Abstract
Background: PlantCrystals are a new concept to produce plant-based formulations. Their principle is based on the diminution of parts of or whole plants. In this study, the effect of a surfactant on stinging nettle leaf PlantCrystals was investigated. Secondly, the contents of bulk material and the PlantCrystals formulation were compared. In addition, for the very first time, the skin penetration of PlantCrystals was investigated. Methods: Stinging nettle leaves were milled with high-pressure homogenization. Sizes were analyzed via light microscopy and static light scattering. To investigate the effect of the milling, the flavonoid and total carotenoid content were determined, and the antioxidant capacity of the formulation was measured via total polyphenol content and DPPH (1,1-diphenyl-2-picrylhydrazyl) assay. Finally, the impact on skin penetration was investigated. Results: Size analysis showed a stabilizing effect of the surfactant, and the chemical analysis revealed higher flavonoid and polyphenol contents for PlantCrystals. The penetration of the formulation into the stratum corneum was shown to be promising; PlantCrystals possessed a visually perceived higher fluorescence and homogeneity compared to the bulk material. Conclusion: The concept of PlantCrystals improved the availability of valuable constituents and the penetration efficacy. The utilization of the natural chlorophyll fluorescence for skin penetration analysis of plant-based formulations proved itself highly effective.
1. Introduction
Eternal youth and beauty have always been desirable for humanity, and attempts to achieve them have taken place since ancient times. Today, the idea has been translated into anti-aging strategies. The target is no longer the conservation of overall health for all time, but rather the comprehensive support of a healthy body and the delaying of age-related metabolic mechanisms [1,2]. From a cosmetic point of view, anti-aging is predominantly related to the visual perception of the human skin, because aging can easily be recognized, e.g., wrinkles or sagging in the skin [1,3].
Nonetheless, skin should not be considered from a cosmetic point of view only; it is the largest organ and the barrier between the body and the outer world [4]. Intact and healthy skin is therefore also important from a medical perspective. Photo-aging (UV-light exposure) is most commonly known as an exogenous factor responsible for skin aging along with other factors, e.g., smoking or air pollution [5]. These factors are often oxidants themselves or induce processes frequently connected to the production of reactive oxygen species (ROS), respectively [1,6]. Moreover, an impaired stratum corneum (SC) with a lost barrier function can cause the generation of ROS, thus damaging underlying cell layers, which results in, e.g., extensive aging, inflammation, or skin cancer. Therefore, the delivery of antioxidants to the skin can delay or prevent the ROS damaging effect, strengthen the SC, and increase its protective function [7,8]. Nowadays, the anti-aging effect of antioxidants has raised their popularity, and the demands of consumers are not just focused on the efficacy and price of a product but on the sustainability, too [9]. Recent studies have shown that plant materials could sustainably be refined by nanoization, which can stimulate undiscovered potential [10,11,12].
Based on these results, PlantCrystals are a new concept for the processing of plants by downsizing the whole material into the lower micro- or even nanosized range without the need for organic solvents or the production of organic waste. Another advantage of PlantCrystals compared to ordinary extracts is the cell disruption of the organic material. This leads to the liberation of all actives of the used material and their retention inside the formulation. On the contrary, an extract is limited to a single or a fraction of ingredients with similar chemical properties (e.g., water or ethanol-soluble). Accordingly, in this study, stinging nettle (SN) leaves (Urtica dioica), also called the “Queen of Herbs,” were used for the production of PlantCrystals and applied on skin [13].
Usually, SN is known as a diuretic, but the pharmaceutical effect is not restricted to that. Numerous health benefits can be attributed to the SN and it is still investigated for many pharmacological effects, e.g., antioxidant, antimicrobial, antimitotic, painkiller, anti-inflammatory, and anticancerogenic [14,15]. Considering skin as a target, SN has a great variety of constituents with beneficial effects, e.g., chlorophyll, carotenoids, polyphenols [15,16]. The literature reports several favorable pharmacological properties after dermal application of SN extracts, e.g., anti-inflammatory and anti-oxidative [17].
Up until now, PlantCrystals have never been applied and analyzed on the skin. As PlantCrystals can be produced with countless numbers of plants, an analysis without the need of an individualized method for every sample would be beneficial. The approach to overcome this problem was the utilization of one thing most plants have in common: Chlorophyll (a and b) (Figure 1). Chlorophyll fluorescence is well known and has been investigated [18]. If histological sections exhibit penetrated chlorophyll in a sufficient amount, which can be recognized via fluorescence microscopy, the skin penetration of plant-based formulations could easily be analyzed.
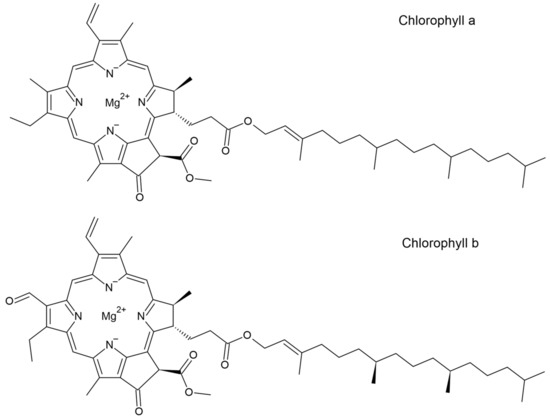
Figure 1.
Structural formula of chlorophyll. Top: Chlorophyll a. Bottom: Chlorophyll b.
The main objective of this study was the development of an SN PlantCrystals formulation for dermal application and the estimation of the SC penetration. SN had not been processed into PlantCrystals in the past; consequently, the first aim was the establishment of a process that is able to downsize the coarse material into the lower micrometer or even smaller size range. Furthermore, the impact of an additional surfactant was evaluated on the obtained sizes and agglomeration of the formulation. Secondly, a bulk suspension and the SN PlantCrystals was investigated with different assays to compare the polyphenol, flavonoid, and carotenoid content, and the antioxidant capacity. Abraham et al. showed already that the impact of the diminution on the activity depends on the chosen plant and the production process [11]. Therefore, a comparison of processed material and bulk material is inevitable to prove that the applied process is beneficial for the formulated plant. After achieving these prerequisites, the dermal application was checked with the new concept of the “fingerprint of nature” by detecting the natural chlorophyll fluorescence to estimate the SC penetration. This would lead to a method that enables a fast, easy, and cost-effective possibility to estimate the penetration of plant-based formulations into the skin.
2. Materials and Methods
2.1. Production of PlantCrystals
Dried SN leaves of organic quality were kindly provided by Blank’s GmbH and Co. KG (Uplengen, Germany) (Figure 2A). The coarse material was downsized with a coffee mill (CM) (CG9100, AICOK, Guangdong, China) to obtain a powdered sample for further processing. Subsequently, the powdered sample (Figure 2B) was dispersed in water at a concentration of 3% (w/w). Additionally, a sample containing 3% (w/w) of the FDA-approved surfactant TPGS (α-Tocopherol Polyethylene Glycol Succinate, Gustav Parmentier GmbH, Frankfurt a. M., Germany) was produced. Both samples were stirred overnight at room temperature to let the particles swell for better processability and to ensure that no change in size will occur later on (CM+S). In the next step, the samples were deagglomerated with an Ultra-Turrax (T 25 digital, IKA-Werke GmbH & Co. KG, Staufen, Germany) for one minute with increasing rotational speed from 3000 to 25,000. The next premilling step was conducted with a Miccra D27 (MICCRA GmbH, Heitersheim, Germany) to further reduce the size of bigger particles. The samples were processed four times with increasing rotational speed for 5 min with 7000, 12,000, 18,000, and 24,000 rpm. The final particle diminution was done via high-pressure homogenization (HPH) with a LAB 40 (APV Gaulin, Lübeck, Germany) at 500, 750, 1000, and 1500 bar five times each (Figure 2C). For content analysis, samples without TPGS were analyzed to exclude the impact of TPGS during the measurements or a possible additional extraction or protection of active ingredients due to TPGS micelle formulation [19]. For skin penetration studies, the samples containing TPGS were used to guarantee a good wettability and spreadability of the PlantCrystals formulation. As a control, to show the effect of the diminution, a bulk suspension of overnight-swelled SN leaves (Figure 2A) with the same concentration was used.
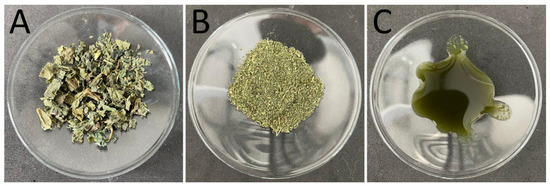
Figure 2.
Macroscopic images of the samples. (A): Coarse stinging nettle leaves (bulk). (B): Dry milled powder (coffee mill (CM)). (C): PlantCrystals suspension (high-pressure homogenization (HPH)).
2.2. Particle Size Analysis
Light microscopy (Olympus BX53, Olympus Cooperation, Tokyo, Japan) was used for visual examination of the samples. Further particle size analysis was carried out via static light scattering (SLS, Mastersizer 3000, Malvern Instruments, Kassel, Germany), with a refractive index of 1.47 and absorption index of 0.01. Results were calculated as a volumetric (D(v)) and numeric (D(n)) distribution. The volume-based size distribution D(v)0.x means that x % of the total volume (summarized volume of all particles) is represented by particles with a size equal to or lower than the given number. Therefore, the D(v)-value emphasizes larger-sized particles to the power of three and is important for checking for large agglomerates and particles. A numerically based size distribution D(n)0,x means that x% of the particles are equal to or smaller than the given value. The D(n)-value is of great importance regarding the skin study, as it emphasizes the main fraction of particle sizes present in the sample, which are mainly involved in the penetration process. A combination of volumetric and numeric results should give further insights into the sample processing and impact of the surfactant on large-sized particle fractions (D(v)) and on the main particle size fraction (D(n)).
2.3. Total Polyphenol Content
The Folin–Ciocalteu colorimetric assay was used to assess the total polyphenol content and subsequently the antioxidant capacity of the samples [20]. First, 100 μL of the SN suspension was mixed with 200 µL of Folin–Ciocalteu’s reagent (Merck KGaA, Darmstadt, Germany) and 2 mL purified water, and was afterward incubated at room temperature for 5 min. After that, 1 mL of 20% (w/v) Na2CO3 was added and incubated in the dark for 1 h. Finally, the obtained reaction mixture was spectroscopically measured at 765 nm (Multiskan GO, Thermo Scientific, Dreieich, Germany). The calculation of the content was done with respect to gallic acid as standard, and results are expressed as gallic acid equivalent (mg GAE).
2.4. Flavonoid Content
For the determination of the flavonoid content, an aluminum complex formation reaction was used, which is a widely used spectrophotometric method to determine the different flavonoid compounds in food and plant samples [21]. Further, 100 µL of each diluted suspension was added to 100 µL of 2% (w/v) AlCl3 ethanol solution in a 96-well plate and incubated in the dark for 1 h. After that, the absorbance of the samples was measured at 420 nm. The calculation of the content was done with respect to quercetin (Biomol GmbH, Hamburg, Germany) as standard and the results are expressed as quercetin equivalent (µg QE).
2.5. Total Carotenoid Content
The carotenoid content of the samples was determined by measuring the carotenoids spectral absorption at a specific wavelength, using the method after Rodriguez-Amaya [22]. The absorbance of each sample was measured at 450 nm. The calculation was done with respect to the standard curve of β-carotene (TCI Deutschland GmbH, Eschborn, Germany), and results are expressed as µg β-carotene equivalent (µg βCE).
2.6. Antioxidant Capacity
The antioxidant capacity was assessed using DPPH assay, which employs 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radicals as an indicator [23]. Briefly, 0.2 mM methanolic solution of DPPH (Sigma-Aldrich Corporation, St. Louis, MO, USA) was prepared, and 100 µL of the diluted samples was added into a 96-well plate and mixed with 100 µL of the DPPH solution. The plate was incubated in the dark for 30 min, and absorbance was measured at 517 nm [23]. The free radical scavenging activity was calculated according to the following equation:
where %RSA is the radical scavenging activity in percentage, ADPPH is the DPPH absorbance, and Asample is the sample absorbance. The sample concentrations and their corresponding %RSA were plotted on a graph to calculate the IC50 value.
%RSA = (ADPPH − Asample/ADPPH) × 100%
2.7. Statistical Analysis
Statistical analysis of the data was done using GraphPad Prism® software (v. 8.3, GraphPad Software, Inc., La Jolla, CA, USA), and a paired t-test was applied for comparison, where p-values < 0.05 were considered statistically significant.
2.8. Determination of the Skin Penetration
The skin penetration study was conducted on fresh porcine ears, obtained from a local slaughterhouse as by-products of the meat production. Pig ears are used as an ex-vivo skin model, due to the similarity between pig and human skin [24,25,26]. After cleaning with purified water and careful drying with paper towels, 50 mg of each sample was applied to an examination area of 2 × 2 cm. Subsequently, the formulations were massaged into the skin for 1 min using a saturated glove [27]. All samples were incubated at 32 °C for 6 h and the skin surface was carefully wiped with wet paper towels to remove any sample residues. Subsequently, punch biopsies of 15 mm diameter were taken, embedded in Tissue-Tek® (Sakura Finetek Europe B.V., Alphen aan den Rijn, The Netherlands), and immediately frozen at −80 °C. Cryosectioning in 20 µm thick vertical cuts was carried out with a cryomicrotome (Mod. 2700, Reichert-Jung, Nußloch, Germany). Fluorescence imaging of all cuts was performed with the Olympus CKX53 (Olympus, Japan), equipped with an Olympus U-HGLGPS light source and 200-fold magnification. The intensity of the fluorescence light source was set to 50%. The exposure time was kept constant to 50 ms for all images. The filter selected for analysis was the DAPI HC filter block system (excitation filter: 540–560 nm, dichroic mirror: 570 nm, emission filter: Starting at 580 nm (LP)).
3. Results and Discussion
3.1. Production of PlantCrystals and Particle Size Analysis
The production of PlantCrystals from coarse SN leaf bulk material was successful. HPH resulted in very small particles, and no intact cells were observed via light microscopy. A distinct differentiation of the samples processed without additional stabilization (Figure 3A) and TPGS (Figure 3B) as surfactant could not visually be observed after light microscopic examination. Only a slightly lower tendency of agglomeration could be perceived for the sample with TPGS as a surfactant.
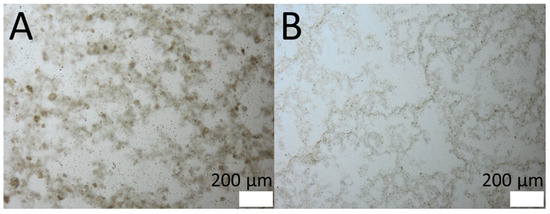
Figure 3.
Light microscopic images of processed stinging nettle (SN) PlantCrystals samples. Magnification: 100-fold. (A): Without additional surfactant. (B): With TPGS as surfactant.
The volumetrically based particle size analysis via SLS revealed that the milling process was able to reduce the sizes from several 100 µm to a lower double-digit µm size range (D(v)0.50). A comparison of the samples showed that the surfactant had a recognizable impact on the milled and swollen sample (CM+S). It could be recognized that the sample without surfactant had the same D(v)0.10-value as the sample stabilized with TPGS (Figure 4), but for the higher D(v) values, the sizes were roughly doubled. As both samples were composed and treated identically except for the addition of surfactant, this shows that TPGS was able to prevent larger sample agglomerations during the swelling. The processed samples showed similar results for all D(v)-values except for the D(v)0.99, which could be interpreted as a sign of a pronounced agglomeration for the sample stabilized with TPGS (Figure 4). Nonetheless, it has to be considered that the D(v)0.99 is considerably variable and not always the most reliable parameter, as one or very few larger-sized particles could have a major influence, e.g., due to sample preparation [28].
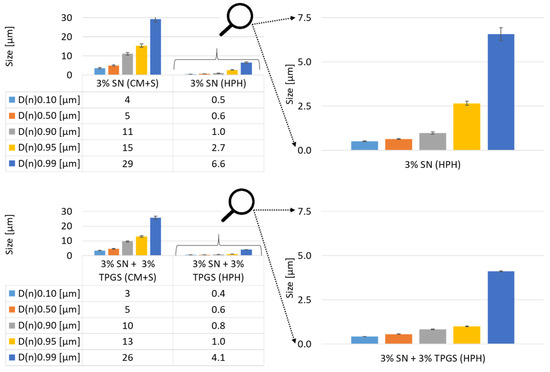
Figure 4.
Volumetrically based size distribution of unprocessed and processed SN samples. Top, left: Without surfactant. Bottom, left: With surfactant. Right: Rescaled results for the corresponding PlantCrystals samples for better visualization and comparison.
The obtained sizes are very promising compared to the results for leaf-based material achieved by Abraham et al. [11]. SLS measurements resulted in nearly the same sizes and size distributions for the final PlantCrystals formulation. A possible drawback of this study compared to Abraham et al. is that the pre-milling was not as effective in the present study and that the Miccra D27 was used, which introduced a lot of heat and air into the sample. On the other hand, the final diminution of the particles by HPH was gentler in this study, as the number of HPH cycles was reduced from 18 to 15 cycles with pressures ≤ 1000 bar and from 10 to 5 cycles with a pressure of 1500 bar [11]. In fact, the similar sizes obtained for PlantCrystals in both studies lead consequently to an interesting theory: Two tremendously different processes were applied, and on top of that, two different surfactants were used (or none), but in the end, similar sizes were obtained. This leads to the assumption that the diminution of plant material via HPH is limited to a certain extent. At least for SN leaves (this study) and sage/laura leaves (Abraham et al.), the indications seem to be very strong and should be investigated in future studies [11]. Overall, both studies show that HPH is very efficient in producing PlantCrystals, but premilling is unavoidable to prevent a blockage of the piston gap. There is still potential for an optimization of the production process, which should on the one hand, be most effective, but also introduce as less heat and air into the sample as possible to preserve as much of the ingredients as achievable, on the other.
The numeric size distribution analysis showed again that the swollen sample included slightly bigger sizes for the samples without surfactant compared to samples containing TPGS (Figure 5). The processed sample possessed similar sizes for lower D-values (D(n)0.10 and D(n)0.50), and large differences could be recognized for higher D-values (D(n)0.95 to D(n)0.99).
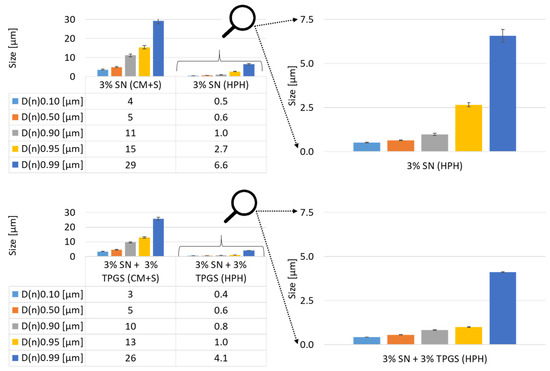
Figure 5.
Numerically based size distribution of unprocessed and processed SN samples. Top, left: Without surfactant. Bottom, left: With surfactant. Right: Rescaled results for the corresponding PlantCrystals samples for better visualization and comparison.
To get a better idea of the process, the effect of the surfactant, and the resulting processed samples, all size analysis (D(v), D(n), light microscopy) must be considered and combined. In general, the measurement setup for SLS cannot distinguish between one big-sized particle and an agglomeration of particles. It is possible to do additional measurements to check for agglomerations, if the agglomeration is not too strong and can be decomposed, e.g., changing the stirring speed or sonication [29,30]. Nonetheless, these procedures are not commonly used and it is possible that they do not have the desired effect. A fast and easy solution is the use of light microscopy, which enables a fast assumption, even for tight agglomerations. Regarding the microscopic analysis, it could be recognized that for the sample without TPGS, agglomerations were darker, and overall, the sample seemed cloudier. On the other hand, single particles of both samples seemed to be in the same size range (Figure 3). This means that differences in the size of SLS measurements are most likely related to agglomeration and not the “real” sizes of the particles.
For an interpretation of the SLS data, it is important to know that the D(v)-values represent and emphasize larger-sized particles exponentially to the power of three, whereas the D(n)-value represents the quantity of the particles [28]. To compare the results, a ratio of the D-values was done by dividing the D(v)- or D(n)-value of the sample without surfactant by the corresponding D-value with additional surfactant (Table 1). A ratio greater than 1.0 would mean that the surfactant had a stabilizing impact, whereas a ratio lower than 1.0 would indicate a destabilizing effect, and a ratio of 1.0 precisely indicates no effect. For the dry-milled and swollen sample (CM+S), the strong increase in the ratio of the D(v)-value shows that preferentially big agglomerates could be prevented by TPGS, whereas the constant ratio of the D(n)-value reveals that the stabilization with TPGS had only a minor impact on smaller agglomerates or single particles. For the processed samples, the opposite can be observed; the ratio of the D(v)-value is nearly constant, but the ratio of the D(n)-value increases with higher D(n)-values. This means that in the case of the processed samples, larger agglomerates occur with and without TPGS (D(v)). On the other hand, the surfactant did inhibit the agglomeration of single particles, and also, large aggregations could at least be reduced to a certain extent (D(n)).

Table 1.
The size ratio of samples without surfactant and with TPGS (D-value without surfactant divided by D-value stabilized with TPGS).
Overall, it can be summarized that TPGS as a surfactant had no major impact on the size or size distribution, but an effect can be recognized. A self-stabilizing effect of the PlantCrystals formulation could not be observed. On the contrary, the PlantCrystals formulation benefited from the addition of the surfactant. HPH proved itself once again as a highly effective technique to produce PlantCrystals formulations and it was able to reduce the main particle size to the desired range around or below 1 µm. However, it requires extensive pre-milling steps to prevent blockage of the machine and cannot be used as a standalone technique [11].
3.2. Content Analysis and Antioxidant Capacity (AOC) Studies
In this section, the analysis was done to the initial unprocessed sample (bulk suspension) and the PlantCrystals to investigate the impact of the milling on the SN bio-actives, especially the antioxidants. SN is well known for its polyphenol, flavonoid, and carotenoid content, which show great health benefits. They have antioxidant, anti-inflammatory, and antitumor activities, as well as many other activities [23,31,32]. In addition, it contains high amounts of chlorophyll, which possess marked antioxidant and antimutagenic activity; furthermore, it can be used as an antiacne and a tissue-stimulating agent [33,34]. All those bio-actives can serve as a superior natural support to strengthen the barrier function of the SC. Several spectrophotometric studies were conducted to investigate the effect of milling techniques on the SN content and antioxidants. Flavonoid and carotenoid contents were measured before and after the milling. In addition, the total polyphenol content and DPPH assay were used to estimate the antioxidant capacity. All results of the content analysis and assays are displayed in Table 2.

Table 2.
Results of content analysis and Antioxidant Capacity (AOC) studies.
Diminution was expected to improve the availability of the plant constituents by the destruction of plant cells, which should result in a further release of the active compounds, thus improving the pharmacological potential of the formulation. In alignment with that, a significant improvement was observed for the flavonoid content (p-value = 0.0165). For the bulk material, the value was 107 ± 2 µg QE and could be improved to 288 ± 38 µg QE for the PlantCrystals suspension. Therefore, it can be stated that HPH leads to a doubled flavonoid availability of the SN PlantCrystals compared to bulk. This improvement is particularly important for underlining the effectiveness of the PlantCrystals technique because the SN flavonoids like ursolic acid and quercetin are considered the most important actives that are responsible for the SN anti-aging effect on the skin. This effect is based on the ability of the constituents to inhibit elastase and collagenase [17]. Furthermore, the improvement in the flavonoid content was supported by the usage of the hydrophilic media in the PlantCrystals formulation as Bourgeois et al. showed the superiority of a hydrophilic extract in regard to antioxidant and anti-aging activities [17].
The obtained results for the total carotenoid content did not reveal any significant improvement after the homogenization process. A possible reason for this is the exposure to heat, air, and light during the production, in addition to the hydrophilic nature of the formulation, which may not support the free availability of lipophilic actives like carotenoids [22].
Polyphenols are well known for their expanded range of health benefits as antioxidant, antimicrobial, anti-inflammatory, antiulcer, antitumor agents, and many other effects [35,36,37]. SN is considered a rich source of different kinds of polyphenols, such as gallic acid, caffeic acid, ferulic acid, quercetin, and rutin [36,38]. The analysis of the polyphenolic content was done to provide a better understanding of the formulation. This assay is also considered an antioxidant capacity assay and it is able to measure the hydrophilic and lipophilic polyphenols simultaneously [39]. Comparable to the results of the flavonoid contents, the total polyphenol content could be improved significantly (p-value = 0.0013). The PlantCrystals sample showed a 65% higher content compared to the bulk material. This strong increase was not expected, because previous studies by Abraham et al. showed an increase of 1.1% after homogenization for argan seeds only [11]. The explanation for this could be the different antioxidants of the plants: i.e., SN is rich in hydrophilic antioxidants and chlorophyll, whereas argan seeds contain mainly lipophilic antioxidants.
The results of the DPPH assay showed that the IC50 value of the formulation could not be improved by PlantCrystals technology. DPPH assay is an efficient antioxidant assay that is commonly used in the literature, but it has a main drawback regarding carotenoid and chlorophyll-rich formulations. The presence of these constituents overlaps photometrically with the DPPH measurement, leading to a false positive reading. This overlapping could explain the unexpected high IC50 value for the PlantCrystals formulation. This was not expected, as previous PlantCrystals formulations always achieved an improvement in the AOC analyzed via DPPH assay [10,11,40].
To summarize, HPH was able to increase the flavonoid content and the AOC via polyphenol measurements but not the carotenoid content and the AOC measurement via DPPH assay. On the one hand, possible improvements can be assigned to the particle size reduction, which would lead to a larger surface area and consequently an improved diffusion and availability of the actives. The destruction of plant cells with HPH also leads to an improved liberation of active ingredients. On the other hand, the diminution technique has an impact on the physicochemical stability of the constituents. The processes used in this study introduced heat and air into the formulation to a certain extent, which could lead to a loss of sensitive constituents, e.g., due to oxidation. Protection from light, appropriate cooling, and sterilization of the sample could be further improvements to produce PlantCrystals in future studies and guarantee better protection of the valuable ingredients.
3.3. Determination of the Skin Penetration
In the last part of this study, a comparison of the penetration behavior of SN bulk and PlantCrystals suspensions was investigated. Fluorescence microscopy enabled visualization of the SN leaf sample on the skin, by tracking the penetration of chlorophyll type a and b. The SN is known for its large quantities of these natural dyes, which are responsible for the green color of the plant. Both substances are able to absorb blue (400–420 nm) and red light (640–680 nm), resulting in a chlorophyll fluorescence at about 690 and 740 nm [41]. This red light fluoresced by chlorophyll can be tracked using fluorescence microscopy.
The results of the skin penetration study of SN leaf samples are displayed in Figure 6. Initially, the autofluorescence of the skin was adjusted to a minimum (Figure 6A). It must be mentioned that even the chlorophyll derived from the unprocessed bulk material showed a visually detectable fluorescence in the SC. However, the skin penetration is inconsistent and inhomogeneous (Figure 6B). The PlantCrystals formulation indicated a more consistent and visually perceived higher fluorescence in the SC (Figure 6C).
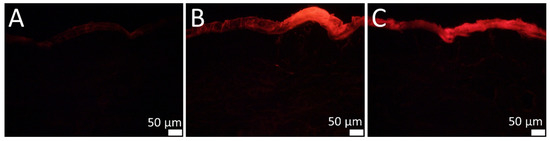
Figure 6.
Fluorescence microscopic images. Magnification: 200-fold. (A): Untreated skin. (B): Skin treated with SN bulk material. (C): Skin treated with SN PlantCrystals stabilized with TPGS.
It has to be emphasized that the concentration of the plant material is the same, and the main difference is the particle size and the amount of destroyed plant cells. This means that differences in penetration can be explained by two effects. First, the disruption of cells will lead to a higher content of free chloroplasts, resulting in a higher concentration and consequently a higher concentration gradient and improved penetration [42,43]. Second, unsolvable material and constituents are much smaller for the PlantCrystals formulation, and this would be comparable to effects known from nanocrystals, e.g., improved adhesion and penetration efficacy. In addition, the homogeneity would be improved due to the smaller particle size and higher total contact area of particles [44,45].
Nonetheless, a penetration into the SC was the target of this study, and it can be recognized that a distinct effect on the penetration depth could not be visually observed for the PlantCrystals compared to the bulk material. The fact that chlorophyll showed no transdermal penetration into living skin layers, even after HPH, can possibly be explained by applying the Lipinski rule of five to the molecule. This rule is originally used to predict the absorption or permeability behavior of drugs after oral administration. The application of this rule in regard to the prediction of the skin penetration of a drug is currently under discussion [46]. It states that poor permeability of a drug can be predicted if more than one criterion applies for the molecule: More than five hydrogen-bond donor groups, ten hydrogen-bond acceptor groups, a molecular weight of more than 500 Da, or an octanol-water partition coefficient (logP value) greater than 5 [47]. Chlorophyll a and b both match all the mentioned criteria besides the hydrogen-bond donor groups (Figure 1). This indicates poor penetration properties per se for the molecules. Thus, PlantCrystals can only improve the consistency and skin penetration depth of chlorophyll to a certain limited extent. Nevertheless, the production of PlantCrystals via HPH increased the amount of chlorophyll a and b in the SC. It can be assumed that if chlorophyll penetrates more homogenously and pronounced into the SC, other plant actives with a higher permeability will be delivered into the skin to a great extent.
Given that the SC was the intended target of this study, PlantCrystals technology is a very promising and versatile formulation approach to exploit the benefits of many natural herbs on skin properties. Thus, for example, many plants possess a moisturizing effect coherent with the principles of corneotherapy to generate healthier skin [48,49]. In addition, components such as essential fatty acids also possess positive effects, e.g., strengthening and repairing the skin barrier, and can even be used as penetration enhancers [50,51]. All these positive effects of plant-derived actives and excipients are naturally incorporated into a PlantCrystals formulation without producing any wastes.
In general, it could be shown that fluorescence microscopy is able to detect the skin penetration of plant-based formulations. This is not only interesting from a scientific point of view, but also for marketing and customer satisfaction, as an effect of a plant-based formulation (e.g., household remedy or skin masks) can be simply proven, e.g., with a special UV lamp after treatment in cosmetic studios. From a scientific point of view, this method is a very fast and cost-effective method to estimate the efficacy of a formulation without the addition of any fluorescence markers. Besides the drawback that only active fluorescence components (for the most part chlorophyll, but others cannot be excluded) can be analyzed, a general assumption about the homogeneity and amount can be done. Moreover, for skin studies with PlantCrystals or plant-based materials, the concept of the “fingerprint of nature” enables the analysis of the effect of a formulation (e.g., used base (gels, creams, etc.) or penetration enhancers) without altering the original formulation or chemical(s) addition.
4. Conclusions
The concept of the “fingerprint of nature” proved its value in this study. The detection of the naturally occurring fluorescent chlorophyll in the stratum corneum was achieved for PlantCrystals, as well as for bulk material. The main target of developing a SN PlantCrystals formulation with an improved SC penetration was achieved and the SC penetration of the PlantCrystals formulation was homogenous compared to the inhomogeneous penetration of the bulk material. The concept of the “fingerprint of nature” should be beneficial for other chlorophyll-rich plant-formulations as an easy and fast technique to estimate their penetration.
During preparation of SN PlantCrystals, described here for the first time, the main particle size of the coarse material could be reduced to the lower micro-sized range. TPGS as additional surfactant prevented agglomeration to a certain extent. The key to activate the full potential of PlantCrystals is the disruption of the plant cells, which was achieved according to the size analysis. This theory was emphasized by the improved liberation of actives and a pronounced antioxidant activity. Higher values of flavonoid content and a better AOC measured utilizing the total phenol content make the SN PlantCrystals a promising formulation for anti-oxidative skin therapy.
Future studies have to prove that these results are not only achievable with “the queen of herbs.” Additional methods to measure the AOC should also be tested for their applicability to chlorophyll-rich PlantCrystals. The commonly used DDPH assay, also utilized in this study, unfortunately could not be used in this study, due to the spectrophotometric overlap between the used reagents and the carotenoid and chlorophyll of the sample.
Author Contributions
Conceptualization, D.K. and C.M.K.; methodology, D.K., R.M.A. and S.W.; formal analysis, D.K., R.M.A. and S.W.; investigation, D.K., R.M.A. and S.W.; resources, C.M.K.; data curation, D.K., R.M.A. and S.W.; writing—original draft preparation, D.K., R.M.A. and S.W.; writing—review and editing, D.K., R.M.A. and J.B.; visualization, D.K., R.M.A. and S.W.; supervision, J.B.; project administration, C.M.K.; funding acquisition, C.M.K. All authors have read and agreed to the published version of the manuscript.
Funding
The work was partly funded by ZIM project: KF ZF4114903AJ8.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data included within the manuscript.
Acknowledgments
The authors would like to kindly thank Blank’s GmbH & Co.KG for the donation of the stinging nettle samples, used in this study. We also like to thank Udo Bakowsky who granted free-of-charge usage of the fluorescence microscope. The authors also thank Soma Sengupta for help with draft reviewing. Reem M. Alnemari would like to thank Taif University in Saudi Arabia for the financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kohl, E.; Steinbauer, J.; Landthaler, M.; Szeimies, R.-M. Skin ageing. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 873–884. [Google Scholar] [CrossRef]
- Mohiuddin, A.K. Skin Aging & Modern Edge Anti-Aging Strategies. Int. J. Dermatol. Skin Care 2019, 1, 8–62. [Google Scholar] [CrossRef]
- Trojahn, C.; Dobos, G.; Lichterfeld, A.; Blume-Peytavi, U.; Kottner, J. Characterizing Facial Skin Ageing in Humans: Disentangling Extrinsic from Intrinsic Biological Phenomena. BioMed Res. Int. 2015, 2015, 318586. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Cannarozzo, G.; Fazia, G.; Bennardo, L.; Tamburi, F.; Amoruso, G.F.; Del Duca, E.; Nisticò, S.P. A New 675 nm Laser Device in the Treatment of Facial Aging: A Prospective Observational Study. Photobiomodul. Photomed. Laser Surg. 2021, 39, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Trouba, K.J.; Hamadeh, H.K.; Amin, R.P.; Germolec, D.R. Oxidative Stress and Its Role in Skin Disease. Antioxid. Redox Signal. 2002, 4, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Vergou, T.; Darvin, M.E.; Patzelt, A.; Meinke, M.C.; Voit, C.; Papakostas, D.; Zastrow, L.; Sterry, W.; Doucet, O. Influence of Topical, Systemic and Combined Application of Antioxidants on the Barrier Properties of the Human Skin. Skin Pharmacol. Physiol. 2016, 29, 41–46. [Google Scholar] [CrossRef]
- Amberg, N.; Fogarassy, C. Green Consumer Behavior in the Cosmetics Market. Resources 2019, 8, 137. [Google Scholar] [CrossRef]
- Griffin, S.; Sarfraz, M.; Farida, V.; Nasim, M.J.; Ebokaiwe, A.P.; Keck, C.M.; Jacob, C. No time to waste organic waste: Nanosizing converts remains of food processing into refined materials. J. Environ. Manag. 2018, 210, 114–121. [Google Scholar] [CrossRef]
- Abraham, A.M.; Alnemari, R.M.; Jacob, C.; Keck, C.M. PlantCrystals—Nanosized Plant Material for Improved Bioefficacy of Medical Plants. Materials 2020, 13, 4368. [Google Scholar] [CrossRef]
- Abraham, A.M.; Alnemari, R.M.; Brüßler, J.; Keck, C.M. Improved Antioxidant Capacity of Black Tea Waste Utilizing PlantCrystals. Molecules 2021, 26, 592. [Google Scholar] [CrossRef]
- Mehegan, M.A. Stinging Nettles—Queen of Herbs. Alchemical Herbal Healing, 1st ed.; Bookbaby: Pennsauken Township, NJ, USA, 2011. [Google Scholar]
- Upton, R. Stinging nettles leaf (Urtica dioica L.): Extraordinary vegetable medicine. J. Herb. Med. 2013, 3, 9–38. [Google Scholar] [CrossRef]
- Semalty, M.; Adhikari, L.; Semwal, D.; Chauhan, A.; Mishra, A.; Kotiyal, R.; Semalty, A. A Comprehensive Review on Phytochemistry and Pharmacological Effects of Stinging Nettle (Urtica dioica). Curr. Tradit. Med. 2017, 3, 156–167. [Google Scholar] [CrossRef]
- Kregiel, D.; Pawlikowska, E.; Antolak, H. Urtica spp.: Ordinary Plants with Extraordinary Properties. Molecules 2018, 23, 1664. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, C.; Leclerc, É.A.; Corbin, C.; Doussot, J.; Serrano, V.; Vanier, J.-R.; Seigneuret, J.-M.; Auguin, D.; Pichon, C.; Lainé, É.; et al. Nettle (Urtica dioica L.) as a source of antioxidant and anti-aging phytochemicals for cosmetic applications. Comptes Rendus Chim. 2016, 19, 1090–1100. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, R.; Khorsandi, K.; Hemmaty, S. Study of the Effect of Surfactants on Extraction and Determination of Polyphenolic Compounds and Antioxidant Capacity of Fruits Extracts. PLoS ONE 2013, 8, e57353. [Google Scholar] [CrossRef]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in Antioxidant Effects and Their Relationship to Phytonutrients in Fruits of Sea Buckthorn (Hippophae rhamnoides L.) during Maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef]
- Ordonez, A.; Gomez, J.; Vattuone, M.; Isla, M.I. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI Press: Washington, DC, USA, 2001. [Google Scholar]
- Katsube, T.; Tabata, H.; Ohta, Y.; Yamasaki, Y.; Anuurad, E.; Shiwaku, K.; Yamane, Y. Screening for Antioxidant Activity in Edible Plant Products: Comparison of Low-Density Lipoprotein Oxidation Assay, DPPH Radical Scavenging Assay, and Folin−Ciocalteu Assay. J. Agric. Food Chem. 2004, 52, 2391–2396. [Google Scholar] [CrossRef]
- Summerfield, A.; Meurens, F.; Ricklin, M.E. The immunology of the porcine skin and its value as a model for human skin. Mol. Immunol. 2015, 66, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Ranamukhaarachchi, S.A.; Lehnert, S.; Ranamukhaarachchi, S.L.; Sprenger, L.; Schneider, T.; Mansoor, I.; Rai, K.; Häfeli, U.O.; Stoeber, B. A micromechanical comparison of human and porcine skin before and after preservation by freezing for medical device development. Sci. Rep. 2016, 6, 32074. [Google Scholar] [CrossRef]
- Jacobi, U.; Kaiser, M.; Toll, R.; Mangelsdorf, S.; Audring, H.; Otberg, N.; Sterry, W.; Lademann, J. Porcine ear skin: An in vitro model for human skin. Skin Res. Technol. 2007, 13, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Nagelreiter, C.; Mahrhauser, D.; Wiatschka, K.; Skipiol, S.; Valenta, C. Importance of a suitable working protocol for tape stripping experiments on porcine ear skin: Influence of lipophilic formulations and strip adhesion impairment. Int. J. Pharm. 2015, 491, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Brar, S.K.; Verma, M. Measurement of nanoparticles by light-scattering techniques. Trends Anal. Chem. 2011, 30, 4–17. [Google Scholar] [CrossRef]
- Kübart, S.A.; Keck, C.M. Laser diffractometry of nanoparticles: Frequent pitfalls & overlooked opportunities. J. Pharm. Technol. Drug Res. 2013, 2, 17. [Google Scholar] [CrossRef]
- Kukric, Z.; Topalic-Trivunovic, L.; Kukavica, B.; Matos, S.; Pavicic, S.; Boroja, M.; Savic, A. Characterization of antioxidant and antimicrobial activities of nettle leaves (Urtica dioica L.). Acta Period. Technol. 2012, 2012, 257–272. [Google Scholar] [CrossRef]
- Pereira, L. Seaweeds as Source of Bioactive Substances and Skin Care Therapy—Cosmeceuticals, Algotheraphy, and Thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef]
- Hayes, M.; Ferruzzi, M.G. Update on the bioavailability and chemopreventative mechanisms of dietary chlorophyll derivatives. Nutr. Res. 2020, 81, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Song, B.H.; Lee, D.H.; Kim, B.C.; Ku, S.H.; Park, E.J.; Kwon, I.H.; Kim, K.H.; Kim, K.J. Photodynamic therapy using chlorophyll-a in the treatment of acne vulgaris: A randomized, single-blind, split-face study. J. Am. Acad. Dermatol. 2014, 71, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I.; Küfrevioglu, O.I.; Oktay, M.; Büyükokuroglu, M.E. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J. Ethnopharmacol. 2004, 90, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Đurović, S.; Pavlić, B.; Šorgić, S.; Popov, S.; Savić, S.; Petronijević, M.; Radojković, M.; Cvetanović, A.; Zeković, Z. Chemical composition of stinging nettle leaves obtained by different analytical approaches. J. Funct. Foods 2017, 32, 18–26. [Google Scholar] [CrossRef]
- Ghaima, K.; Hashim, N.M.; Ali, S.A. Antibacterial and Antioxidant Activities of Ethyl Acetate Extract of Nettle (Urtica dioica) and Dandelion (Taraxacum officinale). J. Appl. Pharm. Sci. 2013, 3, 96–99. [Google Scholar] [CrossRef]
- Orčić, D.; Francišković, M.; Bekvalac, K.; Svirčev, E.; Beara, I.; Lesjak, M.; Mimica-Dukić, N. Quantitative determination of plant phenolics in Urtica dioica extracts by high-performance liquid chromatography coupled with tandem mass spectrometric detection. Food Chem. 2014, 143, 48–53. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough Study of Reactivity of Various Compound Classes toward the Folin−Ciocalteu Reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Griffin, S.; Tittikpina, N.K.; Al-Marby, A.; Alkhayer, R.; Denezhkin, P.; Witek, K.; Gbogbo, K.A.; Batawila, K.; Duval, R.E.; Nasim, M.J.; et al. Turning Waste into Value: Nanosized Natural Plant Materials of Solanum incanum L. and Pterocarpus erinaceus Poir with Promising Antimicrobial Activities. Pharmaceutics 2016, 8, 11. [Google Scholar] [CrossRef]
- Guidi, L.; Tattini, M.; Landi, M. How Does Chloroplast Protect Chlorophyll Against Excessive Light? In Chlorophyll; Jacob-Lopes, E., Zepka, L.Q., Queiroz, M.I., Eds.; InTech: London, UK, 2017. [Google Scholar]
- Scheuplein, R.J.; Blank, I.H. Permeability of the skin. Physiol. Rev. 1971, 51, 702–747. [Google Scholar] [CrossRef] [PubMed]
- Godin, B.; Touitou, E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Deliv. Rev. 2007, 59, 1152–1161. [Google Scholar] [CrossRef]
- Pyo, S.; Meinke, M.C.; Keck, C.M.; Müller, R.H. Rutin—Increased Antioxidant Activity and Skin Penetration by Nanocrystal Technology (smartCrystals). Cosmetics 2016, 3, 9. [Google Scholar] [CrossRef]
- Vidlářová, L.; Romero, G.B.; Hanuš, J.; Štěpánek, F.; Müller, R.H. Nanocrystals for dermal penetration enhancement—Effect of concentration and underlying mechanisms using curcumin as model. Eur. J. Pharm. Biopharm. 2016, 104, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Chaulagain, B.; Jain, A.; Tiwari, A.; Verma, A.; Jain, S.K. Passive delivery of protein drugs through transdermal route. Artif. Cells Nanomed. Biotechnol. 2018, 46, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Gediya, S.; Mistry, R.; Patel, U.; Blessy, M.; Jain, H. Herbal Plants: Used as a Cosmetics. J. Nat. Prod. Plant Resour. 2011, 1, 24–32. [Google Scholar]
- Kapoor, S.; Saraf, S. Formulation and Evaluation of Moisturizer Containing Herbal Extracts for the Management of Dry Skin. Pharmacogn. J. 2010, 2, 409–417. [Google Scholar] [CrossRef]
- Dorni, A.C.; Amalraj, A.; Gopi, S.; Varma, K.; Anjana, S. Novel cosmeceuticals from plants—An industry guided review. J. Appl. Res. Med. Aromat. Plants 2017, 7, 1–26. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: A review. J. Pharm. Pharmacol. 2014, 67, 473–485. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).