Facial Treatment with 3-O-Cetyl Ascorbic Acid for Improvement of Skin Texture: Uptake, Effectiveness, and In Vitro Carcinogenicity Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. 3-CetylAA
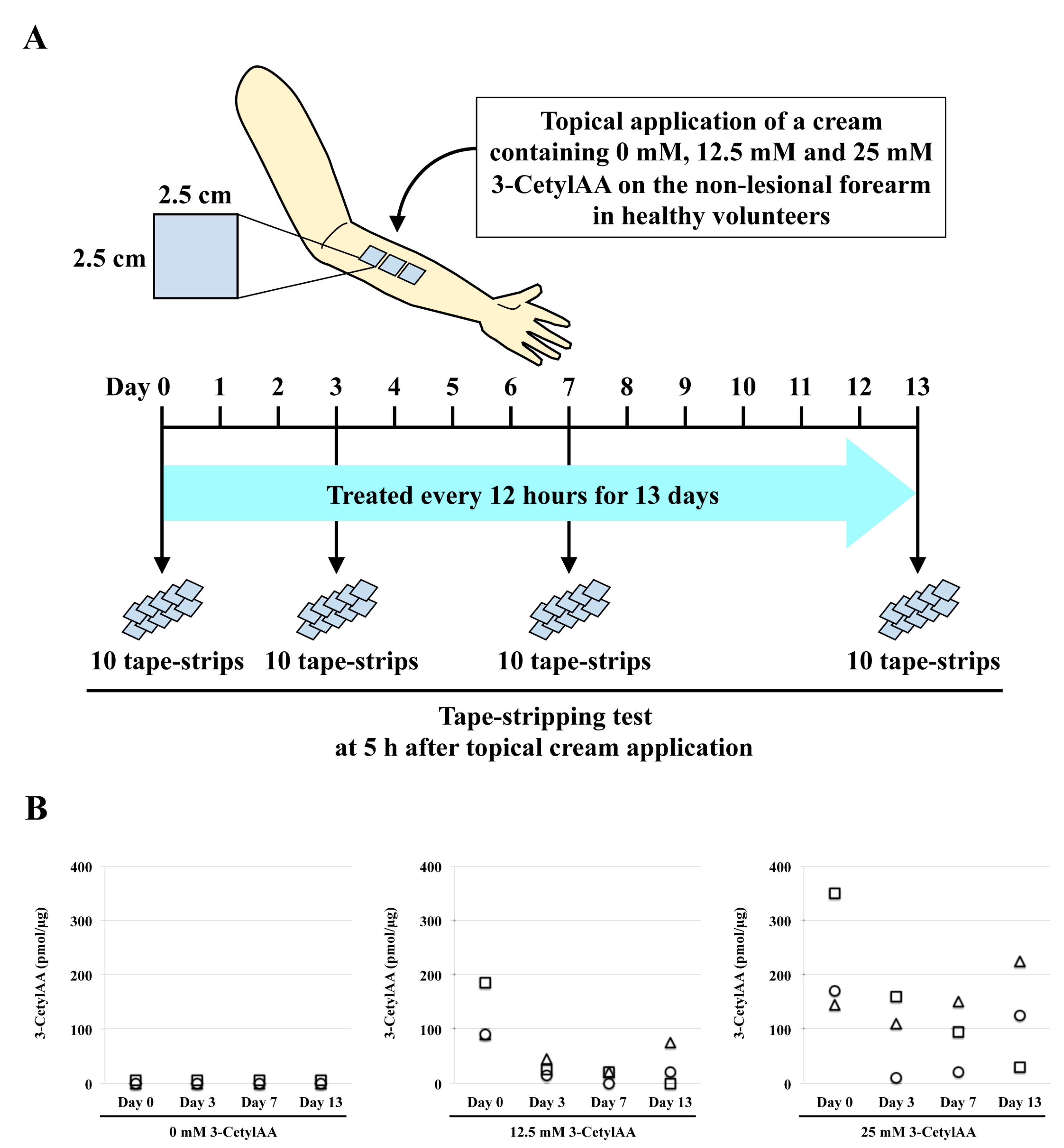
2.2. Tape-Stripping Analysis
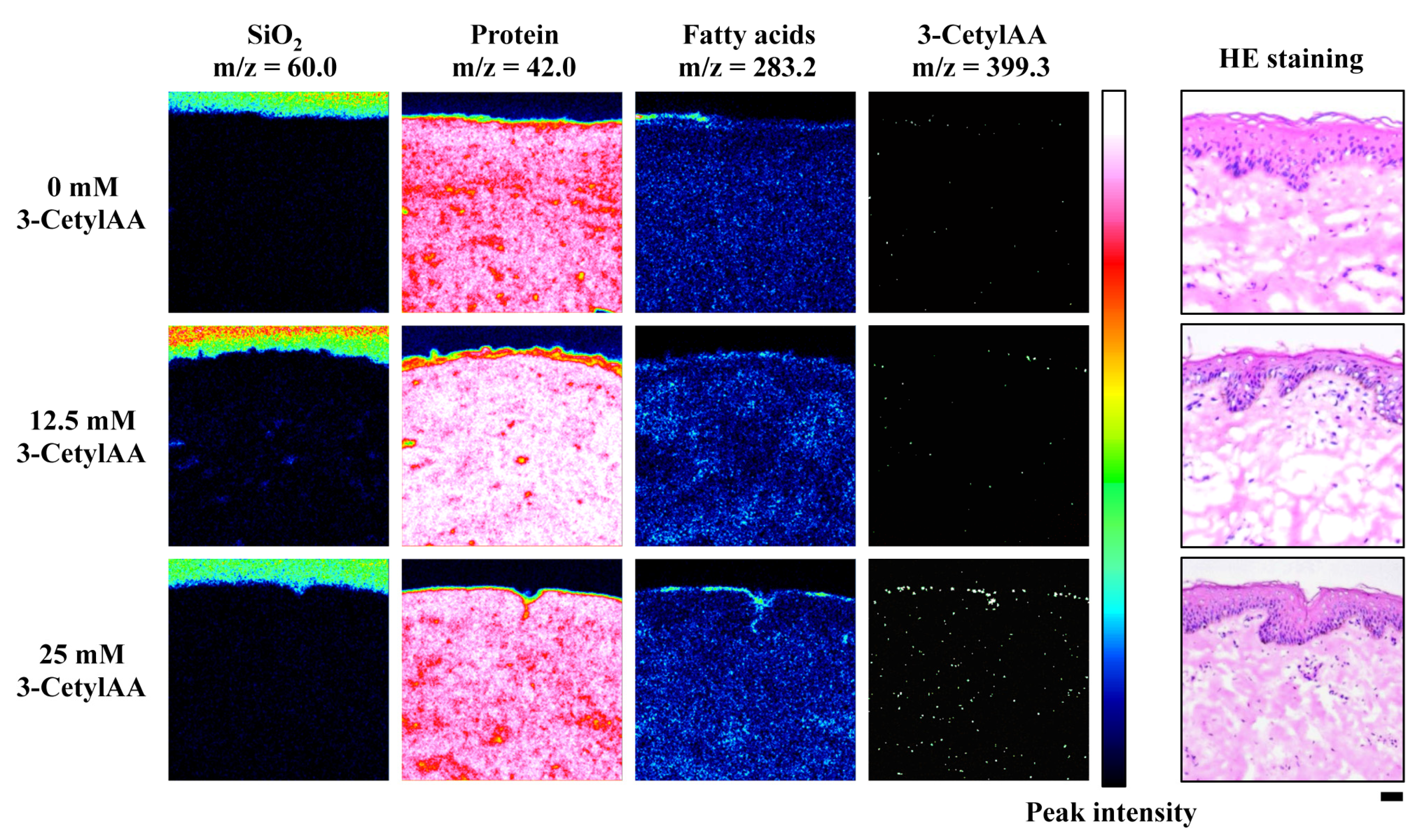
2.3. Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS) Imaging Analysis
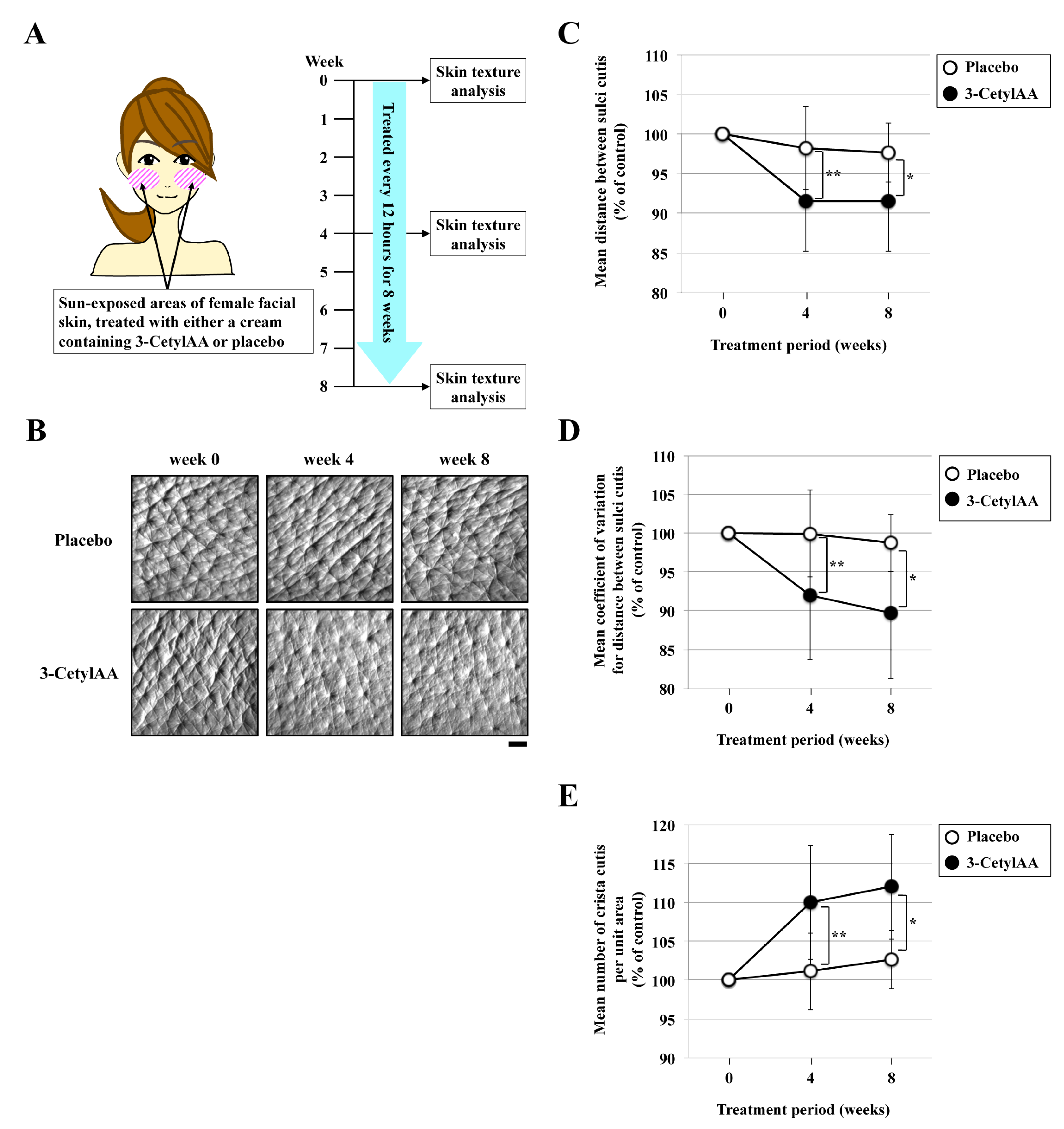
2.4. Skin Texture Analysis
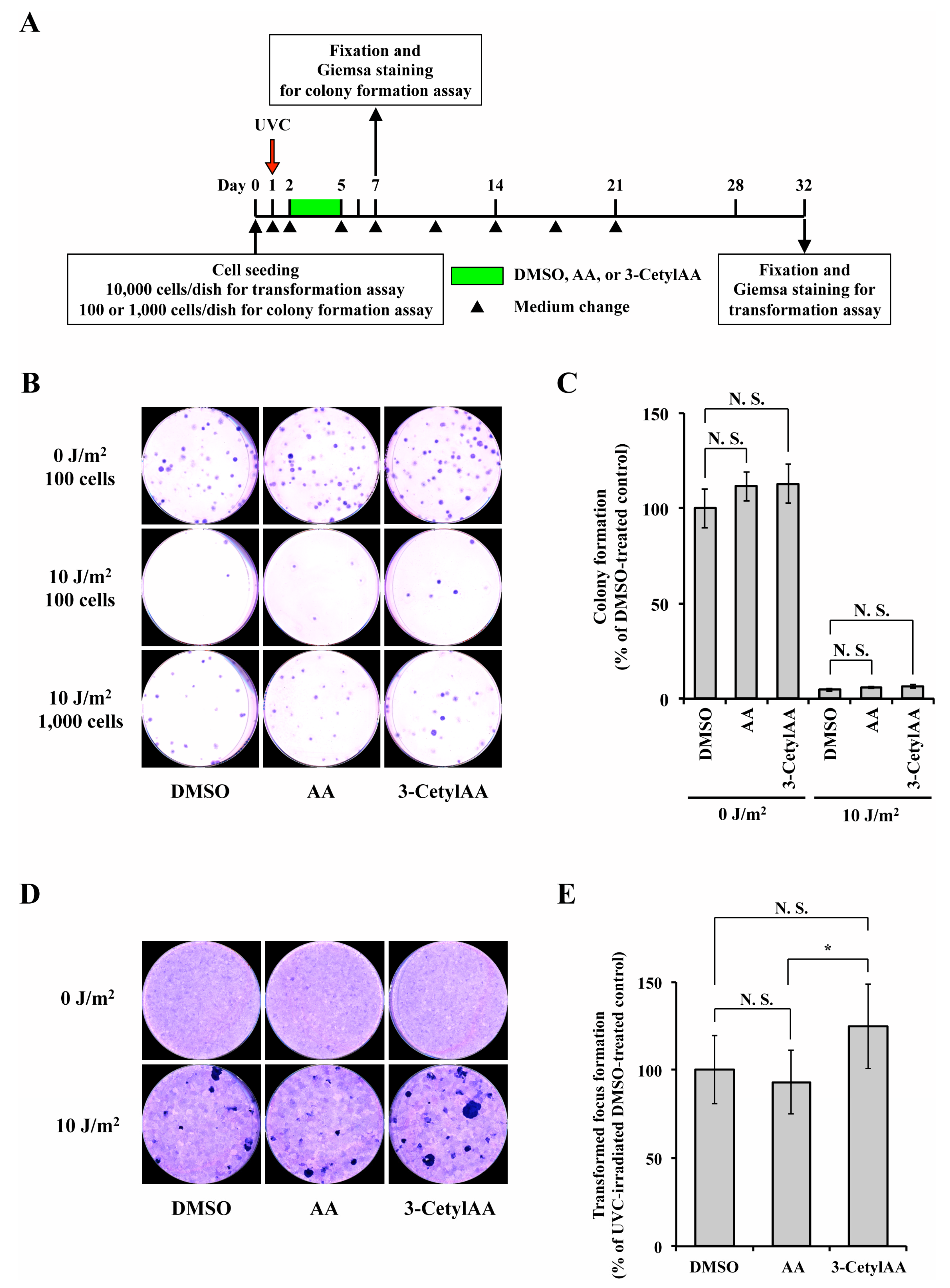
2.5. In Vitro Transformation Assay
2.6. Statistics
2.7. Ethical Considerations
3. Results
3.1. Tape-Stripping of 3-CetylAA in Human Skin
3.2. ToF-SIMS Imaging of 3-CetylAA in Pig Skin
3.3. Effects of 3-CetylAA on Skin Texture in Sun-Exposed Areas
3.4. Toxic and Carcinogenic Effects of 3-CetylAA
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Telang, P.S. Vitamin C in dermatology. Indian Dermatol. Online J. 2013, 4, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrition 2017, 9, 866. [Google Scholar] [CrossRef]
- Ravetti, S.; Clemente, C.; Brignone, S.; Hergert, L.; Allemandi, D.; Palma, S. Ascorbic Acid in Skin Health. Cosmetics 2019, 6, 58. [Google Scholar] [CrossRef]
- Shindo, Y.; Witt, E.; Han, D.; Epstein, W.; Packer, L. Enzymic and Non-Enzymic Antioxidants in Epidermis and Dermis of Human Skin. J. Investig. Dermatol. 1994, 102, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Rhie, G.-E.; Shin, M.H.; Seo, J.Y.; Choi, W.W.; Cho, K.H.; Kim, K.H.; Park, K.C.; Eun, H.C.; Chung, J.H. Aging- and Photoaging-Dependent Changes of Enzymic and Nonenzymic Antioxidants in the Epidermis and Dermis of Human Skin In Vivo. J. Investig. Dermatol. 2001, 117, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- McArdle, F.; Rhodes, L.; Parslew, R.; Jack, C.; Friedmann, P.; Jackson, M. UVR-induced oxidative stress in human skin in vivo: Effects of oral vitamin C supplementation. Free. Radic. Biol. Med. 2002, 33, 1355–1362. [Google Scholar] [CrossRef]
- Shindo, Y.; Witt, E.; Packer, L. Antioxidant Defense Mechanisms in Murine Epidermis and Dermis and Their Responses to Ultraviolet Light. J. Investig. Dermatol. 1993, 100, 260–265. [Google Scholar] [CrossRef]
- Shindo, Y.; Witt, E.; Han, D.; Packer, L. Dose-Response Effects of Acute Ultraviolet Irradiation on Antioxidants and Molecular Markers of Oxidation in Murine Epidermis and Dermis. J. Investig. Dermatol. 1994, 102, 470–475. [Google Scholar] [CrossRef]
- Gref, R.; Deloménie, C.; Maksimenko, A.; Gouadon, E.; Percoco, G.; Lati, E.; Desmaële, D.; Zouhiri, F.; Couvreur, P. Vitamin C–squalene bioconjugate promotes epidermal thickening and collagen production in human skin. Sci. Rep. 2020, 10, 16883. [Google Scholar] [CrossRef]
- Abdullah, M.; Jamil, R.T.; Attia, F.N. Vitamin c (ascorbic acid). In Statpearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Duarte, T.L.; Cooke, M.S.; Jones, G.D. Gene expression profiling reveals new protective roles for vitamin C in human skin cells. Free. Radic. Biol. Med. 2009, 46, 78–87. [Google Scholar] [CrossRef]
- Lee, S.-A.; Son, Y.-O.; Kook, S.-H.; Choi, K.-C.; Lee, J.-C. Ascorbic acid increases the activity and synthesis of tyrosinase in B16F10 cells through activation of p38 mitogen-activated protein kinase. Arch. Dermatol. Res. 2011, 303, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Panich, U.; Tangsupa-a-nan, V.; Onkoksoong, T.; Kongtaphan, K.; Kasetsinsombat, K.; Akarasereenont, P.; Wongkajornsilp, A. Inhibition of UVA-mediated melanogenesis by ascorbic acid through modulation of antioxidant defense and nitric oxide system. Arch. Pharmacal Res. 2011, 34, 811–820. [Google Scholar] [CrossRef] [PubMed]
- De Tullio, M.C. Beyond the Antioxidant: The Double Life of Vitamin C. Prokaryotic Cytoskelet. 2011, 56, 49–65. [Google Scholar] [CrossRef]
- Sanadi, R.M.; Deshmukh, R.S. The effect of Vitamin C on melanin pigmentation A systematic review. J. Oral Maxillofac. Pathol. 2020, 24, 374–382. [Google Scholar] [CrossRef]
- Pinnell, S.R.; Yang, H.; Omar, M.; Riviere, N.M.; DeBuys, H.V.; Walker, L.C.; Wang, Y.; Levine, M. Topical L-Ascorbic Acid: Percutaneous Absorption Studies. Dermatol. Surg. 2001, 27, 137–142. [Google Scholar] [CrossRef]
- Al-Niaimi, F.; Chiang, N.Y.Z. Topical Vitamin C and the Skin: Mechanisms of Action and Clinical Applications. J. Clin. Aesthetic Dermatol. 2017, 10, 14–17. [Google Scholar]
- Stamford, N.P.J. Stability, transdermal penetration, and cutaneous effects of ascorbic acid and its derivatives. J. Cosmet. Dermatol. 2012, 11, 310–317. [Google Scholar] [CrossRef]
- Duarah, S.; Durai, R.D.; Narayanan, V.B. Nanoparticle-in-gel system for delivery of vitamin C for topical application. Drug Deliv. Transl. Res. 2017, 7, 750–760. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.; Huang, J.; Xia, N.; Li, T.; Xia, Q. Self-double-emulsifying drug delivery system incorporated in natural hydrogels: A new way for topical application of vitamin C. J. Microencapsul. 2018, 35, 90–101. [Google Scholar] [CrossRef]
- Macan, A.M.; Kraljević, T.G.; Raić-Malić, S. Therapeutic Perspective of Vitamin C and Its Derivatives. Antioxidants 2019, 8, 247. [Google Scholar] [CrossRef]
- Machado, B.H.B.; Frame, J.; Zhang, J.; Najlah, M. Comparative Study on the Outcome of Periorbital Wrinkles Treated with Laser-Assisted Delivery of Vitamin C or Vitamin C Plus Growth Factors: A Randomized, Double-blind, Clinical Trial. Aesthetic Plast. Surg. 2020, 1–13. [Google Scholar] [CrossRef]
- Caritá, A.C.; de Azevedo, J.R.; Buri, M.V.; Bolzinger, M.-A.; Chevalier, Y.; Riske, K.A.; Leonardi, G.R. Stabilization of vitamin C in emulsions of liquid crystalline structures. Int. J. Pharm. 2021, 592, 120092. [Google Scholar] [CrossRef] [PubMed]
- Elhabak, M.; Ibrahim, S.; Abouelatta, S.M. Topical delivery of l-ascorbic acid spanlastics for stability enhancement and treatment of UVB induced damaged skin. Drug Deliv. 2021, 28, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-S.; Yang, W.-H.; Gao, W.; Zhang, L.; Wei, F.; Liu, H.; Wang, S.-Y.; Li, Y.-Y.; Zhao, W.-M.; Ma, T.; et al. Combination and efficiency: Preparation of dissolving microneedles array loaded with two active ingredients and its anti-pigmentation effects on guinea pigs. Eur. J. Pharm. Sci. 2021, 160, 105749. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.; Goto, S.; Ishiguro, Y.; Suzuki, K.; Nitoda, T.; Yamamoto, I. Permeation and metabolism of a series of novel lipophilic ascorbic acid derivatives, 6-O-acyl-2-O-α-d-glucopyranosyl-l-ascorbic acids with a branched-acyl chain, in a human living skin equivalent model. Bioorganic Med. Chem. Lett. 2004, 14, 623–627. [Google Scholar] [CrossRef]
- Tripathi, R.; Singh, B.; Bisht, S.; Pandey, J. L-Ascorbic Acid in Organic Synthesis: An Overview. Curr. Org. Chem. 2009, 13, 99–122. [Google Scholar] [CrossRef]
- Díaz, Y.D.L.M.Z.; Menghi, K.; Guerrero, M.L.; Nocelli, N.; Fanani, M.L. l-Ascorbic acid alkyl esters action on stratum corneum model membranes: An insight into the mechanism for enhanced skin permeation. Colloids Surfaces B Biointerfaces 2020, 185, 110621. [Google Scholar] [CrossRef]
- Park, C.M.; Lee, S.Y.; Joung, M.S.; Choi, J.W. Anti-wrinkle effect of 3-o-cetyl-l-ascorbic acid. J. Soc. Cosmet. Sci. Korea 2008, 34, 303–309. [Google Scholar]
- Kaburagi, S. The active ingredient of stimulating the biosynthesis of dermal component. Fragr. J. 2008, 34, 42–45. [Google Scholar]
- Hoppel, M.; Tabosa, M.A.M.; Bunge, A.L.; Delgado-Charro, M.B.; Guy, R.H. Assessment of Drug Delivery Kinetics to Epidermal Targets In Vivo. AAPS J. 2021, 23, 1–11. [Google Scholar] [CrossRef]
- N’Dri-Stempfer, B.; Navidi, W.C.; Guy, R.H.; Bunge, A.L. Improved Bioequivalence Assessment of Topical Dermatological Drug Products Using Dermatopharmacokinetics. Pharm. Res. 2008, 26, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Sahle, F.F.; Gebre-Mariam, T.; Dobner, B.; Wohlrab, J.; Neubert, R.H. Skin Diseases Associated with the Depletion of Stratum Corneum Lipids and Stratum Corneum Lipid Substitution Therapy. Ski. Pharmacol. Physiol. 2015, 28, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lim, S.H.; Song, J.Y.; Kim, M.-Y.; Lee, J.H.; Park, J.G.; Kim, H.O.; Park, Y.M. Skin barrier function recovery after diamond microdermabrasion. J. Dermatol. 2009, 36, 529–533. [Google Scholar] [CrossRef]
- Milewski, M.; Brogden, N.K.; Stinchcomb, A.L. Current aspects of formulation efforts and pore lifetime related to microneedle treatment of skin. Expert Opin. Drug Deliv. 2010, 7, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Kang, H.A.; Kim, M.-Y.; Park, Y.M.; Kim, H.O. Damage and Recovery of Skin Barrier Function After Glycolic Acid Chemical Peeling and Crystal Microdermabrasion. Dermatol. Surg. 2004, 30, 390–394. [Google Scholar] [CrossRef]
- Andrews, S.; Lee, J.W.; Prausnitz, M. Recovery of Skin Barrier After Stratum Corneum Removal by Microdermabrasion. AAPS PharmSciTech 2011, 12, 1393–1400. [Google Scholar] [CrossRef][Green Version]
- Weigmann, H.-J.; Lademann, J.; Meffert, H.; Schaefer, H.; Sterry, W. Determination of the Horny Layer Profile by Tape Stripping in Combination with Optical Spectroscopy in the Visible Range as a Prerequisite to Quantify Percutaneous Absorption. Ski. Pharmacol. Physiol. 1999, 12, 34–45. [Google Scholar] [CrossRef]
- Lademann, J.; Ilgevicius, A.; Zurbau, O.; Liess, H.-D.; Schanzer, S.; Weigmann, H.-J.; Antoniou, C.; Von Pelchrzim, R.; Sterry, W. Penetration studies of topically applied substances: Optical determination of the amount of stratum corneum removed by tape stripping. J. Biomed. Opt. 2006, 11, 054026. [Google Scholar] [CrossRef]
- Ishikawa, A.; Fujii, M.; Morimoto, K.; Yamada, T.; Koizumi, N.; Kondoh, M.; Watanabe, Y. Oil-in-water emulsion lotion providing controlled release using 2-methacryloyloxyethyl phosphorylcholine n-butyl methacrylate copolymer as emulsifier. Results Pharma Sci. 2012, 2, 16–22. [Google Scholar] [CrossRef]
- Benedict, W.F.; Chouroulinkov, I.; Fisher, P.B.; Kakunaga, T.; Marquardt, H.; Pienta, R.J.; Yamasaki, H. Transformation assay of established cell lines: Mechanisms and application. In Proceedings of the A Workshop Organized by Iarc in Collaboration with the Us National Cancer Institute and the Us Environmental Protection Agency, Lyon, France, 15–17 February 1984; International Agency for Research on Cancer: Lyon, France, 1985; Volume 67, pp. 1–255. [Google Scholar]
- Doi, N.; Togari, H.; Minagi, K.; Iwaoka, Y.; Tai, A.; Nakaoji, K.; Hamada, K.; Tatsuka, M. 2-O-Octadecylascorbic acid represses RhoGDIβ expression and ameliorates DNA damage-induced abnormal spindle orientations. J. Cell. Biochem. 2021. [Google Scholar] [CrossRef] [PubMed]
- Doi, N.; Togari, H.; Minagi, K.; Nakaoji, K.; Hamada, K.; Tatsuka, M. Protective Effects of Salicornia europaea on UVB-Induced Misoriented Cell Divisions in Skin Epithelium. Cosmetics 2020, 7, 44. [Google Scholar] [CrossRef]
- Liu, N.; Matsumura, H.; Kato, T.; Ichinose, S.; Takada, A.; Namiki, T.; Asakawa, K.; Morinaga, H.; Mohri, Y.; De Arcangelis, A.; et al. Stem cell competition orchestrates skin homeostasis and ageing. Nat. Cell Biol. 2019, 568, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Fushimi, T.; Uchino, T.; Miyazaki, Y.; Hatta, I.; Asano, M.; Fujino, H.; Suzuki, R.; Fujimori, S.; Kamiya, D.; Kagawa, Y. Development of phospholipid nanoparticles encapsulating 3-O-cetyl ascorbic acid and tocopherol acetate (TA-Cassome) for improving their skin accumulation. Int. J. Pharm. 2018, 548, 192–205. [Google Scholar] [CrossRef]
| Volunteer #1 | Volunteer #2 | Volunteer #3 | |
|---|---|---|---|
| 5 h uptake | 14.5 1 (0%) 2 | 12 (0%) | 35 (0%) |
| 7 h clearance | 5.5 (63%) | 0.5 (96%) | 8 (77%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doi, N.; Yamada, Y.; Toyoshima, M.; Kondo, Y.; Nakaoji, K.; Hamada, K.; Tatsuka, M. Facial Treatment with 3-O-Cetyl Ascorbic Acid for Improvement of Skin Texture: Uptake, Effectiveness, and In Vitro Carcinogenicity Assessment. Cosmetics 2021, 8, 38. https://doi.org/10.3390/cosmetics8020038
Doi N, Yamada Y, Toyoshima M, Kondo Y, Nakaoji K, Hamada K, Tatsuka M. Facial Treatment with 3-O-Cetyl Ascorbic Acid for Improvement of Skin Texture: Uptake, Effectiveness, and In Vitro Carcinogenicity Assessment. Cosmetics. 2021; 8(2):38. https://doi.org/10.3390/cosmetics8020038
Chicago/Turabian StyleDoi, Natsumi, Yoshifumi Yamada, Misaki Toyoshima, Yuki Kondo, Koichi Nakaoji, Kazuhiko Hamada, and Masaaki Tatsuka. 2021. "Facial Treatment with 3-O-Cetyl Ascorbic Acid for Improvement of Skin Texture: Uptake, Effectiveness, and In Vitro Carcinogenicity Assessment" Cosmetics 8, no. 2: 38. https://doi.org/10.3390/cosmetics8020038
APA StyleDoi, N., Yamada, Y., Toyoshima, M., Kondo, Y., Nakaoji, K., Hamada, K., & Tatsuka, M. (2021). Facial Treatment with 3-O-Cetyl Ascorbic Acid for Improvement of Skin Texture: Uptake, Effectiveness, and In Vitro Carcinogenicity Assessment. Cosmetics, 8(2), 38. https://doi.org/10.3390/cosmetics8020038






