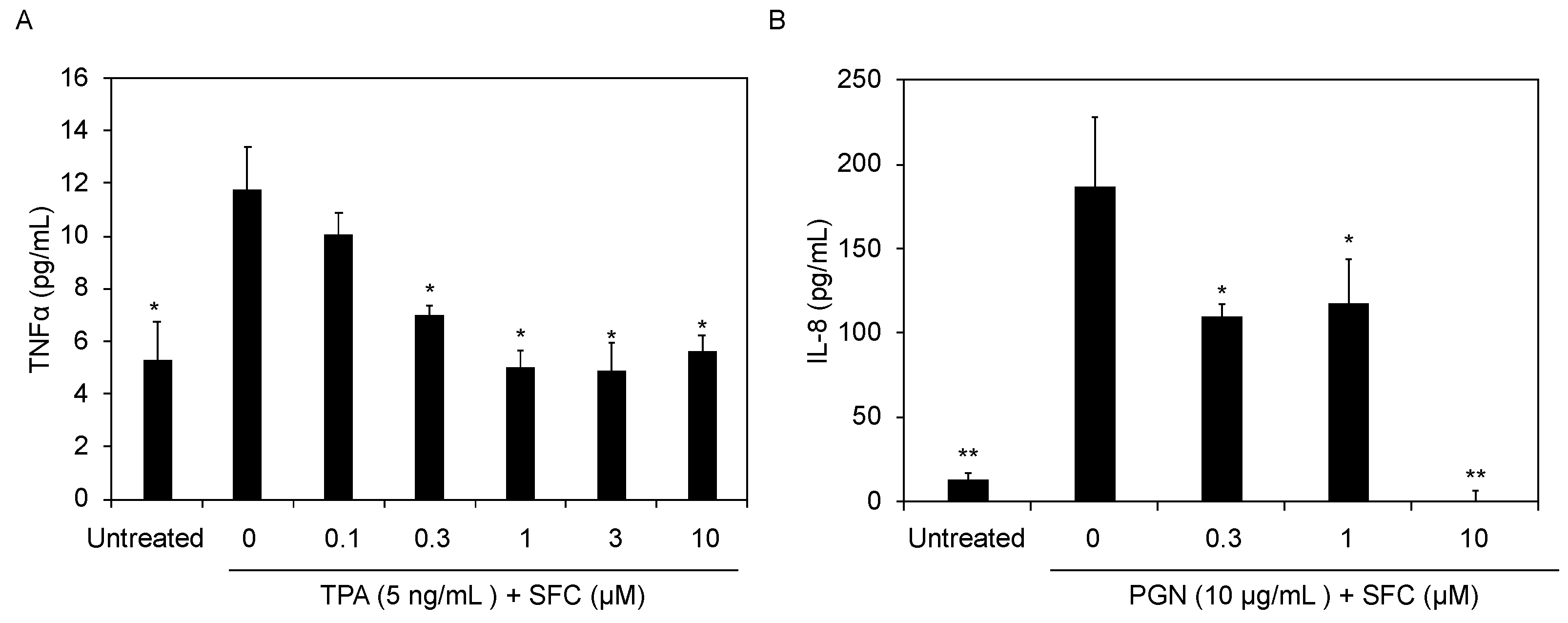
3.3. SFC Protects against Chemical and Bacteria-Induced Inflammation
3.4. SFC Reduces Wrinkles and Improves Several Other Skin Parameters in Human Subjects
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Marshall, C.J. Protein prenylation: A mediator of protein-protein interactions. Science 1993, 259, 1865–1866. [Google Scholar] [CrossRef]
- Kloog, Y.; Cox, A.D. Prenyl-binding domains: Potential targets for Ras inhibitors and anti-cancer drugs. Semin. Cancer Biol. 2004, 14, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Fogg, V.C.; Azpiazu, I.; Linder, M.E.; Smrcka, A.; Scarlata, S.; Gautam, N. Role of the gamma subunit prenyl moiety in G protein beta gamma complex interaction with phospholipase Cbeta. J. Biol. Chem. 2001, 276, 41797–41802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietrich, A.; Scheer, A.; Illenberger, D.; Kloog, Y.; Henis, Y.I.; Gierschik, P. Studies on G-protein alpha.betagamma heterotrimer formation reveal a putative S-prenyl-binding site in the alpha subunit. Biochem. J. 2003, 376 Pt 2, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Regazzi, R.; Sasaki, T.; Takahashi, K.; Jonas, J.C.; Volker, C.; Stock, J.B.; Takai, Y.; Wollheim, C.B. Prenylcysteine analogs mimicking the C-terminus of GTP-binding proteins stimulate exocytosis from permeabilized HIT-T15 cells: Comparison with the effect of Rab3AL peptide. Biochim. Biophys. Acta 1995, 1268, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Volker, C.; Miller, R.A.; McCleary, W.R.; Rao, A.; Poenie, M.; Backer, J.M.; Stock, J.B. Effects of farnesylcysteine analogs on protein carboxyl methylation and signal transduction. J. Biol. Chem. 1991, 266, 21515–21522. [Google Scholar] [CrossRef]
- Philips, M.R.; Pillinger, M.H.; Staud, R.; Volker, C.; Rosenfeld, M.G.; Weissmann, G.; Stock, J.B. Carboxyl methylation of Ras-related proteins during signal transduction in neutrophils. Science 1993, 259, 977–980. [Google Scholar] [CrossRef]
- Huzoor-Akbar; Wang, W.; Kornhauser, R.; Volker, C.; Stock, J.B. Protein prenylcysteine analog inhibits agonist-receptor-mediated signal transduction in human platelets. Proc. Natl. Acad. Sci. USA 1993, 90, 868–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, J.S.; Wolanin, P.M.; Gonzalez, A.V.; Fela, D.A.; Sarngadharan, G.; Rouzard, K.; Perez, E.; Stock, J.B.; Stock, M.B. Topical N-acetyl-S-farnesyl-L-cysteine inhibits mouse skin inflammation, and unlike dexamethasone, its effects are restricted to the application site. J. Investig. Dermatol 2008, 128, 643–654. [Google Scholar] [CrossRef] [Green Version]
- Fernández, J.R.; Webb, C.; Rouzard, K.; Voronkov, M.; Huber, K.L.; Stock, J.B.; Stock, M.; Gordon, J.S.; Perez, E. N-Acetylglutaminoyl-S-farnesyl-L-cysteine (SIG-1191): An anti-inflammatory molecule that increases the expression of the aquaglyceroporin, aquaporin-3, in human keratinocytes. Arch. Dermatol. Res. 2017, 309, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Fernandéz, J.R.; Rouzard, K.; Voronkov, M.; Huber, K.L.; Stock, J.B.; Stock, M.; Gordon, J.S.; Pérez, E. Anti-inflammatory and anti-bacterial properties of tetramethylhexadecenyl succinyl cysteine (TSC): A skin-protecting cosmetic functional ingredient. Int. J. Cosmet. Sci. 2015, 37, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.R.; Webb, C.; Rouzard, K.; Healy, J.; Tamura, M.; Voronkov, M.; Huber, K.L.; Stock, J.B.; Stock, M.; Gordon, J.S.; et al. SIG1459: A novel phytyl-cysteine derived TLR2 modulator with in vitro and clinical anti-acne activity. Exp. Dermatol. 2018, 27, 993–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandéz, J.R.; Rouzard, K.; Voronkov, M.; Feng, X.; Stock, J.B.; Stock, M.; Gordon, J.S.; Shroot, B.; Christensen, M.S.; Pérez, E. SIG1273: A new cosmetic functional ingredient to reduce blemishes and Propionibacterium acnes in acne prone skin. J. Cosmet. Dermatol. 2012, 11, 272–278. [Google Scholar] [CrossRef]
- Fernández, J.R.; Rouzard, K.; Voronkov, M.; Huber, K.L.; Webb, C.; Stock, J.B.; Stock, M.; Gordon, J.S.; Pérez, E. In vitro and clinical evaluation of SIG1273: A cosmetic functional ingredient with a broad spectrum of anti-aging and antioxidant activities. J. Cosmet. Dermatol. 2016, 15, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Gierschik, P.; Steisslinger, M.; Sidiropoulos, D.; Herrmann, E.; Jakobs, K.H. Dual Mg2+ control of formyl-peptide-receptor--G-protein interaction in HL 60 cells. Evidence that the low-agonist-affinity receptor interacts with and activates the G-protein. Eur. J. Biochem. 1989, 183, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.; Rotrosen, D.; Nagauker, O.; Leto, T.L.; Malech, H.L. Induction of the respiratory burst in HL-60 cells. Correlation of function and protein expression. J. Immunol. 1990, 145, 2595–2601. [Google Scholar]
- Dorward, D.A.; Lucas, C.D.; Chapman, G.B.; Haslett, C.; Dhaliwal, K.; Rossi, A.G. The role of formylated peptides and formyl peptide receptor 1 in governing neutrophil function during acute inflammation. Am. J. Pathol. 2015, 185, 1172–1184. [Google Scholar] [CrossRef] [Green Version]
- Su, S.B.; Gong, W.; Gao, J.L.; Shen, W.; Murphy, P.M.; Oppenheim, J.J.; Wang, J.M. A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J. Exp. Med. 1999, 189, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Marzano, A.V.; Ortega-Loayza, A.G.; Heath, M.; Morse, D.; Genovese, G.; Cugno, M. Mechanisms of Inflammation in Neutrophil-Mediated Skin Diseases. Front Immunol. 2019, 10, 1059. [Google Scholar] [CrossRef] [PubMed]
- Reinholz, M.; Ruzicka, T.; Schauber, J. Cathelicidin LL-37: An antimicrobial peptide with a role in inflammatory skin disease. Ann. Dermatol. 2012, 24, 126–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, J.; Lu, D.J.; Pérez-Sala, D.; Ma, Y.T.; Maddox, J.F.; Gilbert, B.A.; Badwey, J.A.; Rando, R.R. Farnesyl-L-cysteine analogs can inhibit or initiate superoxide release by human neutrophils. J. Biol. Chem. 1994, 269, 16837–16844. [Google Scholar] [CrossRef]
- Brennan, M.; Bhatti, H.; Nerusu, K.C.; Bhagavathula, N.; Kang, S.; Fisher, G.J.; Varani, J.; Voorhees, J.J. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem. Photobiol. 2003, 78, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef]
- Paz, M.L.; Ferrari, A.; Weill, F.S.; Leoni, J.; Maglio, D.H.G. Time-course evaluation and treatment of skin inflammatory immune response after ultraviolet B irradiation. Cytokine 2008, 44, 70–77. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.; Kim, J.E.; Cho, K.H.; Chung, J.H. Berberine inhibits TPA-induced MMP-9 and IL-6 expression in normal human keratinocytes. Phytomedicine 2008, 15, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Cataisson, C.; Pearson, A.J.; Tsien, M.Z.; Mascia, F.; Gao, J.-L.; Pastore, S.; Yuspa, S.H. CXCR2 ligands and G-CSF mediate PKCalpha-induced intraepidermal inflammation. J. Clin. Investig. 2006, 116, 2757–2766. [Google Scholar] [CrossRef]
- Miller, L.S. Toll-like receptors in skin. Adv. Dermatol. 2008, 24, 71–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bissett, D.L.; Oblong, J.E.; Berge, C.A. Niacinamide: AB vitamin that improves aging facial skin appearance. Dermatol. Surg. 2005, 31 Pt 2, 860–865, discussion 865. [Google Scholar] [CrossRef]
- Tan, E.W.; Pérez-Sala, D.; Cañada, F.J.; Rando, R.R. Identifying the recognition unit for G protein methylation. J. Biol. Chem. 1991, 266, 10719–10722. [Google Scholar] [CrossRef]
- Bhalla, K.; Hwang, B.J.; Choi, J.H.; Dewi, R.; Ou, L.; Mclenithan, J.; Twaddel, W.; Pozharski, E.; Stock, J.; Girnun, G.D. N-Acetylfarnesylcysteine is a novel class of peroxisome proliferator-activated receptor gamma ligand with partial and full agonist activity in vitro and in vivo. J. Biol. Chem. 2011, 286, 41626–41635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhami, K.; Lee, J.; Levin, L.; Moquete, R.; Stohl, L.L.; Ding, W.; Wong, J.; Schierl, M.; Zhou, X.K.; Gordon, J.S.; et al. N-acetyl-S-farnesyl-l-cysteine suppresses chemokine production by human dermal microvascular endothelial cells. Exp. Dermatol. 2012, 21, 700–705. [Google Scholar] [CrossRef]
- Johnson, B.Z.; Stevenson, A.W.; Prêle, C.M.; Fear, M.W.; Wood, F.M. The Role of IL-6 in Skin Fibrosis and Cutaneous Wound Healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Hwang, I.; Lee, E.; Cho, H.J.; Ryu, J.H.; Kim, T.G.; Yu, J.W. Antimicrobial Peptide LL-37 Drives Rosacea-Like Skin Inflammation in an NLRP3-Dependent Manner. J. Investig. Dermatol. 2021, 131, 2885–2894. [Google Scholar] [CrossRef] [PubMed]
- Morizane, S.; Gallo, R.L. Antimicrobial peptides in the pathogenesis of psoriasis. J. Dermatol. 2012, 39, 225–230. [Google Scholar] [CrossRef]
- Kim, J.; Ochoa, M.T.; Krutzik, S.R.; Takeuchi, O.; Uematsu, S.; Legaspi, A.J.; Brightbill, H.D.; Holland, D.; Cunliffe, W.J.; Akira, S.; et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J. Immunol. 2002, 169, 1535–1541. [Google Scholar] [CrossRef] [Green Version]
- Kaesler, S.; Volz, T.; Skabytska, Y.; Köberle, M.; Hein, U.; Chen, K.M.; Guenova, E.; Wölbing, F.; Röcken, M.; Biedermann, T. Toll-like receptor 2 ligands promote chronic atopic dermatitis through IL-4-mediated suppression of IL-10. J. Allergy Clin. Immunol. 2014, 134, 92–99. [Google Scholar] [CrossRef]
- Yamasaki, K.; Kanada, K.; Macleod, D.T.; Borkowski, A.W.; Morizane, S.; Nakatsuji, T.; Cogen, A.L.; Gallo, R.L. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J. Investig. Dermatol. 2011, 131, 688–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.Y.; Bi, Z.G. UVB-irradiated human keratinocytes and interleukin-1alpha indirectly increase MAP kinase/AP-1 activation and MMP-1 production in UVA-irradiated dermal fibroblasts. Chin. Med. J. 2006, 119, 827–831. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Grade = 0 | Grade = 9 |
|---|---|---|
| Texture (visual) | Smooth, even-looking skin texture, no roughness | Pronounced, extensive visible skin roughness |
| Hydration (visual) | Skin appears hydrated and full/rounded w/no scaling and/or flaking | Skin appears dehydrated and flattened/shriveled w/severe scaling and/or flaking |
| Radiance, luminosity, brightness | Radiant, luminous, or glowing appearance | Dull/matte and/or sallow skin appearance |
| Overall appearance of skin condition (healthy) | Excellent, healthy skin tone free from skin abnormalities | Poor, unhealthy skin tone; extensive skin abnormalities |
| Texture (tactile) | Smooth, even-feeling skin texture | Rough, uneven-feeling skin texture |
| Firmness (tactile) | Skin feels thick, dense, and firm | Skin feels thin and loose |
| Compound | Inducer | Pro-MMP1 IC50 (nM) * | Inducer | IL-6 IC50 (nM) * | TNF-α IC50 (nM) * |
|---|---|---|---|---|---|
| SFC | UVA | 0.01 | UVB | 0.01 | 0.1 |
| Ascorbic acid | >1000 | 100 | 100 | ||
| α-tocopherol | >1000 | 100 | 100 |
| Endpoint | Treatment | Time Point | Subject Improved % | p Value * |
|---|---|---|---|---|
| Texture (visual) | SFC 1% | Week 8 | 6.5% | 1.000 |
| Week 12 | 38.7% | 0.003 | ||
| Niacinamide 5% | Week 8 | 5.6% | 1.000 | |
| Week 12 | 38.9% | 0.070 | ||
| Vehicle | Week 8 | 2.0% | 1.000 | |
| Week 12 | 16.3% | 0.388 | ||
| Hydration (visual) | SFC 1% | Post-application | 90.3% | <0.001 |
| Week 8 | 41.9% | 0.002 | ||
| Week 12 | 80.6% | <0.001 | ||
| Niacinamide 5% | Post-application | 83.3% | <0.001 | |
| Week 8 | 33.3% | 0.289 | ||
| Week 12 | 66.7% | 0.010 | ||
| Vehicle | Post-application | 87.8% | <0.001 | |
| Week 8 | 32.7% | 0.016 | ||
| Week 12 | 59.2% | <0.001 | ||
| Radiance, luminosity, brightness | SFC 1% | Post-application | 45.2% | <0.001 |
| Week 8 | 19.4% | 0.125 | ||
| Week 12 | 51.6% | <0.001 | ||
| Niacinamide 5% | Post-application | 44.4% | 0.008 | |
| Week 8 | 0% | - | ||
| Week 12 | 22.4% | 0.125 | ||
| Vehicle | Post-application | 40.8% | <0.001 | |
| Week 8 | 4.1% | 1.000 | ||
| Week 12 | 24.5% | 0.003 | ||
| Overall appearance of skin condition (healthy) | SFC 1% | Post-application | 32.3% | 0.002 |
| Week 8 | 3.2% | 1.000 | ||
| Week 12 | 22.6% | 0.070 | ||
| Niacinamide 5% | Post-application | 33.3% | 0.031 | |
| Week 8 | 0% | - | ||
| Week 12 | 22.2% | 0.125 | ||
| Vehicle | Post-application | 30.6% | <0.001 | |
| Week 8 | 0% | 1.000 | ||
| Week 12 | 8.2% | 0.375 | ||
| Texture (tactile) | SFC 1% | Post-application | 90.3% | <0.001 |
| Week 8 | 80.6% | <0.001 | ||
| Week 12 | 93.5% | <0.001 | ||
| Niacinamide 5% | Post-application | 100.0% | 0.031 | |
| Week 8 | 66.7% | 0.004 | ||
| Week 12 | 72.2% | <0.001 | ||
| Vehicle | Post-application | 95.9% | <0.001 | |
| Week 8 | 71.4% | <0.001 | ||
| Week 12 | 75.5% | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, J.R.; Rouzard, K.; Fitzgerald, C.; Healy, J.; Tamura, M.; Voronkov, M.; Stock, J.B.; Stock, M.; Pérez, E. N-Succinyl-S-Farnesyl-L-Cysteine (SFC): A Novel Isoprenylcysteine Analog with In Vitro Anti-Inflammatory Activity and Clinical Skin Protecting Properties. Cosmetics 2021, 8, 110. https://doi.org/10.3390/cosmetics8040110
Fernández JR, Rouzard K, Fitzgerald C, Healy J, Tamura M, Voronkov M, Stock JB, Stock M, Pérez E. N-Succinyl-S-Farnesyl-L-Cysteine (SFC): A Novel Isoprenylcysteine Analog with In Vitro Anti-Inflammatory Activity and Clinical Skin Protecting Properties. Cosmetics. 2021; 8(4):110. https://doi.org/10.3390/cosmetics8040110
Chicago/Turabian StyleFernández, José R., Karl Rouzard, Corey Fitzgerald, Jason Healy, Masanori Tamura, Michael Voronkov, Jeffry B. Stock, Maxwell Stock, and Eduardo Pérez. 2021. "N-Succinyl-S-Farnesyl-L-Cysteine (SFC): A Novel Isoprenylcysteine Analog with In Vitro Anti-Inflammatory Activity and Clinical Skin Protecting Properties" Cosmetics 8, no. 4: 110. https://doi.org/10.3390/cosmetics8040110
APA StyleFernández, J. R., Rouzard, K., Fitzgerald, C., Healy, J., Tamura, M., Voronkov, M., Stock, J. B., Stock, M., & Pérez, E. (2021). N-Succinyl-S-Farnesyl-L-Cysteine (SFC): A Novel Isoprenylcysteine Analog with In Vitro Anti-Inflammatory Activity and Clinical Skin Protecting Properties. Cosmetics, 8(4), 110. https://doi.org/10.3390/cosmetics8040110







