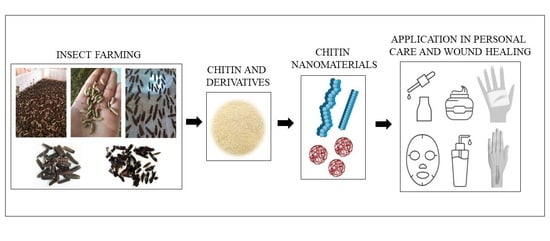
Insect Chitin-Based Nanomaterials for Innovative Cosmetics and Cosmeceuticals
Abstract
1. Introduction
2. Chitin
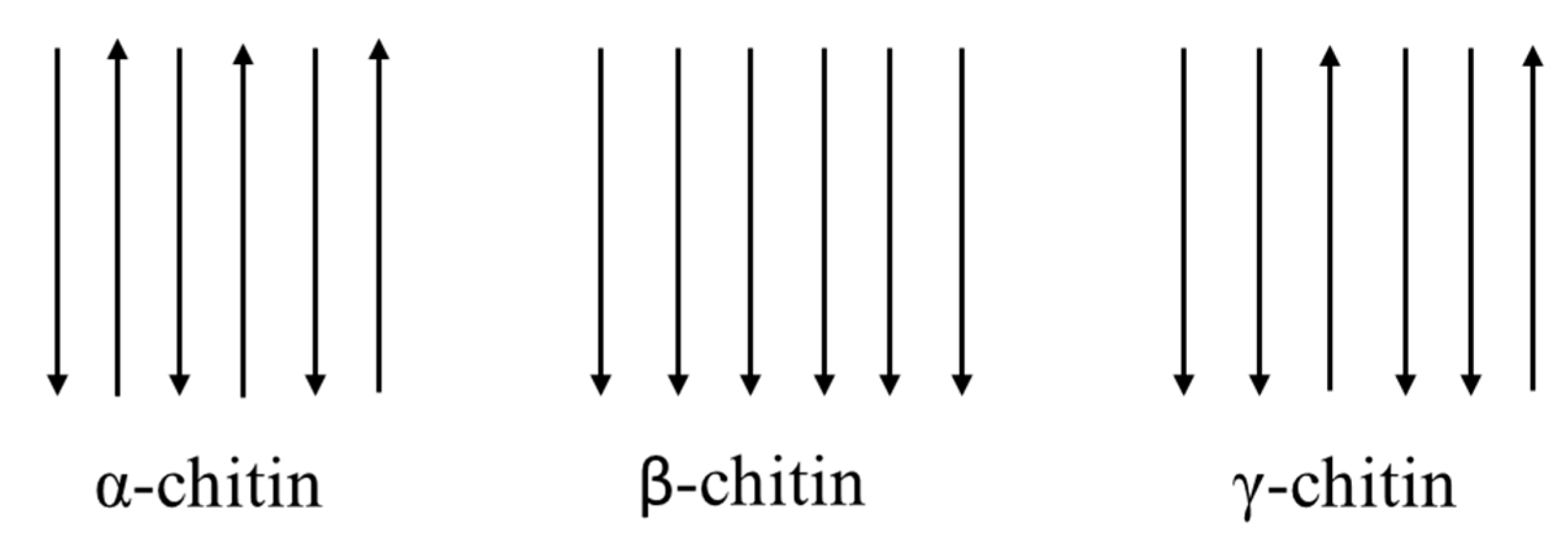
2.1. Structure and Properties
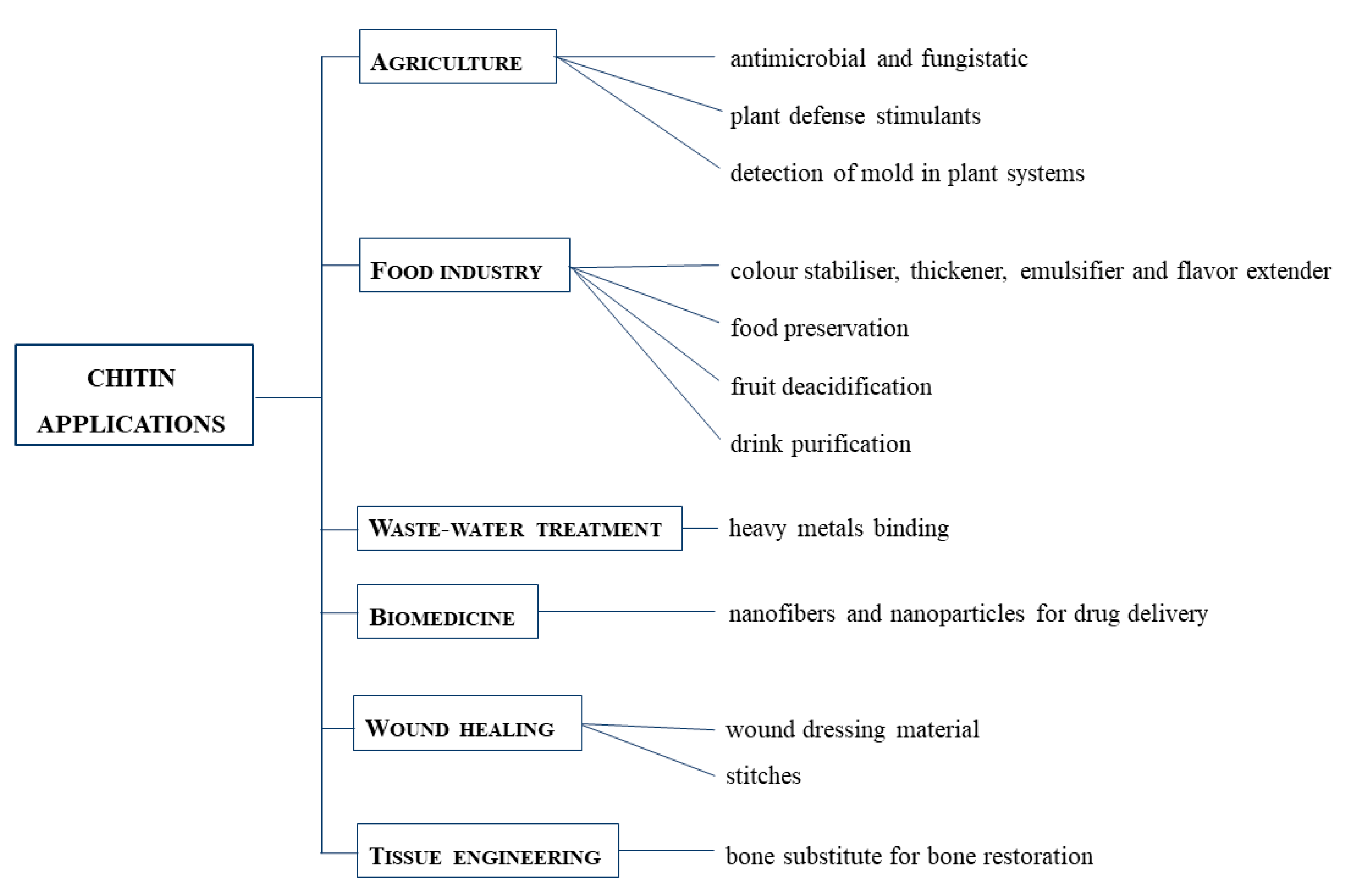
2.2. Chitin Fields of Application
- Tissue engineering, as a bone substitute for bone restoration through modification with hydroxyapatite or bioactive glass ceramics [43].
- Wound dressing, thanks to the antimicrobial, blood clotting, swelling, cell attachment and cytocompatibility aspects of chitin-based composites [44].
- Wastewater treatments, thanks to their non-toxicity. They can bind to water pollutants, especially heavy metals [45].
2.3. Chitin Sources
3. Insect-Based Chitin
3.1. Chitin Extraction from Insects
3.2. Insect Chitin Derivatives
4. Application of Chitin and Its Derivatives in Cosmetics and Cosmeceuticals
5. Chitin-Based Nanomaterials in Personal Care
5.1. Innovation of Chitin Nanofibers and Nanofibrils
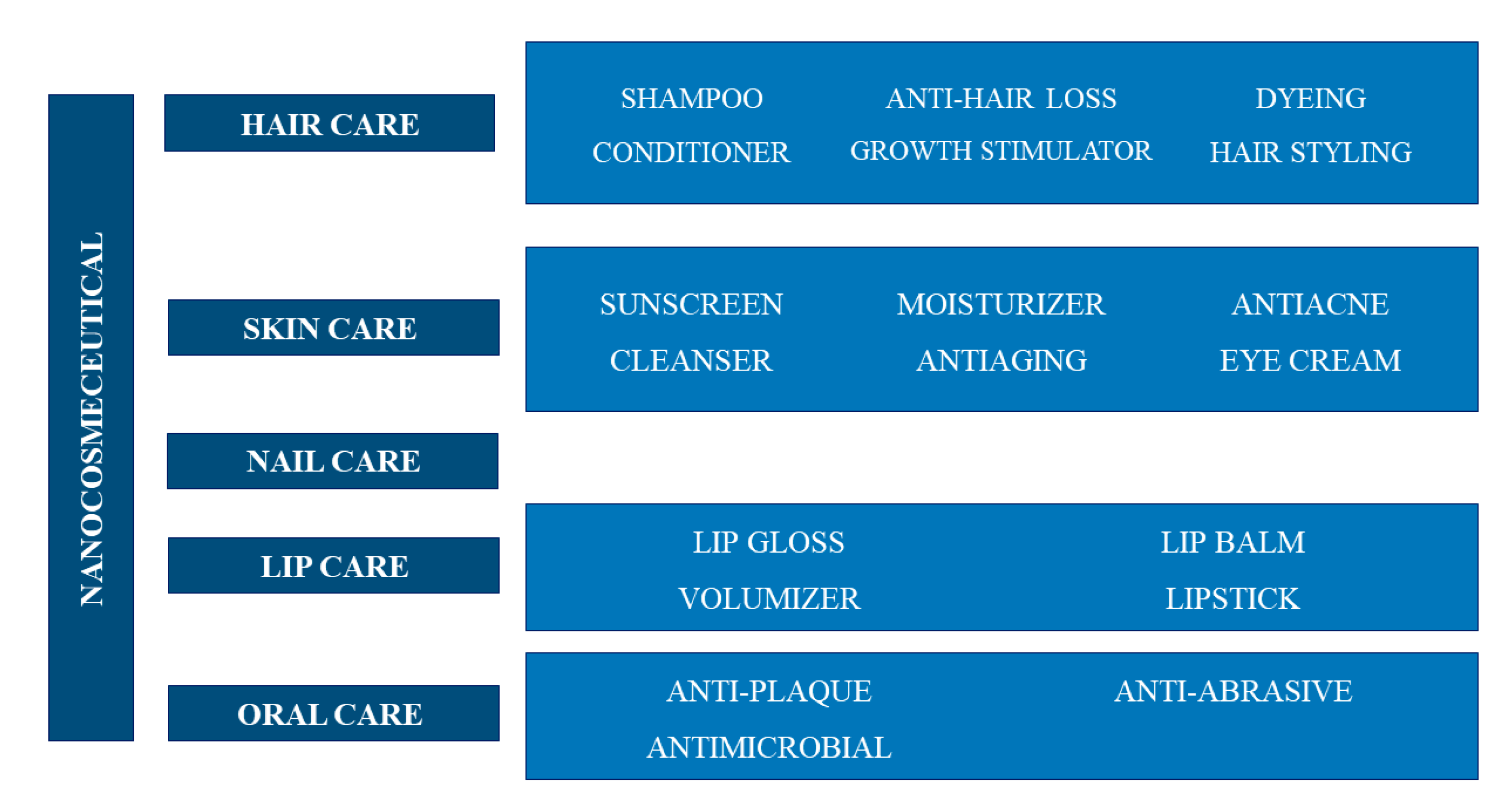
5.2. Cosmetic and Cosmeceutical Applications of Chitin-Based Nanomaterial
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morganti, P.; del Ciotto, P.; Morganti, G.; Fabien-Soule, V. Application of Chitin Nanofibrils and Collagen of Marine Origin as Bioactive Ingredients. In Marine Cosmeceuticals: Trends and Prospects; Kim, S.K., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 267–289. [Google Scholar]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.D.L.L.; Caballero, A.H. Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef]
- Regulation (CE) No. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off. J. Eur. Union 2009, 342, 59–209.
- Elsner, P.; Maibach, H.I. (Eds.) . Cosmeceuticals: Drugs vs. Cosmetics; Marcel Dekker, Inc.: New York, NY, USA, 2000; Volume 23. [Google Scholar]
- Morganti, P. Cosmeceuticals. Clin. Dermatol. 2008, 26, 317. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 2018, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Preparation of chitin nanofibers with a uniform width as α-chitin from crab shells. Biomacromolecules 2009, 10, 1584–1588. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Simple preparation method of chitin nanofibers with a uniform width of 10–20 nm from prawn shell under neutral conditions. Carbohydr. Polym. 2011, 84, 762–764. [Google Scholar] [CrossRef]
- Kamble, P.; Sadarani, B.; Majumdar, A.; Bhullar, S. Nanofiber based drug delivery systems for skin: A promising therapeutic approach. J. Drug Deliv. Sci. Technol. 2017, 41, 124–133. [Google Scholar] [CrossRef]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and chitosans: Characteristics, eco-friendly processes, and applications in cosmetic science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Crini, G. Historical review on chitin and chitosan biopolymers. Environ. Chem. Lett. 2019, 17, 1623–1643. [Google Scholar] [CrossRef]
- Khoushab, F.; Yamabhai, M. Chitin Research Revisited. Mar. Drugs 2010, 8, 1988–2012. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Dutta, P.K.; Dutta, J.; Tripathi, V.S. Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Goy, R.C.; De Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polimeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Austin, P.R. Chitin solutions and purification of chitin. Methods Enzym. 1988, 161, 403–407. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Singh, S.K. Solubility of lignin and chitin in ionic liquids and their biomedical applications. Int. J. Biol. Macromol. 2019, 132, 265–277. [Google Scholar] [CrossRef]
- Jaworska, M.M.; Kozlecki, T.; Gorak, A. Review of the application of ionic liquids as solvents for chitin. J. Polym. Eng. 2012, 32, 67–69. [Google Scholar] [CrossRef]
- Roy, J.C.; Salaün, F.; Giraud, S.; Ferri, A.; Chen, G.; Guan, J. Solubility of Chitin: Solvents, Solution Behaviors and Their Related Mechanisms. Solubility Polysacch. 2017, 10. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, Á. Functional Characterization of Chitin and Chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Rasul, R.M.; Tamilarasi Muniandy, M.; Zakaria, Z.; Shah, K.; Chee, C.F.; Dabbagh, A.; Rahman, N.A.; Wong, T.W. A review on chitosan and its development as pulmonary particulate anti-infective and anti-cancer drug carriers. Carbohydr. Polym. 2020, 250, 116800. [Google Scholar] [CrossRef]
- Vaz, J.M.; Taketa, T.B.; Hernandez-Montelongo, J.; Chevallier, P.; Cotta, M.A.; Mantovani, D.; Beppu, M.M. Antibacterial properties of chitosan-based coatings are affected by spacer-length and molecular weight. Appl. Surf. Sci. 2018, 445, 478–487. [Google Scholar] [CrossRef]
- De Queiroz Antonino, R.S.C.M.; Lia Fook, B.R.P.; De Oliveira Lima, V.A.; De Farias Rached, R.Í.; Lima, E.P.N.; Da Silva Lima, R.J.; Peniche Covas, C.A.; Lia Fook, M.V. Preparation and Characterization of Chitosan Obtained from Shells of Shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef]
- Hossain, M.; Iqbal, A. Production and characterization of chitosan from shrimp waste. J. Bangladesh Agric. Univ. 2014, 12, 153–160. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Jang, M.K.; Kong, B.G.; Il Jeong, Y.; Lee, C.H.; Nah, J.W. Physicochemical characterization of α-chitin, β-chitin, and γ-chitin separated from natural resources. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3423–3432. [Google Scholar] [CrossRef]
- Minke, R.; Blackwell, J. The structure of α-chitin. J. Mol. Biol. 1978, 120, 167–181. [Google Scholar] [CrossRef]
- Pighinelli, L. Methods of Chitin Production a Short Review. Am. J. Biomed. Sci. Res. 2019, 3, 307–314. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kawakami, K.; Miyatake, K.; Morimoto, M.; Shigemasa, Y.; Minami, S. Analgesic effects of chitin and chitosan. Carbohydr. Polym. 2002, 49, 249–252. [Google Scholar] [CrossRef]
- Azuma, K.; Nishihara, M.; Shimizu, H.; Itoh, Y.; Takashima, O.; Osaki, T.; Itoh, N.; Imagawa, T.; Murahata, Y.; Tsuka, T.; et al. Biological adhesive based on carboxymethyl chitin derivatives and chitin nanofibers. Biomaterials 2015, 42, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Nair, S.V.; Tokura, S.; Tamura, H.; Selvamurugan, N. Novel carboxymethyl derivatives of chitin and chitosan materials and their biomedical applications. Prog. Mater. Sci. 2010, 55, 675–709. [Google Scholar] [CrossRef]
- Barikani, M.; Oliaei, E.; Seddiqi, H.; Honarkar, H. Preparation and application of chitin and its derivatives: A review. Iran. Polym. J. 2014, 23, 307–326. [Google Scholar] [CrossRef]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in plant protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Da Silva, S.B.; De Souza, D.; Lacerda, L.D. Food applications of chitosan and its derivatives. Chitin Chitosan Prop. Appl. 2010, 55, 675–709. [Google Scholar]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as A Preservative for Fruits and Vegetables: A Review on Chemistry and Antimicrobial Properties. Chitosan A Preserv. Fruits Veg. A Rev. Chem. Antimicrob. Prop. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Al-Manhel, A.J.; Al-Hilphy, A.R.S.; Niamah, A.K. Extraction of chitosan, characterisation and its use for water purification. J. Saudi Soc. Agric. Sci. 2018, 17, 186–190. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Yang, Y.M.; Hu, W.; Wang, X.D.; Gu, X.S. The controlling biodegradation of chitosan fibers by N-acetylation in vitro and in vivo. J. Mater. Sci. Mater. Med. 2007, 18, 2117–2121. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef] [PubMed]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a wound dressing starting material: Antimicrobial properties and mode of action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed]
- Sarode, S.; Upadhyay, P.; Khosa, M.A.; Mak, T.; Shakir, A.; Song, S.; Ullah, A. Overview of wastewater treatment methods with special focus on biopolymer chitin-chitosan. Int. J. Biol. Macromol. 2019, 121, 1086–1100. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Gallert, C.; Winter, J. Chitin purification from shrimp wastes by microbial deproteination and decalcification. Appl. Microbiol. Biotechnol. 2008, 79, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Gortari, M.C.; Hours, R.A. Biotechnological processes for chitin recovery out of crustacean waste: A mini-review. Electron. J. Biotechnol. 2013, 16, 14. [Google Scholar]
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef]
- Pittman, S.J.; McAlpine, C.A. Movements of marine fish and decapod crustaceans: Process, theory and application. Adv. Mar. Biol. 2003, 44, 205–294. [Google Scholar] [CrossRef]
- Gillett, R. Global study of shrimp fisheries. In Food and Agriculture Organization of the United Nations Fisheries; Technical Paper No. 475; FAO: Rome, Italy, 2008; p. 331. [Google Scholar]
- Abidin, N.A.Z.; Kormin, F.; Abidin, N.A.Z.; Anuar, N.A.F.M.; Bakar, M.F.A. The potential of insects as alternative sources of chitin: An overview on the chemical method of extraction from various sources. Int. J. Mol. Sci. 2020, 21, 1–25. [Google Scholar]
- Philibert, T.; Lee, B.H.; Fabien, N. Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337. [Google Scholar] [CrossRef]
- Abdel-Gawad, K.M.; Hifney, A.F.; Fawzy, M.A.; Gomaa, M. Technology optimization of chitosan production from Aspergillus niger biomass and its functional activities. Food Hydrocoll. 2017, 63, 593–601. [Google Scholar] [CrossRef]
- Waśko, A.; Bulak, P.; Polak-Berecka, M.; Nowak, K.; Polakowski, C.; Bieganowski, A. The first report of the physicochemical structure of chitin isolated from Hermetia illucens. Int. J. Biol. Macromol. 2016, 92, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Nemtsev, S.V.; Zueva, O.Y.; Khismatullin, M.R.; Albulov, A.I.; Varlamov, V.P. Isolation of chitin and chitosan from honeybees. Appl. Biochem. Microbiol. 2004, 40, 39–43. [Google Scholar] [CrossRef]
- Zhang, M.; Haga, A.; Sekiguchi, H.; Hirano, S. Structure of insect chitin isolated from beetle larva cuticle and silkworm (Bombyx mori) pupa exuvia. Int. J. Biol. Macromol. 2000, 27, 99–105. [Google Scholar] [CrossRef]
- Badawy, R.; Mohamed, H. Chitin extration, Composition of Different Six Insect Species and Their Comparable Characteristics with That of the Shrimp. J. Am. Sci. 2015, 11, 127. [Google Scholar]
- Meky, N.; Badawy, R.; Mohamed, H.; Samir, S. Extraction of chitin from six different insect species as alternative source for biological applications. Afr. J. Biol. Sci. 2017, 13, 197–205. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, Y.; Han, Q.; Ji, L.; Zhang, H.; Fei, Z.; Wang, Y. Comparison of the physicochemical, rheological, and morphologic properties of chitosan from four insects. Carbohydr. Polym. 2019, 209, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Yu, H.; Zhang, M.; Yang, Y.; Zhang, G. Physicochemical properties and antioxidant activity of chitosan from the blowfly Chrysomya megacephala larvae. Int. J. Biol. Macromol. 2013, 60, 347–354. [Google Scholar] [CrossRef]
- Ai, H.; Wang, F.; Yang, Q.; Zhu, F.; Lei, C. Preparation and biological activities of chitosan from the larvae of housefly, Musca domestica. Carbohydr. Polym. 2008, 72, 419–423. [Google Scholar] [CrossRef]
- Cortes Ortiz, J.A.; Ruiz, A.T.; Morales-Ramos, J.A.; Thomas, M.; Rojas, M.G.; Tomberlin, J.K.; Yi, L.; Han, R.; Giroud, L.; Jullien, R.L. Insect Mass Production Technologies. In Insects as Sustainable Food Ingredients; Elsevier: Amsterdam, The Netherlands, 2016; pp. 153–201. [Google Scholar]
- Ganguly, S.; Neog, P.; Gogoi, M.; Bordoloi, P.L.; Para, P.A. Edible Insects as Sources of Novel Bioactive Compounds. In Recent Research Trends in Veterinary Sciences and Animal Husbandry; AkiNik Publications: Delhi, India, 2018; pp. 55–69. [Google Scholar]
- Moretta, A.; Salvia, R.; Scieuzo, C.; Di Somma, A.; Vogel, H.; Pucci, P.; Sgambato, A.; Wolff, M.; Falabella, P. A bioinformatic study of antimicrobial peptides identified in the Black Soldier Fly (BSF) Hermetia illucens (Diptera: Stratiomyidae). Sci. Rep. 2020, 10, 16875. [Google Scholar] [CrossRef]
- Manniello, M.D.; Moretta, A.; Salvia, R.; Scieuzo, C.; Lucchetti, D.; Vogel, H.; Sgambato, A.; Falabella, P. Insect antimicrobial peptides: Potential weapons to counteract the antibiotic resistance. Cell. Mol. Life Sci. 2021, 1, 3. [Google Scholar]
- Davies, R.G. Insect structure and function. In Outlines of Entomology; Springer: Dordrecht, The Netherlands, 1988; pp. 7–96. [Google Scholar] [CrossRef]
- Gunderson, S.; Schiavone, R. The insect exoskeleton: A natural structural composite. JOM 1989, 41, 60–63. [Google Scholar] [CrossRef]
- Vincent, J.F.V.; Wegst, U.G.K. Design and mechanical properties of insect cuticle. Arthropod Struct. Dev. 2004, 33, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Chandran, R.; Williams, L.; Hung, A.; Nowlin, K.; La Jeunesse, D. SEM characterization of anatomical variation in chitin organization in insect and arthropod cuticles. Micron 2016, 82, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Jantzen da Silva Lucas, A.; Menegon de Oliveira, L.; da Rocha, M.; Prentice, C. Edible insects: An alternative of nutritional, functional and bioactive compounds. Food Chem. 2020, 311, 126022. [Google Scholar] [CrossRef]
- Derrien, C.; Boccuni, A. Current status of the insect producing industry in Europe. In Edible Insects in Sustainable Food Systems; Springer: Cham, Switzerland, 2018; pp. 471–479. [Google Scholar]
- Scala, A.; Cammack, J.A.; Salvia, R.; Scieuzo, C.; Franco, A.; Bufo, S.A.; Tomberlin, J.K.; Falabella, P. Rearing substrate impacts growth and macronutrient composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) larvae produced at an industrial scale. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Bava, L.; Jucker, C.; Gislon, G.; Lupi, D.; Savoldelli, S.; Zucali, M.; Colombini, S. Rearing of hermetia illucens on different organic by-products: Influence on growth, waste reduction, and environmental impact. Animals 2019, 9, 289. [Google Scholar] [CrossRef]
- Jucker, C.; Lupi, D.; Moore, C.D.; Leonardi, M.G.; Savoldelli, S. Nutrient recapture from insect farm waste: Bioconversion with Hermetia illucens (L.)(Diptera: Stratiomyidae). Sustainability 2020, 12, 362. [Google Scholar] [CrossRef]
- Schmitt, E.; de Vries, W. Potential benefits of using Hermetia illucens frass as a soil amendment on food production and for environmental impact reduction. Curr. Opin. Green Sustain. Chem. 2020, 25, 100335. [Google Scholar] [CrossRef]
- Mohammed, M.H.; Williams, P.A.; Tverezovskaya, O. Extraction of chitin from prawn shells and conversion to low molecular mass chitosan. Food Hydrocoll. 2013, 31, 166–171. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Rath, P.; Sri Hari Kumar, A.; Tiwari, T.N. Extraction and characterization of chitin and chitosan from fishery waste by chemical method. Environ. Technol. Innov. 2015, 3, 77–85. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Kaya, M.; Lelešius, E.; Nagrockaite, R.; Sargin, I.; Arslan, G.; Mol, A.; Baran, T.; Can, E.; Bitim, B. Differentiations of Chitin content and surface morphologies of chitins extracted from male and female grasshopper species. PLoS ONE 2015, 10, e115531. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Bulut, E.; Mujtaba, M.; Sivickis, K.; Sargin, I.; Akyuz, B.; Erdogan, S. Gender Influences Differentiation of Chitin Among Body Parts. Arch. Insect Biochem. Physiol. 2016, 93, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Baran, T.; Erdoğan, S.; Menteş, A.; Aşan Özüsağlam, M.; Çakmak, Y.S. Physicochemical comparison of chitin and chitosan obtained from larvae and adult Colorado potato beetle (Leptinotarsa decemlineata). Mater. Sci. Eng. C 2014, 45, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.; Kaya, M. High similarity in physicochemical properties of chitin and chitosan from nymphs and adults of a grasshopper. Int. J. Biol. Macromol. 2016, 89, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Tharanathan, R.N.; Kittur, F.S. Chitin—The Undisputed Biomolecule of Great Potential. Crit. Rev. Food Sci. Nutr. 2003, 43, 61–87. [Google Scholar] [CrossRef]
- Thirunavukkarasu, N.; Shanmugam, A. Extraction of Chitin and Chitosan From Mud Crab Scylla Tranquebarica (Fabricius, 1798). Int. J. Appl. Bio-Eng. 2009, 3, 31–33. [Google Scholar] [CrossRef]
- Kaya, M.; Baublys, V.; Can, E.; Šatkauskienė, I.; Bitim, B.; Tubelytė, V.; Baran, T. Comparison of physicochemical properties of chitins isolated from an insect (Melolontha melolontha) and a crustacean species (Oniscus asellus). Zoomorphology 2014, 133, 285–293. [Google Scholar] [CrossRef]
- Oduor-Odeto, P.M.; Struszezyk, M.H.; Peter, M.G. Characterisation of Chitosan from Blowfly Larvae and Some Crustacean Species from Kenyan Marin Waters Prepared Under Different Conditions. West. Indian Ocean J. Mar. Sci. 2007, 4, 99–108. [Google Scholar] [CrossRef][Green Version]
- Bolat, Y.; Bilgin, Ş.; Günlü, A.; Izci, L.; Koca, S.B.; Çetinkaya, S.; Koca, H.U. Chitin-Chitosan Yield of Freshwater Crab (Potamon potamios, Olivier 1804) Shell. Pak. Vet. J. 2010, 30, 227–231. [Google Scholar]
- Liu, S.; Sun, J.; Yu, L.; Zhang, C.; Bi, J.; Zhu, F.; Qu, M.; Jiang, C.; Yang, Q. Extraction and characterization of chitin from the beetle Holotrichia parallela motschulsky. Molecules 2012, 17, 4604–4611. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Baran, T.; Asan-Ozusaglam, M.; Cakmak, Y.S.; Tozak, K.O.; Mol, A.; Mentes, A.; Sezen, G. Extraction and characterization of chitin and chitosan with antimicrobial and antioxidant activities from cosmopolitan Orthoptera species (Insecta). Biotechnol. Bioprocess Eng. 2015, 20, 168–179. [Google Scholar] [CrossRef]
- Kaya, M.; Bağriaçik, N.; Seyyar, O.; Baran, T. Comparison of chitin structures derived from three common wasp species (vespa crabro linnaeus, 1758, vespa orientalis linnaeus, 1771 and vespula germanica (FABRICIUS, 1793)). Arch. Insect Biochem. Physiol. 2015, 89, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Draczynski, Z. Honeybee corpses as an available source of chitin. J. Appl. Polym. Sci. 2008, 109, 1974–1981. [Google Scholar] [CrossRef]
- Sajomsang, W.; Gonil, P. Preparation and characterization of α-chitin from cicada sloughs. Mater. Sci. Eng. C 2010, 30, 357–363. [Google Scholar] [CrossRef]
- Ibitoye, E.B.; Lokman, I.H.; Hezmee, M.N.M.; Goh, Y.M.; Zuki, A.B.Z.; Jimoh, A.A. Extraction and physicochemical characterization of chitin and chitosan isolated from house cricket. Biomed. Mater. 2018, 13, 25009. [Google Scholar] [CrossRef]
- Majtán, J.; Bíliková, K.; Markovič, O.; Gróf, J.; Kogan, G.; Šimúth, J. Isolation and characterization of chitin from bumblebee (Bombus terrestris). Int. J. Biol. Macromol. 2007, 40, 237–241. [Google Scholar] [CrossRef]
- Kaya, M.; Mujtaba, M.; Bulut, E.; Akyuz, B.; Zelencova, L.; Sofi, K. Fluctuation in physicochemical properties of chitins extracted from different body parts of honeybee. Carbohydr. Polym. 2015, 132, 9–16. [Google Scholar] [CrossRef]
- Kaya, M.; Sofi, K.; Sargin, I.; Mujtaba, M. Changes in physicochemical properties of chitin at developmental stages (larvae, pupa and adult) of Vespa crabro (wasp). Carbohydr. Polym. 2016, 145, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Yeul, V.S.; Rayalu, S.S. Unprecedented Chitin and Chitosan: A Chemical Overview. J. Polym. Environ. 2013, 21, 606–614. [Google Scholar] [CrossRef]
- Win, N.N.; Stevens, W.F. Shrimp chitin as substrate for fungal chitin deacetylase. Appl. Microbiol. Biotechnol. 2001, 57, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Wattjes, J.; Niehues, A.; Cord-Landwehr, S.; Hoßbach, J.; David, L.; Delair, T.; Moerschbacher, B.M. Enzymatic Production and Enzymatic-Mass Spectrometric Fingerprinting Analysis of Chitosan Polymers with Different Nonrandom Patterns of Acetylation. J. Am. Chem. Soc. 2019, 141, 3137–3145. [Google Scholar] [CrossRef]
- Martinou, A.; Kafetzopoulos, D.; Bouriotis, V. Chitin deacetylation by enzymatic means: Monitoring of deacetylation processes. Carbohydr. Res. 1995, 273, 235–242. [Google Scholar] [CrossRef]
- Cortizo, M.S.; Berghoff, C.F.; Alessandrini, J.L. Characterization of chitin from Illex argentinus squid pen. Carbohydr. Polym. 2008, 74, 10–15. [Google Scholar] [CrossRef]
- De Castro, R.J.S.; Ohara, A.; Aguilar, J.G.D.S.; Domingues, M.A.F. Nutritional, functional and biological properties of insect proteins: Processes for obtaining, consumption and future challenges. Trends Food Sci. Technol. 2018, 76, 82–89. [Google Scholar] [CrossRef]
- Jaworska, M.; Sakurai, K.; Gaudon, P.; Guibal, E. Influence of chitosan characteristics on polymer properties. I: Crystallographic properties. Polym. Int. 2003, 52, 198–205. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Zivanovic, S.; Basurto, C.C.; Chi, S.; Davidson, P.M.; Weiss, J. Molecular weight of chitosan influences antimicrobial activity in oil-in-water emulsions. J. Food Prot. 2004, 67, 952–959. [Google Scholar] [CrossRef]
- Vishu Kumar, A.B.; Varadaraj, M.C.; Gowda, L.R.; Tharanathan, R.N. Characterization of chito-oligosaccharides prepared by chitosanolysis with the aid of papain and Pronase, and their bactericidal action against Bacillus cereus and Escherichia coli. Biochem. J. 2005, 391, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.W.; Song, Y.S.; Han, Y.S.; Jo, Y.H.; Choi, M.H.; Park, Y.K.; Kang, S.H.; Kim, S.A.; Choi, C.; Jung, W.J. Production of chitin and chitosan from the exoskeleton of adult two-spotted field crickets (Gryllus bimaculatus). Entomol. Res. 2017, 47, 279–285. [Google Scholar] [CrossRef]
- Lohani, A.; Verma, A.; Joshi, H.; Yadav, N.; Karki, N. Nanotechnology-Based Cosmeceuticals. ISRN Dermatol. 2014, 1–14. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G. Chitin nanofibrils for advanced cosmeceuticals. Clin. Dermatol. 2008, 26, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Sajna, K.V.; Gottumukkala, L.D.; Sukumaran, R.K.; Pandey, A. White Biotechnology in Cosmetics. In Industrial Biorefineries and White Biotechnology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 607–652. [Google Scholar]
- Morganti, P.; Muzzarelli, R.A.A.; Muzzarelli, C. Multifunctional use of innovative chitin nanofibrils for skin care. J. Appl. Cosmetol. 2006, 24, 105–114. [Google Scholar]
- Ngo, D.H.; Kim, S.K. Antioxidant Effects of Chitin, Chitosan, and Their Derivatives, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 73, pp. 15–31. [Google Scholar]
- Azuma, K.; Ifuku, S.; Osaki, T.; Okamoto, Y.; Minami, S. Preparation and biomedical applications of chitin and chitosan nanofibers. J. Biomed. Nanotechnol. 2014, 10, 2891–2920. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rivas, M.; Guzmán, E.; Fernández-Peña, L.; Akanno, A.; Greaves, A.; Léonforte, F.; Ortega, F.; Rubio, R.G.; Luengo, G.S. Deposition of Synthetic and Bio-Based Polycations onto Negatively Charged Solid Surfaces: Effect of the Polymer Cationicity, Ionic Strength, and the Addition of an Anionic Surfactant. Colloids Interfaces 2020, 4, 33. [Google Scholar] [CrossRef]
- Wang, J.J.; Zeng, Z.W.; Xiao, R.Z.; Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar]
- Panonnummal, R.; Jayakumar, R.; Sabitha, M. Comparative anti-psoriatic efficacy studies of clobetasol loaded chitin nanogel and marketed cream. Eur. J. Pharm. Sci. 2017, 96, 193–206. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-based nanomaterials for drug delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. 2016, 10, 483–507. [Google Scholar] [CrossRef] [PubMed]
- Ntohogian, S.; Gavriliadou, V.; Christodoulou, E.; Nanaki, S.; Lykidou, S.; Naidis, P.; Mischopoulou, L.; Barmpalexis, P.; Nikolaidis, N.; Bikiaris, D.N. Chitosan nanoparticles with encapsulated natural and Uf-purified annatto and saffron for the preparation of UV protective cosmetic emulsions. Molecules 2018, 23, 2107. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A. Carboxymethylated chitins and chitosans. Carbohydr. Polym. 1988, 8, 1–21. [Google Scholar] [CrossRef]
- Chen, L.; Du, Y.; Wu, H.; Xiao, L. Relationship between molecular structure and moisture-retention ability of carboxymethyl chitin and chitosan. J. Appl. Polym. Sci. 2002, 83, 1233–1241. [Google Scholar] [CrossRef]
- Kong, C.S.; Kim, J.A.; Ahn, B.; Byun, H.G.; Kim, S.K. Carboxymethylations of chitosan and chitin inhibit MMP expression and ROS scavenging in human fibrosarcoma cells. Process Biochem. 2010, 45, 179–186. [Google Scholar] [CrossRef]
- Ngo, D.N.; Kim, M.M.; Kim, S.K. Chitin oligosaccharides inhibit oxidative stress in live cells. Carbohydr. Polym. 2008, 74, 228–234. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Muzzarelli, C.; Cosani, A.; Terbojevich, M. 6-Oxychitins, novel hyaluronan-like regiospecifically carboxylated chitins. Carbohydr. Polym. 1999, 39, 361–367. [Google Scholar] [CrossRef]
- Sun, L.; Du, Y.; Yang, J.; Shi, X.; Li, J.; Wang, X.; Kennedy, J.F. Conversion of crystal structure of the chitin to facilitate preparation of a 6-carboxychitin with moisture absorption-retention abilities. Carbohydr. Polym. 2006, 66, 168–175. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Nanochitins and Nanochitosans, Paving the Way to Eco-Friendly and Energy-Saving Exploitation of Marine Resources. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; Volume 10, pp. 153–164. [Google Scholar]
- Gautier, S.; Xhauflaire-Uhoda, E.; Gonry, P.; Piérard, G.E. Chitin-glucan, a natural cell scaffold for skin moisturization and rejuvenation. Int. J. Cosmet. Sci. 2008, 30, 459–469. [Google Scholar] [CrossRef]
- Cho, Y.W.; Cho, Y.N.; Chung, S.H.; Yoo, G.; Ko, S.W. Water-soluble chitin as a wound healing accelerator. Biomaterials 1999, 20, 2139–2145. [Google Scholar] [CrossRef]
- Blasinska, A.; Drobnik, J. Effects of nonwoven mats of di-o-butyrylchitin and related polymers on the process of wound healing. Biomacromolecules 2008, 9, 776–782. [Google Scholar] [CrossRef]
- Chilarski, A.; Szosland, L.; Krucińska, I.; Kiekens, P.; Błasińska, A.; Schoukens, G.; Cisło, R.; Szumilewicz, J. Novel dressing materials accelerating wound healing made from dibutyrylchitin. Fibres Text. East. Eur. 2007, 15, 77–81. [Google Scholar]
- Soetemans, L.; Uyttebroek, M.; Bastiaens, L. Characteristics of chitin extracted from black soldier fly in different life stages. Int. J. Biol. Macromol. 2020, 165, 3206–3214. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Lin, A.Y.M.; McKittrick, J.; Meyers, M.A. Structure and mechanical properties of crab exoskeletons. Acta Biomater. 2008, 4, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Saimoto, H. Chitin nanofibers: Preparations, modifications, and applications. Nanoscale 2012, 4, 3308–3318. [Google Scholar] [CrossRef] [PubMed]
- Raabe, D.; Sachs, C.; Romano, P. The crustacean exoskeleton as an example of a structurally and mechanically graded biological nanocomposite material. Acta Mater. 2005, 53, 4281–4292. [Google Scholar] [CrossRef]
- Raabe, D.; Romano, P.; Sachs, C.; Fabritius, H.; Al-Sawalmih, A.; Yi, S.B.; Servos, G.; Hartwig, H.G. Microstructure and crystallographic texture of the chitin-protein network in the biological composite material of the exoskeleton of the lobster Homarus americanus. Mater. Sci. Eng. A 2006, 421, 143–153. [Google Scholar] [CrossRef]
- Ling, S.; Kaplan, D.L.; Buehler, M.J. Nanofibrils in nature and materials engineering. Nat. Rev. Mater. 2018, 3, 1–15. [Google Scholar] [CrossRef]
- Ifuku, S. Chitin and chitosan nanofibers: Preparation and chemical modifications. Molecules 2014, 19, 18367–18380. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Yoshioka, M.; Morimoto, M.; Yano, H.; Saimoto, H. Fibrillation of dried chitin into 10-20 nm nanofibers by a simple grinding method under acidic conditions. Carbohydr. Polym. 2010, 81, 134–139. [Google Scholar] [CrossRef]
- Ifuku, S.; Nomura, R.; Morimoto, M.; Saimoto, H. Preparation of chitin nanofibers from mushrooms. Materials 2011, 4, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Mavi, S. Preparation of Chitin and Derivatives Thereof for Cosmetic and Therapeutic Use. International Patent PCT/IB2005/053576, 2 November 2005. [Google Scholar]
- Morganti, P. Spray-Dried Chitin Nanofibrils, Method for Production and Uses Thereof. International Patent PCT/IB2006/054403, 23 November 2006. [Google Scholar]
- Morganti, P. Chitin Nanofibrils in Skin Treatment. J. Appl. Cosmetol. 2009, 25, 251–270. [Google Scholar]
- Jayakumar, R.; Prabaharan, M.; Nair, S.V.; Tamura, H. Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol. Adv. 2010, 28, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chwee, T.L.; Ramakrishna, S.; Huang, Z.M. Recent development of polymer nanofibers for biomedical and biotechnological applications. J. Mater. Sci. Mater. Med. 2005, 16, 933–946. [Google Scholar] [CrossRef]
- Jain, T.; Kumar, H.; Dutta, P.K. D-glucosamine and N-acetyl d-glucosamine: Their potential use as regenerative medicine. In Chitin and Chitosan for Regenerative Medicine; Springers: New Delhi, India, 2016; pp. 279–295. [Google Scholar]
- Morganti, P.; Carezzi, F.; Del Ciotto, P.; Morganti, G.; Nunziata, M.L.; Gao, X.; Tishenko, G.; Yudin, V. Chitin Nanofibrils: A Natural Multifunctional Polymer. Nanobiotechnology 2014, 1, 1–31. [Google Scholar]
- Bissett, D.L.; Robinson, L.R.; Raleigh, P.S.; Miyamoto, K.; Hakozaki, T.; Li, J.; Kelm, G.R. Reduction in the appearance of facial hyperpigmentation by topical N-acetyl glucosamine. J. Cosmet. Dermatol. 2007, 6, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Bissett, D.L.; Farmer, T.; McPhail, S.; Reichling, T.; Tiesman, J.P.; Juhlin, K.D.; Hurley, G.J.; Robinson, M.K. Genomic expression changes induced by topical N-acetyl glucosamine in skin equivalent cultures in vitro. J. Cosmet. Dermatol. 2007, 6, 232–238. [Google Scholar] [CrossRef]
- Danti, S.; Trombi, L.; Fusco, A.; Azimi, B.; Lazzeri, A.; Morganti, P.; Coltelli, M.B.; Donnarumma, G. Chitin nanofibrils and nanolignin as functional agents in skin regeneration. Int. J. Mol. Sci. 2019, 20, 2669. [Google Scholar] [CrossRef]
- Morganti, P.; Li, Y.H. Innovation in cosmetic and medical science. The role of chitin nanofibrils composites. J. Appl. Cosmetol. 2015, 33, 9–24. [Google Scholar]
- Ito, I.; Yoneda, T.; Omura, Y.; Osaki, T.; Ifuku, S.; Saimoto, H.; Azuma, K.; Imagawa, T.; Tsuka, T.; Murahata, Y.; et al. Protective effect of chitin urocanate nanofibers against ultraviolet radiation. Mar. Drugs 2015, 13, 7463–7475. [Google Scholar] [CrossRef]
- Ito, I.; Osaki, T.; Ifuku, S.; Saimoto, H.; Takamori, Y.; Kurozumi, S.; Imagawa, T.; Azuma, K.; Tsuka, T.; Okamoto, Y.; et al. Evaluation of the effects of chitin nanofibrils on skin function using skin models. Carbohydr. Polym. 2014, 101, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Palombo, M.; Cardillo, A.; Del Ciotto, P.; Morganti, G.; Gazzaniga, G. Anti-dandruff and anti-oily efficacy of hair formulations with a repairing and restructuring activity. The positive influence of the Zn-chitin nanofibrils complexes. J. Appl. Cosmetol. 2012, 30, 149–159. [Google Scholar]
- Morganti, P.; Fabrizi, G.; Palombo, M.; Cardillo, M.; Cardillo, A.; Del Ciotto, P.; Carezzi, F.; Morganti, G. Activity of chitin nanofibrils block-copolymers entrapping Zn/AI/SA/allantoin on seborrheic dermatitis. A randomized double-blind placebo controlled study. J. Appl. Cosmetol. 2014, 32, 3–19. [Google Scholar]
- Morganti, P.; Palombo, M.; Palombo, P.; Fabrizi, G.; Cardillo, A.; Carezzi, F.; Morganti, G.; Ruocco, E.; Dzierzgowski, S. Cosmetic Science in Skin Aging: Achieving the Efficacy by the Chitin Nano-Structured Crystallites. SÖFW J. 2010, 136, 14–24. [Google Scholar]
- Morganti, P.; Morganti, G.; Fabrizi, G.; Cardillo, A. A new sun to rejuvenate the skin. J. Appl. Cosmetol. 2008, 26, 159–168. [Google Scholar]
- Morganti, P.; Fabrizi, G.; Palombo, P.; Palombo, M.; Ruocco, E.; Cardillo, A.; Morganti, G. Chitin-nanofibrils: A new active cosmetic carrier. J. Appl. Cosmetol. 2008, 26, 113–128. [Google Scholar]
- Morganti, P.; Palombo, P.; Palombo, M.; Fabrizi, G.; Cardillo, A.; Svolacchia, F.; Guevara, L.; Mezzana, P. A phosphatidylcholine hyaluronic acid chitin-nanofibrils complex for a fast skin remodeling and a rejuvenating look. Clin. Cosmet. Investig. Dermatol. 2012, 5, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Fabrizi, G.; Palombo, P.; Palombo, M.; Guarneri, F.; Cardillo, A.; Morganti, G. New chitin complexes and their anti-aging activity from inside out. J. Nutr. Health Aging 2012, 16, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Palombo, M.; Tishchenko, G.; Yudin, V.E.; Guarneri, F.; Cardillo, M.; Del Ciotto, P.; Carezzi, F.; Morganti, G.; Fabrizi, G. Chitin-hyaluronan nanoparticles: A multifunctional carrier to deliver anti-aging active ingredients through the skin. Cosmetics 2014, 1, 140–158. [Google Scholar] [CrossRef]
- Morganti, P.; Palombo, M.; Fabrizi, G.; Guarneri, F.; Svolacchia, F.; Cardillo, A.; Del Ciotto, P.; Carezzi, F.; Morganti, G. New insights on anti-aging activity of chitin nanofibril-hyaluronan block copolymers entrapping active ingredients: In vitro and in vivo study. J. Appl. Cosmetol. 2013, 31, 1–29. [Google Scholar]
- Morganti, P.; Del Ciotto, P.; Carezzi, F.; Guarneri, F.; Yeo, Y.J. Skin lightening efficacy of new formulations enhanced by chitin nanoparticles delivery system. Note I. J. Appl. Cosmetol. 2014, 32, 57–71. [Google Scholar]
- Morganti, P.; Palombo, M.; Carezzi, F.; Nunziata, M.L.; Morganti, G.; Cardillo, M.; Chianese, A. Green nanotechnology serving the bioeconomy: Natural beauty masks to save the environment. Cosmetics 2016, 3, 41. [Google Scholar] [CrossRef]
- Morganti, P.; Fusco, A.; Paoletti, I.; Perfetto, B.; Ciotto, P.; Del Palombo, M.; Chianese, A.; Baroni, A.; Donnarumma, G. Anti-inflammatory, immunomodulatory, and tissue repair activity on human keratinocytes by green innovative nanocomposites. Materials 2017, 10, 843. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G.; Colao, C. Biofunctional Textiles for Aging Skin. Biomedicines 2019, 7, 51. [Google Scholar] [CrossRef]
- Morganti, P.; Yudin, V.E.; Morganti, G.; Colte, M.B. Trends in surgical and beauty masks for a cleaner environment. Cosmetics 2020, 7, 68. [Google Scholar] [CrossRef]
- Donnarumma, G.; Fusco, A.; Morganti, P.; Palombo, M. Advanced medications made by green nanocomposites. Int. J. Res. Pharm. Nano Sci. 2016, 5, 261–270. [Google Scholar]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Maevskaia, E.N.; Shabunin, A.S.; Dresvyanina, E.N.; Dobrovol’skaya, I.P.; Yudin, V.E.; Paneyah, M.B.; Fediuk, A.M.; Sushchinskii, P.L.; Smirnov, G.P.; Zinoviev, E.V.; et al. Influence of the Introduced Chitin Nanofibrils on Biomedical Properties of Chitosan-Based Materials. Nanomaterials 2020, 10, 945. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaya, I.P.; Yudin, V.E.; Popryadukhin, P.V.; Lebedeva, I.O.; Shabunin, A.S.; Stoyanovsky, R.G.; Asadulaev, M.S.; Ivan, E.M.; Morganti, P. In vivo study of the nanofiber-based composite wound dressing intended for treatment of deep skin wounds. J. Appl. Cosmetol. 2016, 34, 1–8. [Google Scholar]
- Mattioli-Belmonte, M.; Zizzi, A.; Lucarini, G.; Giantomassi, F.; Biagini, G.; Tucci, G.; Orlando, F.; Provinciali, M.; Carezzi, F.; Morganti, P. Chitin Nanofibrils Linked to Chitosan Glycolate as Spray, Gel, and Gauze Preparations for Wound Repair. J. Bioact. Compat. Polym. 2007, 22, 525–538. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Morganti, P.; Morganti, G.; Palombo, P.; Palombo, M.; Biagini, G.; Mattioli Belmonte, M.; Giantomassi, F.; Orlandi, F.; Muzzarelli, C. Chitin nanofibrils/chitosan glycolate composites as wound medicaments. Carbohydr. Polym. 2007, 70, 274–284. [Google Scholar] [CrossRef]
- Izumi, R.; Komada, S.; Ochi, K.; Karasawa, L.; Osaki, T.; Murahata, Y.; Tsuka, T.; Imagawa, T.; Itoh, N.; Okamoto, Y.; et al. Favorable effects of superficially deacetylated chitin nanofibrils on the wound healing process. Carbohydr. Polym. 2015, 123, 461–467. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triunfo, M.; Tafi, E.; Guarnieri, A.; Scieuzo, C.; Hahn, T.; Zibek, S.; Salvia, R.; Falabella, P. Insect Chitin-Based Nanomaterials for Innovative Cosmetics and Cosmeceuticals. Cosmetics 2021, 8, 40. https://doi.org/10.3390/cosmetics8020040
Triunfo M, Tafi E, Guarnieri A, Scieuzo C, Hahn T, Zibek S, Salvia R, Falabella P. Insect Chitin-Based Nanomaterials for Innovative Cosmetics and Cosmeceuticals. Cosmetics. 2021; 8(2):40. https://doi.org/10.3390/cosmetics8020040
Chicago/Turabian StyleTriunfo, Micaela, Elena Tafi, Anna Guarnieri, Carmen Scieuzo, Thomas Hahn, Susanne Zibek, Rosanna Salvia, and Patrizia Falabella. 2021. "Insect Chitin-Based Nanomaterials for Innovative Cosmetics and Cosmeceuticals" Cosmetics 8, no. 2: 40. https://doi.org/10.3390/cosmetics8020040
APA StyleTriunfo, M., Tafi, E., Guarnieri, A., Scieuzo, C., Hahn, T., Zibek, S., Salvia, R., & Falabella, P. (2021). Insect Chitin-Based Nanomaterials for Innovative Cosmetics and Cosmeceuticals. Cosmetics, 8(2), 40. https://doi.org/10.3390/cosmetics8020040