Abstract
Inspired by the redox reactions in the preparation of the iron gall ink that has been used in Europe since the Middle Ages, we developed a technology for forming the oil-in-water emulsions, without any surfactants and emulsifiers, by homogenizing a mixture of tannic acid, gallic acid, Fe(D-gluconate)2, and natural oil, which are all approved as cosmetic ingredients. Various plant-derived oils, such as argan oil, olive oil, sunflower oil, grape seed oil, hemp seed oil, peppermint oil, rosemary oil, and ylang-ylang oil, were used as an oil phase for the emulsion formation, and all the fabricated emulsions exhibited the capability of black hair-dyeing. This surfactant-free emulsion technology for combining the hair-dyeing capability of Fe3+–tannin complex with the hair-fortifying property of natural oil would have great impact on the hair-cosmetic industry.
1. Introduction
Gray hair is aesthetically unappealing and can cause psychosocial stress, such as low self-esteem, in some countries, because it is often perceived in conjunction with aging. The detailed etiopathogenesis of hair graying remains unclear, but recent studies suggest that hydrogen peroxide (H2O2), accumulated in scalp hair shafts, induces the oxidative damage in the hair follicle, causing the hair graying [1,2,3,4]. It is reported that the protein expression of catalase and methionine sulfoxide (Met–S=O) reductases A and B is reduced significantly, and the Met residues in tyrosinase, including Met 374 in the active site, are also oxidized to Met–S=O, limiting its catalytic activity for melanogenesis [1]. Another study also indicates the repression of catalase expression and hydroxyl radical-scavenging activities in the unpigmented hair follicle [2]. The oxidative factors have been reported to be multifold, for example, ultraviolet (UV) radiation, pollution, psychological stress, alcohol consumption, smoking, inflammation, and chronic diseases [5,6,7,8,9,10,11], which makes the prevention and treatment of premature graying of hair (PGH) (and hair graying in general) difficult clinically [12].
Temporal hair coloring is widely used for the coverage of premature graying of hair (PGH) [13] and senile gray hair [14], but public concern on the potential side effects of dyes in the hair-dyeing products, such as allergic reactions and tumorigenesis [15,16,17], leads academic researchers and cosmetic companies to seek for alternatives [18,19,20,21,22,23,24,25,26,27,28]. For example, a mixture of graphene sheets, vitamin C, and chitosan was utilized for the black hair-dyeing formulation that had an antistatic property and enhanced thermal conduction [18]. Various biocompatible oxidation conditions for polydopamine deposition to hairs have also been investigated [19,20,21,22,23,24]. In addition, there has been much effort on the functional combination of hair coloring and strengthening (or other hair-boosting functions) based on nature-derived, biofriendly compounds [25]. However, in some cases, hair-boosting compounds are oil-soluble, requiring an emulsion formation, which generally employs surfactants or emulsifiers for emulsion stability. In this work, considering that the surfactants would be potentially irritable to the hair, scalp, and skin, we investigated a surfactant-free, oil-in-water emulsion technology for black hair-dyeing formulation, in which the incorporation of hair-strengthening ingredients was possible. Specifically, we utilized the material-independent coating property of Fe3+–tannin complex [29] for both emulsion stabilization and hair dyeing.
2. Materials and Methods
Gallic acid (3,4,5-trihydroxybenzoic acid, ≥97.5%, Merck), tannic acid (Merck), iron(II) gluconate dihydrate (Fe(D-gluconate)2·2H2O, ≥98%, Merck), and Nile Red (Nile Blue A oxazone, TCI) were used as received. Deionized (DI) water (18.3 MΩ∙cm) from Milli-Q Direct 8 (Millipore) was used. Argan oil, olive oil, sunflower oil, grape seed oil, hemp seed oil, peppermint oil, rosemary oil, and ylang-ylang oil were purchased from local markets in Daejeon, Korea. 1,2-Hexanediol (COSING REF No.: 54,170) was purchased from Dong Bang LB. The oil, as an internal phase (20% v/v), was added to a Fe2+–tannin solution ([Fe2+] = 10 mM; [GA] = 10 mM; [TA] = 1 mM) and broken into small droplets by a homogenizer (T 25 digital ULTRA-TURRAX®, IKA®). After 24 h of reaction with air exposure, the resulting emulsions were characterized by confocal laser-scanning microscopy (CLSM). For the CLSM analysis, the oil was mixed with Nile Red (λmax: 553 nm) for easy visualization before emulsion formation. The bleached blonde hair sample was immersed for 30 min in the emulsion, dried with a hair dryer, shampooed with a commercially available hair shampoo (Amorepacific) in tap water at 20 °C, and dried with a hair dryer. In the long-term spraying experiment, 1,2-hexanediol was added to the emulsion (pH 5) with the final concentration of 2 wt%, and the set of shampooing–drying–spraying–drying was repeated at 24 h intervals. The L* values were measured with a colorimeter (TES-135A, TES Electrical Electronics Corp.). At least 10 measurements per sample (triplicated) were performed, and the averaged L* values with standard deviation were reported.
3. Results
In the traditional recipe of the iron gall ink (IGI), the use of which dates back to the Middle Ages in Europe, ink blackening is achieved by the air oxidation of Fe2+ to Fe3+ ions in the Fe2+–tannin complex that is prepared by mixing tannins (e.g., gallic acid and tannic acid) from oak galls and green vitriol (iron(II) sulfate, FeSO4) [30,31]. Inspired by the IGI, we recently reported the use of the Fe2+–tannin complex as a reaction precursor for interfacial-film formation [32], protein sequestering [33], black hair-dyeing [28], and single-cell nanoencapsulation (SCNE) [34], as an alternative to the direct method that mixes Fe3+ and tannins [29,35]. Especially, we found that the relatively high O2 level in the oil phase—about 100-fold higher than in water at 298.15 K [36]—enabled the surfactant-free emulsion formation with hexadecane as a model of internal oil phase [32]. In this work, we employed various biofriendly plant oils, as models for the oil phase, in the formation of the surfactant-free emulsions based on our reported protocol, and studied the hair-dyeing capability of the emulsions formed (Figure 1).
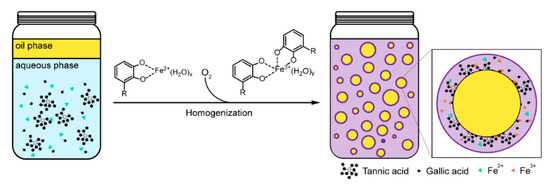
Figure 1.
Schematic of the surfactant-free emulsion formation, inspired by the iron gall ink (IGI).
The main ingredients in the emulsion formation were Fe(D-gluconate)2 (Fe(glu)2, COSING REF No.: 56,253), gallic acid (GA, COSING REF No.: 76,338), and tannic acid (TA, COSING REF No.: 38,472). The final concentrations were set to be 10 mM of Fe(glu)2, 10 mM of GA, and 1 mM of TA, respectively, based on our previous report on the black hair-dyeing capability of the Fe(glu)2/GA/TA combination [28]. The model oils employed in this study were argan oil, olive oil, sunflower oil, grape seed oil, hemp seed oil, peppermint oil, rosemary oil, and ylang-ylang oil. The surfactant-free oil-in-water (o/w) emulsions were formed with 1:4 ratio of the oil to water (χ = 0.2). Note that the use of 20 % (v/v) oils was to investigate the feasibility of emulsion formation by our IGI-based protocol, and the oil percentage, especially for essential oils, would be reduced and/or other oil-soluble compounds be utilized as a main component for the oil phase at the practical setting. The o/w emulsions were blackish to the naked eyes, and no precipitates were observed (Figure 2a and Figure S1). The optical images showed the successful emulsion formation (Figure 2b and Figure S2). The confocal laser-scanning microscopy (CLSM) analysis, with lipophilic Nile Red as an oil-phase marker, directly evidenced the formation and stabilization of the emulsions without any surfactants or emulsifiers (Figure 2c and Figure S3). The hair-coloring capability of the eight different emulsions was investigated by measuring the L* value in the CIELAB L*a*b* color space [37], after immersing a blonde hair sample for 30 min in the emulsion and shampooing (Table 1, Figure 2d and Figure S4). In the CIELAB L*a*b* color space, the L* (lightness) value is defined to be 0 for black and 100 for white. Therefore, lower L* values mean that the samples are darker in the lightness coordinate. Table 1 shows that the shampooing step did not change the L* values noticeably, and the L* values remained below 30 after dyeing and shampooing, confirming the black hair-dyeing capability of our surfactant-free emulsion system.
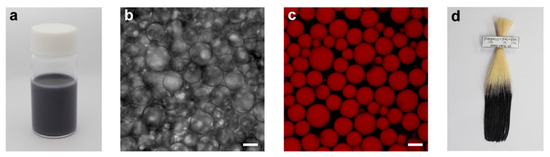
Figure 2.
(a–c) Characterizations of the surfactant-free emulsions with the ylang-ylang oil-based emulsion as a representative. (a) Optical photograph of the emulsion-containing vial. (b) Optical and (c) CLSM images of the emulsion. Scale bar: 10 µm. (d) Optical photograph of the hair sample after dyeing with the ylang-ylang oil-based emulsion. The characterization data for the other emulsions are presented in the Supplementary Materials.

Table 1.
Percent L* value change (%ΔL*) after hair dyeing and shampooing. %ΔL* = (L*sample − L*measured)/ L*sample × 100. AO: argan oil; OO: olive oil; SFO: sunflower oil; GSO: grape seed oil; HSO: hemp seed oil; PO: peppermint oil; RO: rosemary oil; Y-YO: ylang-ylang oil.
We analyzed the emulsion stability at various pH values, because low pH would have damaging effects on the hair and skin. For instance, the average pH of the skin is reported to be 4.9, and the pH of forehead is 4.4 [38]. On the other hand, the pH of the initial emulsion was measured to be around 3.4–3.5 for all the emulsions formed, suggesting the need of pH increase for mild administration to the hair and scalp. In addition, the maintained stability of Fe3+–tannin-based formulation, upon pH increase, is particularly important at the practical setting, because the Fe3+–tannin complex itself is known to precipitate rapidly at the elevated pH [28,39]. We varied the emulsion pH to 4, 5, 6, and 7, and analyzed the emulsion stability by CLSM with Nile Red as a marker. The CLSM images arguably confirmed that all the tested emulsions were stable at the elevated pH values (Figure S5). For example, the initial pH value of the ylang-ylang oil-based emulsion was 3.43, and the structural integrity of the emulsion was maintained at the elevated pH up to 7. We did not test its stability under basic conditions because most hair and skin products are acidic or neutral.
After confirming the emulsion stability at the elevated pH, we investigated the hair-dyeing capability of argan oil-based and ylang-ylang oil-based emulsions, as representatives, after pH adjustment to 5.0. We used the emulsions that had the pH value of 5.0 because previous reports showed that the shampoo with pH of higher than 5.5 had negative effects on the hair and scalp [40]. In the immersion-based dyeing, the argan oil- and ylang-ylang oil-based emulsions gave the L* values of 26.6 ± 4.1 and 28.4 ± 1.9, respectively (L* values of the intact hair samples: 77.3 ± 1.7 and 78.3 ± 1.5), but the L* values increased to 58.1 ± 1.5 and 58.6 ± 2.1 after shampooing, different from the results with the pH-unadjusted emulsions. This result indicated that the increase in pH made the emulsion less persistent to shampooing in the dyeing capability. The hair became oily after emulsion treatment, implying that the emulsion was ruptured on the hair, and the oil coated the hair. We thought that the leaked oil diminished the attractive interactions between the hair and the Fe3+–tannin complex. However, considering the beneficial effects of the essential oils on hair, we thought that our emulsion formulation could be used for natural, surfactant-free hair treatment beyond the dyeing capability. For example, peppermint and rosemary oils have been reported to promote hair growth [41,42].
We tested the long-term hair-dyeing characteristics of the spraying method that is more relevant to and more convenient in the real setting than the emulsion method. It is anticipated that the public prefers simple spraying to immersion for hair coloring on the daily basis. This practice would be beneficial particularly to the formulation for both hair-colorizing and -boosting from Fe3+/TA/GA and functional ingredients in the oil phase, respectively. The emulsion at pH 5 was sprayed onto shampooed blond hair samples, followed by drying. The protocol set of shampooing, drying, spraying, and drying, which would be adopted smoothly to the real-life setting, was repeated at 24 h intervals. Figure 3 clearly showed that the hair sample became darker after shampooing treatment, as spraying was repeated at the daily basis. For example, in the treatment of argan oil-based emulsion, the L* value of the shampooed sample (L*: 76.8 ± 1.0) was 36.3 ± 1.5 after 10 repetitions of the sprayed dyeing, which was much lower to that after the first dyeing and shampooing (55.3 ± 0.7) (Figure 3a). In addition, the L* value after dyeing decreased, although slowly, with repetition of spraying (from 34.4 ± 1.1 to 27.2 ± 1.0 in Figure 3a). The ylang-ylang oil-based emulsion (pH 5) also showed the same trend as the argan oil-based one in the spraying system (Figure 3b). Taken together, these results suggested that our spraying protocol, based on the Fe2+–tannin emulsion, would be used for daily treatment of graying hair.
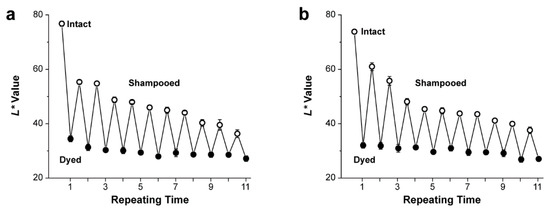
Figure 3.
Graphs of L* values for repeated dyeing and shampooing in the spraying protocol. (a) Argan oil-based emulsion (pH 5). (b) Ylang-ylang oil-based emulsion (pH 5).
4. Conclusions
In this work, we developed a totally surfactant-free fabrication technology for stabilizing the natural oil-containing oil-in-water emulsions, inspired by the iron gall ink, for synergistically utilizing the water-soluble and oil-soluble compounds in the prevention and treatment of hair graying. Our formulation has several advantages in haircare applications: (1) It does not require any surfactants or emulsion-stabilizing chemicals for the emulsion formation, which are potentially irritable to the hair and skin, as well as the unwanted components in the formula to the customers in some countries. (2) The emulsion is stable at the pH values of the skin and therefore not harmful or irritable to the scalp and skin. (3) The color of the Fe3+–tannin complex is innately dark purplish black, which would be useful for black hair-dyeing. We also believe that the advanced combination of therapeutically active, lyophilic compounds, such as Minoxidil [43], in the oil phase and hair-colorizing Fe3+–tannin complex in the form of surfactant-free emulsions would provide a natural formula to the simultaneous treatment for baldness and hair graying, which is our next research thrust.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-9284/8/1/9/s1, Figure S1: Optical photographs of the vials containing the emulsions with argan oil, olive oil, sunflower oil, grape seed oil, hemp seed oil, peppermint oil, rosemary oil, and ylang-ylang oil. Figure S2: Optical photographs of the emulsions with argan oil, olive oil, sunflower oil, grape seed oil, hemp seed oil, peppermint oil, rosemary oil, and ylang-ylang oil. Figure S3: CLSM images of the emulsions with argan oil, olive oil, sunflower oil, grape seed oil, hemp seed oil, peppermint oil, rosemary oil, and ylang-ylang oil. Figure S4: Optical photographs of the hair samples after dyeing with the emulsions having argan oil, olive oil, sunflower oil, grape seed oil, hemp seed oil, peppermint oil, rosemary oil, and ylang-ylang oil. Figure S5: CLSM images of the emulsions, having argan oil, olive oil, sunflower oil, grape seed oil, hemp seed oil, peppermint oil, rosemary oil, and ylang-ylang oil, at various pH values.
Author Contributions
Conceptualization: E.K.K. and I.S.C.; methodology: E.K.K. and I.S.C.; validation: S.Y.H., E.K.K., and I.S.C.; investigation: S.Y.H. and E.K.K.; writing: S.Y.H., E.K.K., and I.S.C.; visualization: S.Y.H.; supervision: I.S.C.; project administration: I.S.C.; funding acquisition: I.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Cannabis Medical, Inc., and Hansol Poultry.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wood, J.M.; Decker, H.; Hartmann, H.; Chavan, B.; Rokos, H.; Spencer, J.D.; Hasse, S.; Thornton, M.J.; Shalbaf, M.; Paus, R.; et al. Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair. FASEB J. 2009, 23, 2065–2075. [Google Scholar] [CrossRef]
- A Shi, Y.; Luo, L.-F.; Liu, X.-M.; Zhou, Q.; Xu, S.-Z.; Lei, T.-C. Premature graying as a consequence of compromised antioxidant activity in hair bulb melanocytes and their precursors. PLoS ONE 2014, 9, e93589. [Google Scholar] [CrossRef]
- Trüeb, R.M. Oxidative stress in ageing of hair. Int. J. Trichology 2009, 1, 6–14. [Google Scholar] [CrossRef]
- Arck, P.C.; Overall, R.; Spatz, K.; Liezman, C.; Handjiski, B.; Klapp, B.F.; Birch-Machin, M.A.; Peters, E.M.J. Towards a “free radical theory of graying”: Melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J. 2006, 20, 1567–1569. [Google Scholar] [CrossRef]
- Emerit, I.; Filipe, P.; Freitas, J.; Vassy, J. Protective effect of superoxide dismutase against hair graying in a mouse model. Photochem. Photobiol. 2004, 80, 579–582. [Google Scholar] [CrossRef]
- Irie, M.; Asami, S.; Nagata, S.; Miyata, M.; Kasai, H. Relationships between perceived workload, stress and oxidative DNA damage. Int. Arch. Occup. Environ. Health 2001, 74, 153–157. [Google Scholar] [CrossRef]
- Epel, E.S.; Blackburn, E.H.; Lin, J.; Dhabhar, F.S.; Adler, N.E.; Morrow, J.D.; Cawthon, R.M. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA 2004, 101, 17312–17315. [Google Scholar] [CrossRef]
- Akin Belli, A.; Etgu, F.; Ozbas Gok, S.; Kara, B.; Dogan, G. Risk factors for premature hair graying in young Turkish adults. Pediatr. Dermatol. 2016, 33, 438–442. [Google Scholar] [CrossRef]
- Shin, H.; Ryu, H.H.; Yoon, J.; Jo, S.; Jang, S.; Choi, M.; Kwon, O.; Jo, S.J. Association of premature hair graying with family history, smoking, and obesity: A cross-sectional study. J. Am. Acad. Dermatol. 2015, 72, 321–327. [Google Scholar] [CrossRef]
- Jo, S.J.; Paik, S.H.; Choi, J.W.; Lee, J.H.; Cho, S.; Kim, K.H.; Eun, H.C.; Kwon, O.S. Hair graying pattern depends on gender, onset age and smoking habits. Acta Derm. Venereol. 2012, 92, 160–161. [Google Scholar] [CrossRef]
- Trüeb, R.M. Association between smoking and hair loss: Another opportunity for health education against smoking? Dermatology 2003, 206, 189–191. [Google Scholar] [CrossRef]
- Kaur, K.; Kaur, R.; Bala, I. Therapeutics of premature hair graying: A long journey ahead. J. Cosmet. Dermatol. 2019, 18, 1206–1214. [Google Scholar] [CrossRef]
- Kumar, A.B.; Shamim, H.; Nagaraju, U. Premature graying of hair: Review with updates. Int. J. Trchology 2018, 10, 198–203. [Google Scholar] [CrossRef]
- Da França, S.A.; Dario, M.F.; Esteves, V.B.; Baby, A.R.; Velasco, M.V.R. Types of hair dye and their mechanisms of action. Cosmetics 2015, 2, 110–126. [Google Scholar] [CrossRef]
- Søsted, H.; Rustemeyer, T.; Gonçalo, M.; Bruze, M.; Goossens, A.; Giménez-Arnau, A.M.; Le Coz, C.J.; White, I.R.; Diepgen, T.L.; Andersen, K.E.; et al. Contact allergy to common ingredients in hair dyes. Contact Dermat. 2013, 69, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Takkouche, B.; Etminan, M.; Montes-Martínez, A. Personal use of hair dyes and risk of cancer: A meta-analysis. JAMA 2005, 293, 2516–2525. [Google Scholar] [CrossRef]
- Zhang, Y.; Sanjose, S.D.; Bracci, P.M.; Morton, L.M.; Wang, R.; Brennan, P.; Hartge, P.; Boffetta, P.; Becker, N.; Maynadie, M.; et al. Personal use of hair dye and the risk of certain subtypes of non-Hodgkin lymphoma. Am. J. Epidemiol. 2008, 167, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhou, L.; Chiou, K.; Huang, J. Multifunctional graphene hair dye. Chem 2018, 4, 784–794. [Google Scholar] [CrossRef]
- Battistella, C.; McCallum, N.C.; Gnanasekaran, K.; Zhou, X.; Caponetti, V.; Montalti, M.; Gianneschi, N.C. Mimicking natural human hair pigmentation with synthetic melanin. ACS Cent. Sci. 2020, 6, 1179–1188. [Google Scholar] [CrossRef]
- Battistella, C.; McCallum, N.C.; Vanthournout, B.; Forman, C.J.; Ni, Q.Z.; La Clair, J.J.; Burkat, M.D.; Shawkey, M.D.; Gianneschi, N.C. Bioinspired chemoenzymatic route to artificial melanin for hair pigmentation. Chem. Mater. 2020, 32, 9201–9210. [Google Scholar] [CrossRef]
- Dong, Y.Y.; Qiu, Y.; Gao, D.; Zhang, K.L.; Zhou, K.; Yin, H.G.; Yi, G.Y.; Li, J.; Xia, Z.N.; Fu, Q.F. Melanin-mimetic multicolor and low-toxicity hair dye. RSC Adv. 2019, 9, 33617–33624. [Google Scholar] [CrossRef]
- Gao, Z.F.; Wang, X.Y.; Gao, J.B.; Xia, F. Rapid preparation of polydopamine coating as a multifunctional hair dye. RSC Adv. 2019, 9, 20492–20496. [Google Scholar] [CrossRef]
- Im, K.M.; Kim, T.W.; Jeon, J.R. Metal-chelation assisted deposition of polydopamine on human hair: A ready-to use eumelanin-based hair dyeing methodology. ACS Biomater. Sci. Eng. 2017, 3, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, W.I.; Youn, W.; Seo, J.; Kim, B.J.; Lee, J.K.; Choi, I.S. Enzymatic film formation of nature-derived phenolic amines. Nanoscale 2018, 10, 13351–13355. [Google Scholar] [CrossRef] [PubMed]
- Tiampasook, P.; Chaiyasut, C.; Sivamaruthi, B.S.; Timudom, T.; Nacapunchai, D. Effect of Phyllanthus emblica Linn. on tensile strength of virgin and bleached hairs. Appl. Sci. 2020, 10, 6305. [Google Scholar] [CrossRef]
- Singh, V.; Ali, M.; Upadhyay, S. Study of colouring effect of herbal hair formulations on graying hair. Pharmacogn. Res. 2015, 7, 259–262. [Google Scholar] [CrossRef]
- Sivaram, G.; Malini, S.; Babu, G. Review of Ayurvedic herbs with Kesharanjana property in the management of canities (Palitya). Int. J. Ayurvedic Med. 2018, 9, 9–12. [Google Scholar]
- Han, S.Y.; Hong, S.-P.; Kang, E.K.; Kim, B.J.; Lee, H.; Kim, W.I.; Choi, I.S. Iron gall ink revisited: Natural formulation for black hair-dyeing. Cosmetics 2019, 6, 23. [Google Scholar] [CrossRef]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; van Koeverden, M.P.; Such, G.K.; Cui, J.; Caruso, F. One-step assembly of coordination complexes for versatile film and particle engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef]
- Bat-Yehouda, M.Z. Les Encres Noires au Moyen Age (jusqu’à 1600) [Black Inks in the Middle Ages (until 1600)]; Centre National de la Recherche Scientifique: Paris, France, 1983; pp. 96–97. [Google Scholar]
- Pone, A.; Brostoff, L.B.; Gibbons, S.K.; Zavalij, P.; Virgah, C.; Hooper, J.; Alnemart, S.; Gaskell, K.J.; Eichhorn, B. Elucidation of the Fe(III) gallate structure in historical iron gall ink. Anal. Chem. 2016, 88, 5152–5258. [Google Scholar] [CrossRef]
- Lee, H.; Kim, W.I.; Youn, W.; Park, T.; Lee, S.; Kim, T.-S.; Mano, J.F.; Choi, I.S. Iron gall ink revisited: In situ oxidation of Fe(II)-tannin complex for fluidic-interface engineering. Adv. Mater. 2018, 30, 1805091. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Lee, J.K.; Choi, I.S. Iron gall ink revisited: Hierarchical formation of Fe(III)-tannic acid coacervate particles in microdroplets for protein condensation. Chem. Commun. 2019, 55, 2142–2145. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, J.; Han, S.Y.; Han, S.; Youn, W.; Choi, H.; Yun, G.; Choi, I.S. Ascorbic acid-mediated reductive disassembly of Fe3+-tannic acid shells in degradable single-cell nanoencapsulation. Chem. Commun. 2020, 56, 13748–13751. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ping, Y.; Ejima, H.; Alt, K.; Meissner, M.; Richardson, J.J.; Yan, Y.; Peter, K.; von Elverfeldt, D.; Hagemeyer, C.E.; et al. Engineering multifunctional capsules through the assembly of metal-phenolic networks. Angew. Chem. Int. Ed. 2014, 53, 5546–5551. [Google Scholar] [CrossRef]
- Battino, R.; Rettich, T.R.; Tominaga, T. The solubility of oxygen and ozone in liquids. J. Phys. Chem. Ref. Data 1983, 12, 163–178. [Google Scholar] [CrossRef]
- Durmus, D. CIELAB color space boundaries under theoretical spectra and 99 test color samples. Color Res. Appl. 2020, 45, 796–802. [Google Scholar] [CrossRef]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, S.; Moon, H.C.; Seo, H.; Kim, J.Y.; Hong, S.-P.; Lee, B.S.; Kang, E.; Lee, J.; Ryu, D.H.; et al. Antimicrobial spray nanocoating of supramolecular Fe(III)-tannic acid metal-organic coordination complex: Applications to shoe insoles and fruits. Sci. Rep. 2017, 7, 6980. [Google Scholar] [CrossRef]
- Dias, M.F.R.G.; de Almeida, A.M.; Cecato, P.M.R.; Adriano, A.R.; Pichler, J. The Shampoo pH can affect the hair: Myth or reality? Int. J. Trichology 2014, 6, 95–99. [Google Scholar] [CrossRef]
- Oh, J.Y.; Park, M.A.; Kim, Y.C. Peppermint oil promotes hair growth without toxic signs. Toxicol. Res. 2014, 30, 297–304. [Google Scholar] [CrossRef]
- Panahi, Y.; Taghizadeh, M.; Marzony, E.T.; Sahebkar, A. Rosemary oil vs Minoxidil 2% for the treatment of androgenetic alopecia: A randomized comparative trial. Skinmed 2015, 13, 15–21. [Google Scholar] [PubMed]
- Abd, E.; Benson, H.A.E.; Roberts, M.S.; Grice, J.E. Minoxidil skin delivery from nanoemulsion formulations containing eucalyptol or oleic acid: Enhanced diffusivity and follicular targeting. Pharmaceutics 2018, 10, 19. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).