Orbicularis Oculi Muscle Size and Function: Exploring the Influence of Aging and Exercise Training
Abstract
:1. Introduction
2. The Search of Studies
3. Influence of Age on the Function of Blinks
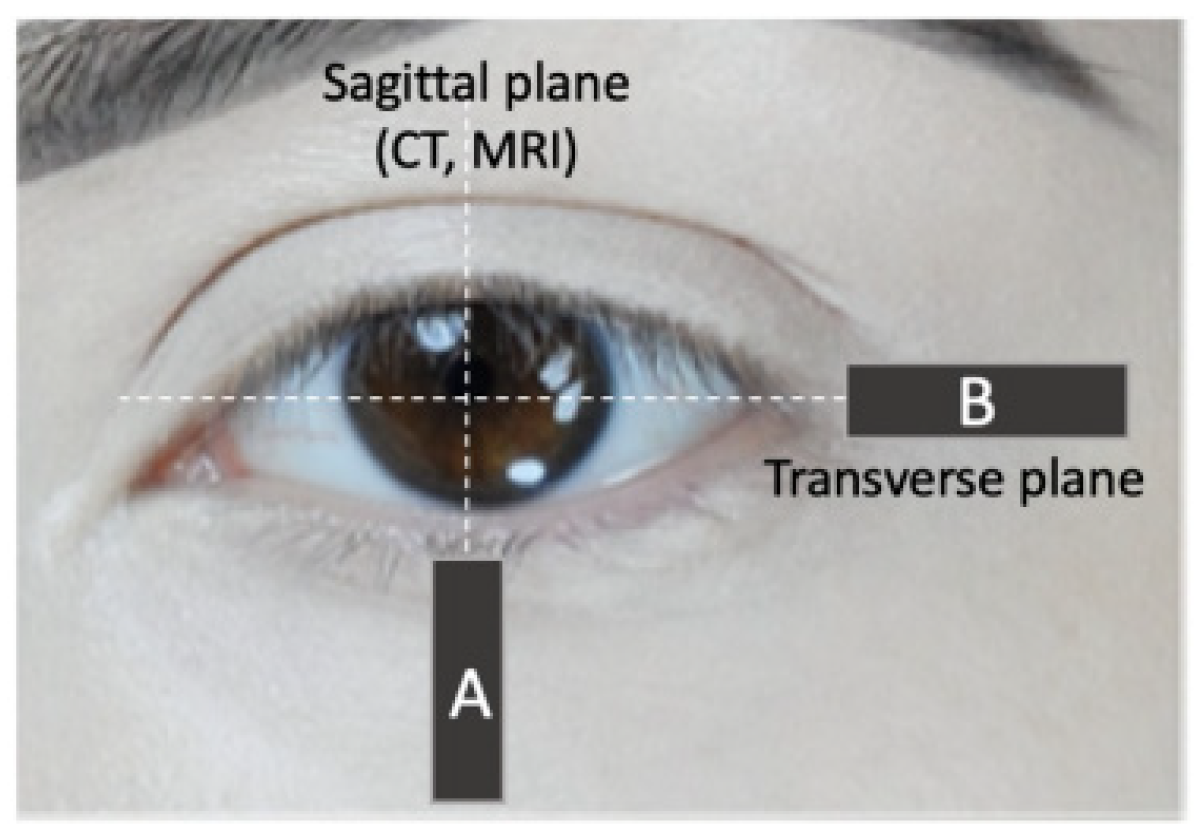
4. Influence of Age on the Orbicularis Oculi Muscle Size
5. Effect of Facial Exercise on Orbicularis Oculi Muscle Size
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geisler, C.; Braun, W.; Pourhassan, M.; Schweitzer, L.; Glüer, C.C.; Bosy-Westphal, A.; Müller, M.J. Gender-specific associations in age-related changes in resting energy expenditure (REE) and MRI measured body composition in healthy Caucasians. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2016, 71, 941–946. [Google Scholar] [CrossRef] [Green Version]
- Fortin, M.; Videman, T.; Gibbons, L.E.; Battie, M.C. Paraspinal muscle morphology and composition: A 15-yr longitudinal mag-netic resonance imaging study. Med. Sci. Sport. Exerc. 2014, 46, 893–901. [Google Scholar] [CrossRef]
- Saladin, K. Anatomy & Physiology: The Unity of Form and Function, 8th ed.; McGrawHill: New York, NY, USA, 2018; p. 319. [Google Scholar]
- Abe, T.; Sakamaki, M.; Yasuda, T.; Bemben, M.G.; Kondo, M.; Kawakami, Y.; Fukunaga, T. Age-related, site-specific muscle loss in 1507 Japanese men and women aged 20 to 95 years. J. Sport. Sci. Med. 2011, 10, 145–150. [Google Scholar]
- Abe, T.; Loenneke, J.P.; Thiebaud, R.S.; Fukunaga, T. Age-related site-specific muscle wasting of upper and lower extremities and trunk in Japanese men and women. Age 2014, 36, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Daboul, A.; Schwahn, C.; Bülow, R.; Kiliaridis, S.; Kocher, T.; Klinke, T.; Mundt, T.; Mourad, S.; Völzke, H.; Habes, M.; et al. Influence of age and tooth loss on masticatory muscles characteristics: A population based MR imaging study. J. Nutr. Health Aging 2018, 22, 829–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, Y.; Hirano, H.; Arai, H.; Morishita, S.; Ohara, Y.; Edahiro, A.; Murakami, M.; Pt, H.S.; Kikutani, T.; Suzuki, T. Relationship Between Frailty and Oral Function in Community-Dwelling Elderly Adults. J. Am. Geriatr. Soc. 2017, 65, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Volk, G.F.; Sauer, M.; Pohlmann, M.; Guntinas-Lichius, O. Reference values for dynamic facial muscle ultrasonography in adults. Muscle Nerve 2014, 50, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Hindman, H.B. Aging: A Predisposition to Dry Eyes. J. Ophthalmol. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Karson, C.N. Spontaneous Eye-Blink Rates and Dopaminergic Systems. Brain 1983, 106, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Windhager, S.; Mitteroecker, P.; Rupić, I.; Lauc, T.; Polašek, O.; Schaefer, K. Facial aging trajectories: A common shape pattern in male and female faces is disrupted after menopause. Am. J. Phys. Anthropol. 2019, 169, 678–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannarozzo, G.; Fazia, G.; Bennardo, L.; Tamburi, F.; Amoruso, G.F.; Del Duca, E.; Nistico, S.P. A new 675 nm laser de-vice in the treatment of facial aging: A prospective observational study. Photobiomodul. Photomed. Laser Surg. 2021, 39, 118–122. [Google Scholar] [CrossRef] [PubMed]
- De Greef, S.; Claes, P.; Vandermeulen, D.; Mollemans, W.; Suetens, P.; Willems, G. Large-scale in-vivo Caucasian facial soft tissue thickness database for craniofacial reconstruction. Forensic Sci. Int. 2006, 159, S126–S146. [Google Scholar] [CrossRef]
- Okuda, I.; Irimoto, M.; Nakajima, Y.; Sakai, S.; Hirata, K.; Shirakabe, Y. Using Multidetector Row Computed Tomography to Evaluate Baggy Eyelid. Aesthet. Plast. Surg. 2012, 36, 290–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peshori, K.R.; Schicatano, E.J.; Gopalaswamy, R.; Sahay, E.; Evinger, C. Aging of the trigeminal blink system. Exp. Brain Res. 2001, 136, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.S.; Baker, R.S.; Chuke, J.C.; Rouholiman, B.R.; A Hasan, S.; Gaza, W.; Stava, M.W.; Porter, J.D. Age-related changes in human blinks. Passive and active changes in eyelid kinematics. Investig. Ophthalmol. Vis. Sci. 1997, 38, 92–99. [Google Scholar]
- Sforza, C.; Rango, M.; Galante, D.; Bresolin, N.; Ferrario, V.F. Spontaneous blinking in healthy persons: An optoelectronic study of eyelid motion. Ophthalmic Physiol. Opt. 2008, 28, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Coors, A.; Merten, N.; Ward, D.D.; Schmid, M.; Breteler, M.M.B.; Ettinger, U. Strong age but weak sex effects in eye movement performance in the general adult population: Evidence from the Phineland Study. Vis. Res. 2021, 178, 124–133. [Google Scholar] [CrossRef]
- DeAngelis, K.D.; Rider, A.; Potter, W.; Jensen, J.; Fowler, B.T.; Fleming, J.C. Eyelid Spontaneous Blink Analysis and Age-Related Changes Through High-Speed Imaging. Ophthalmic Plast. Reconstr. Surg. 2019, 35, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.A.; Shipley, R.J.; Edirisinghe, M.; Ezra, D.G.; Rose, G.; Best, S.M.; Cameron, R.E. High-speed camera characterization of voluntary eye blinking kinematics. J. R. Soc. Interface 2013, 10, 20130227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Happak, W.; Burggasser, G.; Gruber, H. Histochemical characteristics of human mimic muscles. J. Neurol. Sci. 1988, 83, 25–35. [Google Scholar] [CrossRef]
- Goodmurphy, C.W.; Ovalle, W. Morphological study of two human facial muscles: Orbicularis oculi and corrugator supercilia. Clin. Anat. 1999, 12, 1–11. [Google Scholar] [CrossRef]
- Hwang, K.; Huan, F.; Kim, D.J. Muscle Fiber Types of Human Orbicularis Oculi Muscle. J. Craniofac. Surg. 2011, 22, 1827–1830. [Google Scholar] [CrossRef]
- Cheng, N.C.; Liao, S.L.; Wang, I.J.; Lin, I.C.; Tang, Y.B. Fiber type and myosin heavy chain compositions of adult pretarsal orbicularis oculi muscle. J. Mol. Histol. 2007, 38, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Costin, B.R.; Plesec, T.P.; Kopplin, L.J.; Chundury, R.V.; McBride, J.M.; Levine, M.R.; Perry, J.D. Regional variations in orbicularis oculi histology. Ophthalmic Plast. Reconstr. Surg. 2015, 31, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Kajiya, K. Age-related structural alterations of skeletal muscles and associated capillaries. Angiogenesis 2020, 23, 79–82. [Google Scholar] [CrossRef]
- Abe, T.; Spitz, R.W.; Wong, V.; Viana, R.B.; Yamada, Y.; Bell, Z.W.; Chatakondi, R.N.; Loenneke, J.P. Assessments of facial muscle thickness by ultrasound in younger adults: Absolute and relative reliability. Cosmetics 2019, 6, 65. [Google Scholar] [CrossRef] [Green Version]
- Volk, G.F.; Karamyan, I.; Klingner, C.M.; Reichenbach, J.R.; Guntinas-Lichius, O. Quantitative magnetic resonance imaging volumetry of facial muscles in healthy patients with facial palsy. Plast. Reconstr. Surg. Glob. Open 2014, 2, e173. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Wong, V.; Spitz, R.W.; Viana, R.B.; Bell, Z.W.; Yamada, Y.; Chatakondi, R.N.; Loenneke, J.P. Influence of sex and resistance training status on orofacial muscle strength and morphology in healthy adults between the ages of 18 and 40: A cross-sectional study. Am. J. Hum. Biol. 2020, 32, e23401. [Google Scholar] [CrossRef] [PubMed]
- Viana, R.B.; da Sliva, W.F.; de Lira, C.A.B. Effects of chewing training on orofacial and cognitive function in healthy individuals: A systematic review. Cosmetics 2020, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Abe, T.; Viana, R.B.; Wong, V.; Bell, Z.W.; Spitz, R.W.; Yamada, Y.; Thiebaud, R.S.; Loenneke, J.P. The influence of training variables on lingual strength and swallowing in adults with and without dysphagia. JCSM Clin. Rep. 2020, 5, 29–41. [Google Scholar]
- Wong, V.; Abe, T.; Spitz, R.W.; Bell, Z.W.; Yamada, Y.; Chatakondi, R.N.; Loenneke, J.P. Effects of Age, Sex, Disease, and Exercise Training on Lip Muscle Strength. Cosmetics 2020, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Van Borsel, J.; De Vos, M.C.; Bastiaansen, K.; Welvaert, J.; Lambert, J. The effectiveness of facial exercise for facial rejuvenation: A systematic review. Aesthet. Surg. J. 2014, 34, 22–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, T.; Loenneke, J.P. The Influence of Facial Muscle Training on the Facial Soft Tissue Profile: A Brief Review. Cosmetics 2019, 6, 50. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Jeon, S.; Kim, J.-K.; Hwang, J.S. Effects of Kyunghee Facial Resistance Program (KFRP) on mechanical and elastic properties of skin. J. Dermatol. Treat. 2015, 27, 191–196. [Google Scholar] [CrossRef]
- Alam, M.; Walter, A.J.; Geisler, A.; Roongpisuthipong, W.; Sikorski, G.; Tung, R.; Poon, E. Association of Facial Exercise With the Appearance of Aging. JAMA Dermatol. 2018, 154, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.A.; Ramli, N.M.; Fong, K.C.S.; Goh, K.J. MRI findings of orbicularis oculi hypertrophy due to heavy resistance training on the inferior orbital rim. Neurol. Asia 2013, 18, 427–429. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, T.; Loenneke, J.P. Orbicularis Oculi Muscle Size and Function: Exploring the Influence of Aging and Exercise Training. Cosmetics 2021, 8, 29. https://doi.org/10.3390/cosmetics8020029
Abe T, Loenneke JP. Orbicularis Oculi Muscle Size and Function: Exploring the Influence of Aging and Exercise Training. Cosmetics. 2021; 8(2):29. https://doi.org/10.3390/cosmetics8020029
Chicago/Turabian StyleAbe, Takashi, and Jeremy P. Loenneke. 2021. "Orbicularis Oculi Muscle Size and Function: Exploring the Influence of Aging and Exercise Training" Cosmetics 8, no. 2: 29. https://doi.org/10.3390/cosmetics8020029
APA StyleAbe, T., & Loenneke, J. P. (2021). Orbicularis Oculi Muscle Size and Function: Exploring the Influence of Aging and Exercise Training. Cosmetics, 8(2), 29. https://doi.org/10.3390/cosmetics8020029