2.1.1. Study Design
Study 1 was an open-label, randomized, intraindividual comparison study in healthy adult subjects with dry and sensitive skin. Study visits took place on day 0 (baseline) and study days 1, 2, 7, 14, 21, and 28. In a subset of subjects (called “subgroup”), skin barrier dysfunction was experimentally induced at one skin area on each volar forearm by application of 0.8% sodium lauryl sulfate (SLS, 1 mL) under a semiocclusive patch (Tegaderm®, 3M Health Care, Neuss, Germany) for 24 h. Subjects undergoing SLS challenge had an additional study visit on day -1 to have SLS and patch applied.
There was no overnight confinement of study participants, and subjects returned to the study site without having applied Emollient 1 on that day.
All subjects had the test areas A0, A1, A2 marked on their volar forearms. A0 and A1 were always on the same arm while A2 was located on the contralateral arm. The subgroup had two additional marked areas on the volar forearms (A3 and A4) for the SLS challenge. A3 and A4 were to be placed on different forearms. Every test area had a size of 16 cm2, except A0 which had a size of 5 cm2. Emollient 1 was always applied on the same skin areas of one volar forearm (A0, A1, A3). The areas of the contralateral arm (A2, A4) served as controls and remained untreated with Emollient 1. The locations of A0, A1, and A3 on the same forearm were chosen by randomization. Likewise, the allocation of forearms (left or right) to Emollient 1 treatment was carried out according to a balanced randomization scheme.
Approximately 2 mg/cm2 of Emollient 1 was to be applied on the assigned skin areas on a once-daily schedule. The first application was made with the help of a technician at the study site on day 0 following baseline assessments. No study product was applied on days 1 and 2. Thereafter, the application of Emollient 1 was performed by the subjects themselves at home until day 28. In subjects of the subgroup, SLS patches were removed 30 min before first application (day 0, baseline). At that time, the SLS challenge had been completed. Compliance was verified by weighing each container with Emollient 1 (200 mL plastic tubes) before and after the application period.
2.1.2. Subjects and Assessments
Healthy male and female subjects between 18 and 70 years of age and having skin types II–IV on the Fitzpatrick scale [
15] were eligible for study participation. Subjects were required to have very dry, flaky, and sensitive skin as judged clinically by the study’s dermatologist. For inclusion, females had to be nonpregnant and nonbreastfeeding. Female subjects of childbearing age were required to use reliable methods of contraception during the study.
Subjects were excluded if they had: any skin condition at the target area that would interfere with interpretation of study results (e.g., atopic skin, excessive pilosity, scars, irritated skin, pigmentation disorders); a condition requiring the use of drugs interfering with study assessments within 6 months (systemic retinoids), 3 months (other systemic antiacne medication), 2 months (topical retinoids), 1 month (other topical antiacne medication), or within 2 weeks (antibiotics, antiacne cosmetic products, topical or systemic use of anti-inflammatory drugs or antihistamines) before or during the study; started/changed estrogen-progesterone contraception or hormonal treatment within 3 months before or during the trial; allergies to any ingredient of the study product; history of adverse reactions to cosmetic products; vaccination within 2 weeks prior to or during the trial; or desensitization treatment within 6 months before start of the study.
Subjects were not allowed to have had any noninvasive (e.g., scrub, cleansing) or invasive (e.g., laser treatment, peeling) cosmetic interventions on the test areas within 1 month and 2 months, respectively, prior to and during the study. Similarly, intensive exposure of test areas to ultraviolet light was not permitted within 1 month before and throughout the trial. Subjects were also not allowed to use topical preparations other than the study product on the target area during the course of the study. On days of study visits, subjects had to come to the trial center without having applied anything on the forearms. Moreover, the use of detergents was not allowed on test areas since the previous evening; neither was the consumption of hot beverages within 2 h before instrumental measurements.
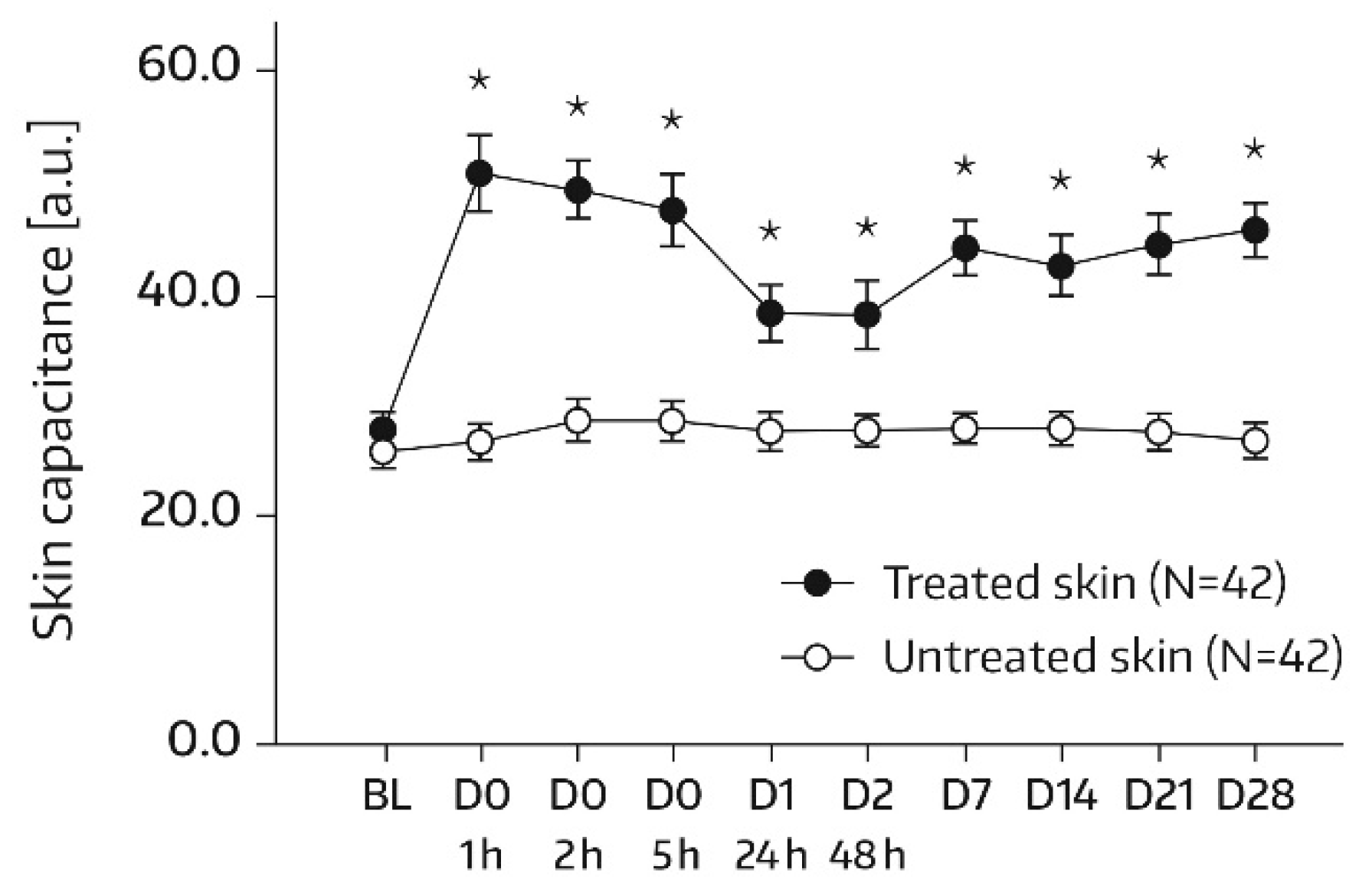
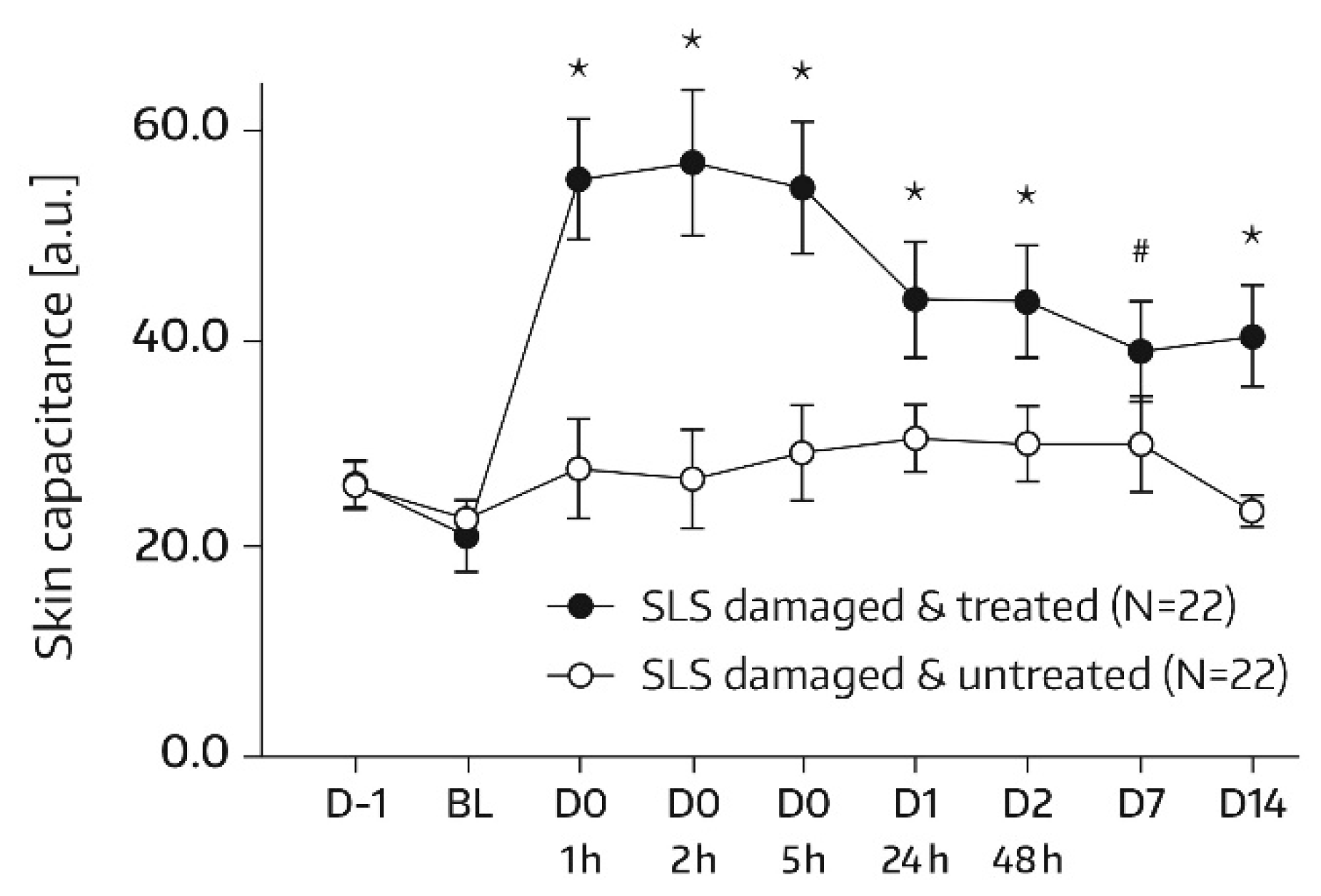
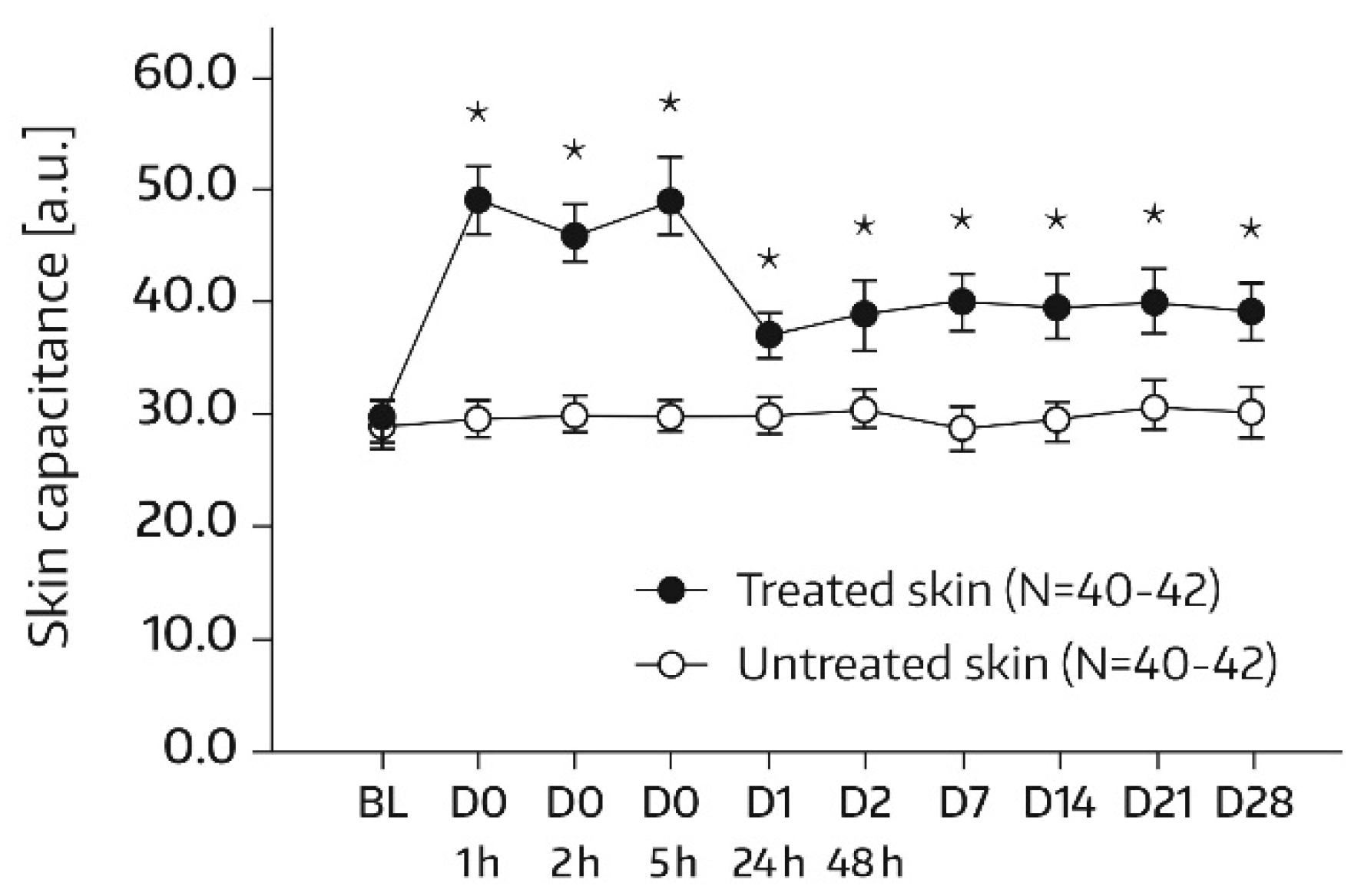
SC hydration was assessed by corneometry (Corneometer
® CM825, Courage & Khazaka, Cologne, Germany), which determines the electrical capacitance of the skin surface. Capacitance is considered a function of the SC water content [
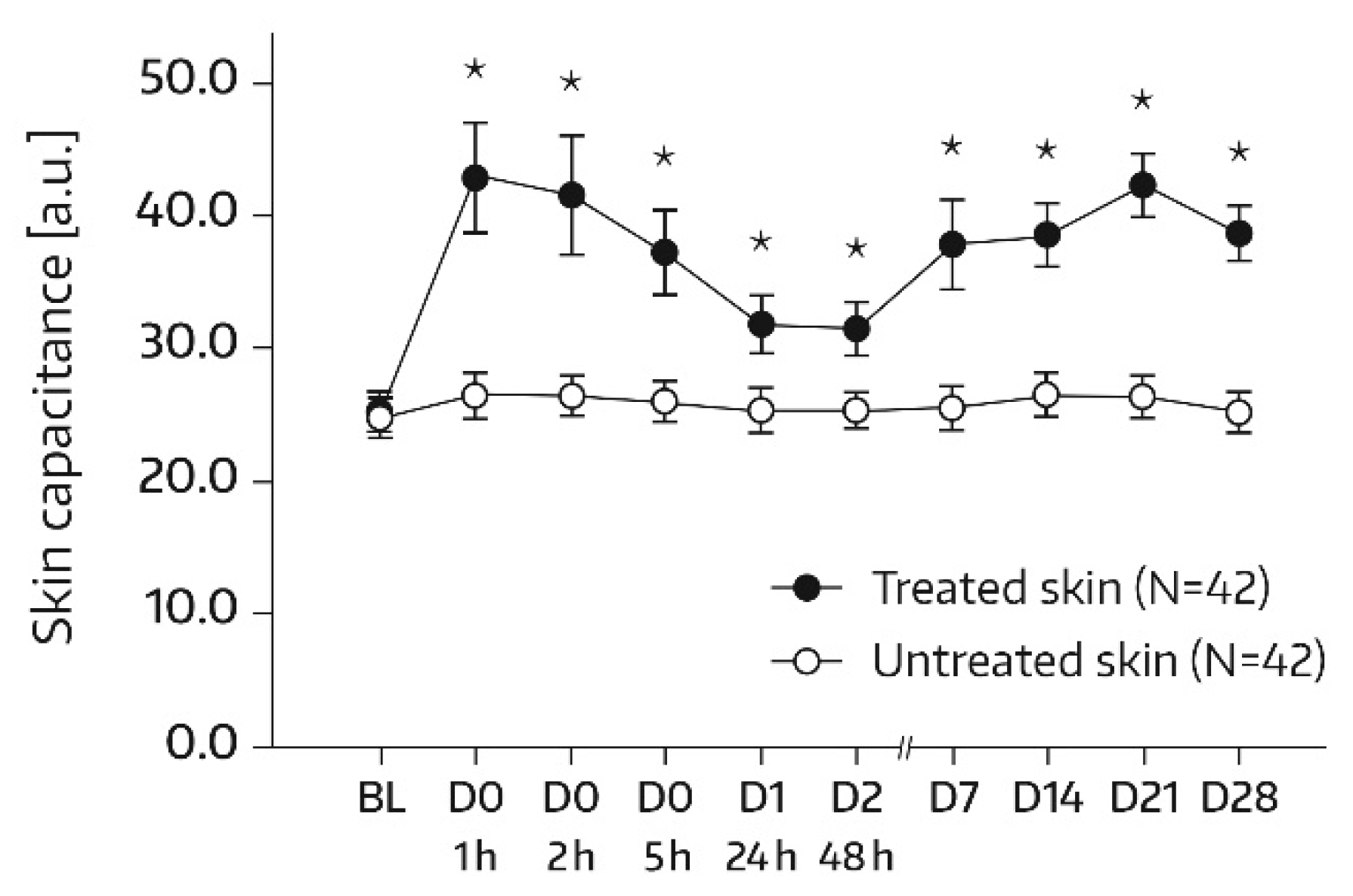
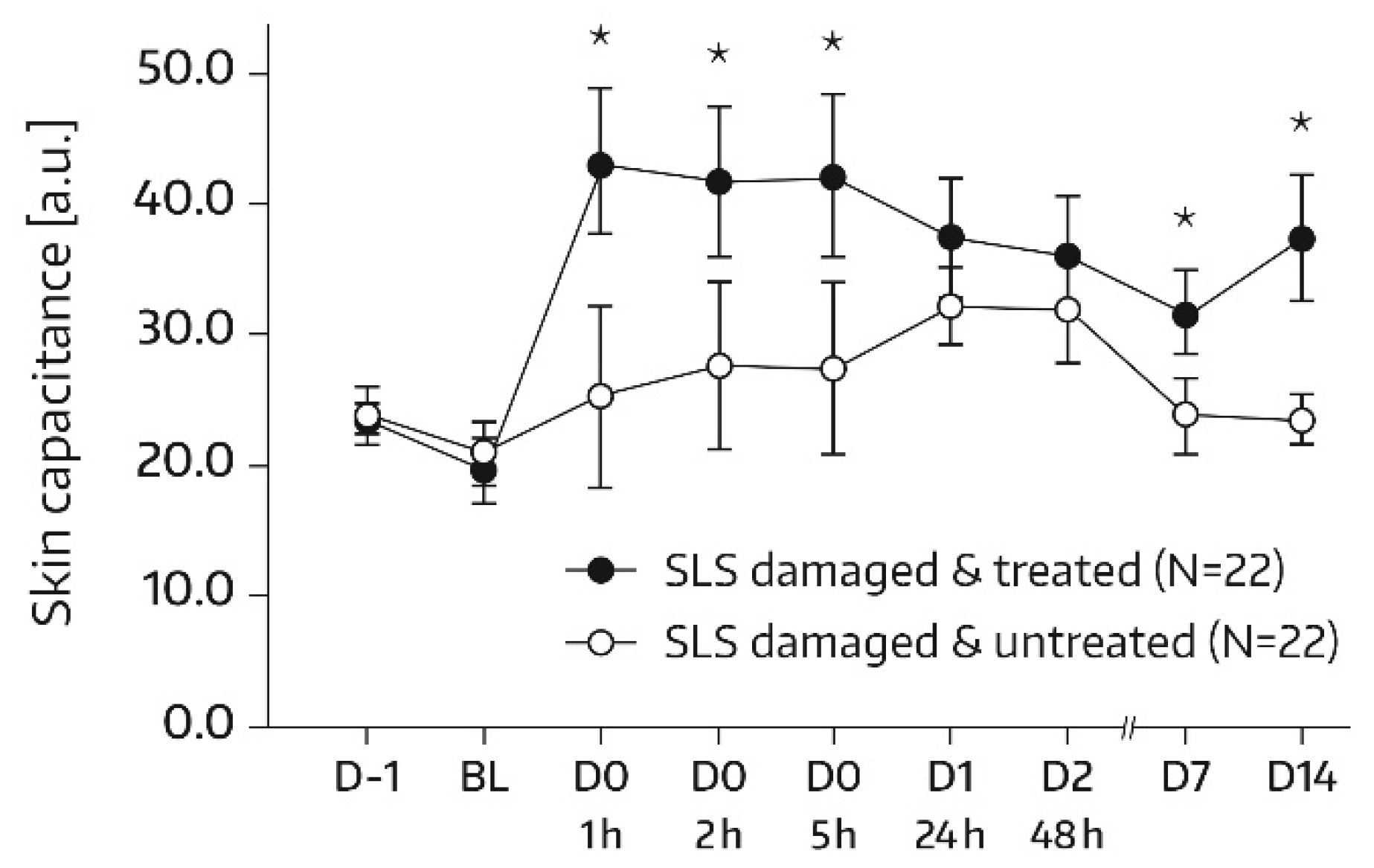
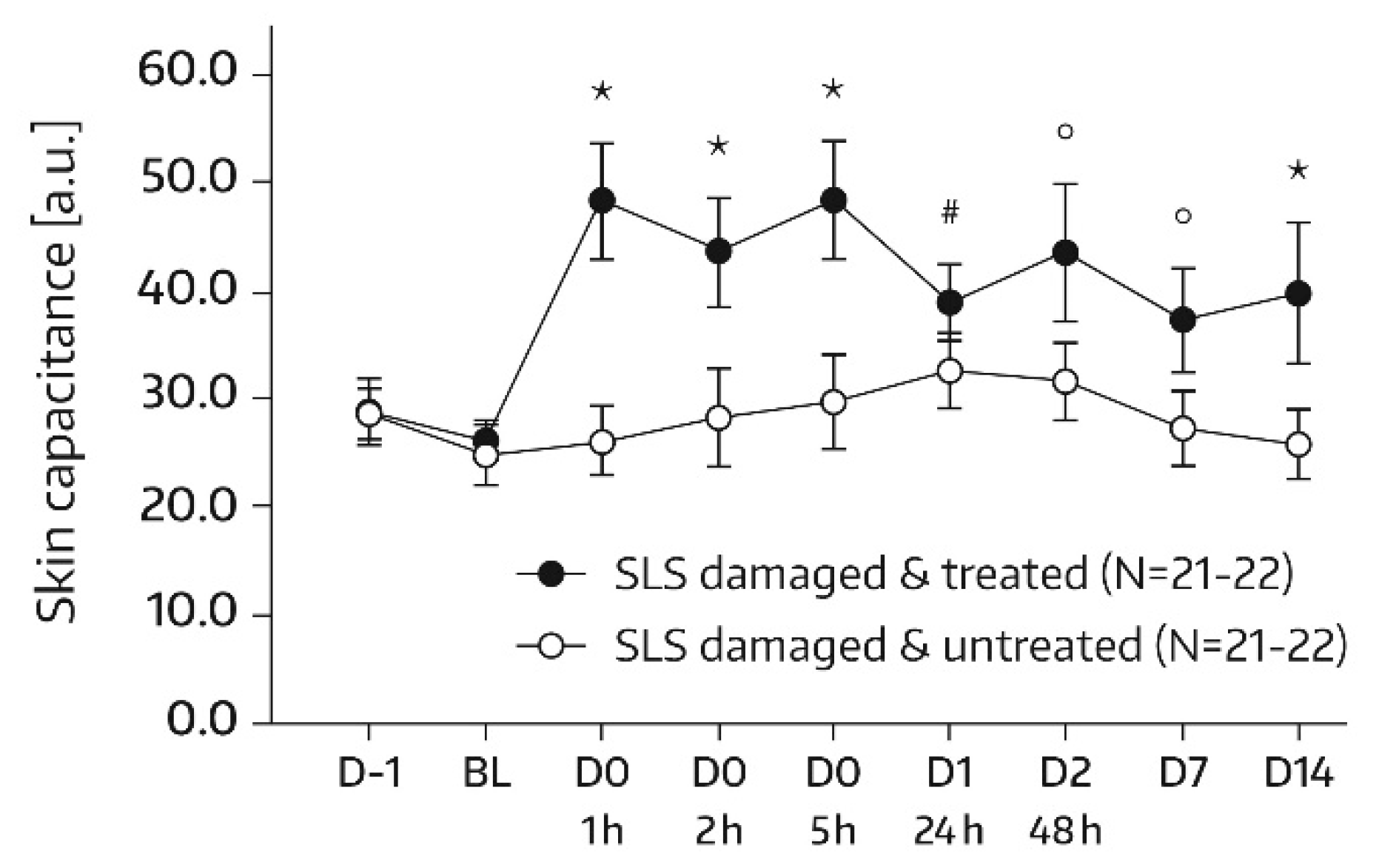
16]. Measurements were conducted on each of the two test areas (A1 and A2) at baseline and at 1, 2, 5, 24 (day 1), and 48 h (day 2) after the first and single application of Emollient 1. Additional measurements took place on study days 7, 14, 21, and 28. In subjects of the subgroup, SC hydration was also quantified in skin areas A3 and A4. For these areas, the assessment time schedule was the same as for areas A1 and A2, except for an additional measurement on day -1 before SLS application and omission of the day‑21 and day‑28 assessments. Three measurements were performed in the skin areas per assessment time. An increase in corneometry/skin capacitance values reflects improved skin hydration [
17].
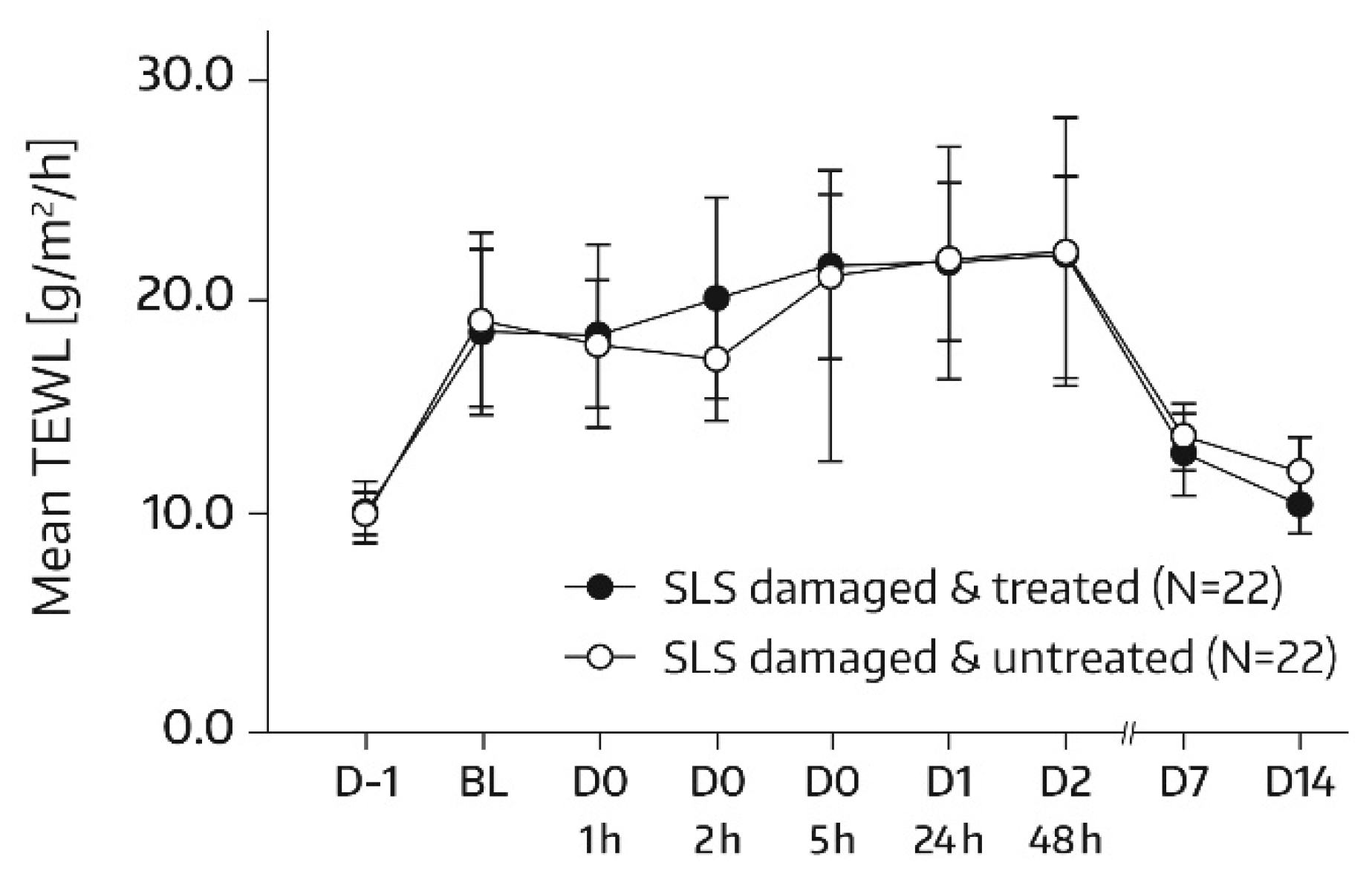
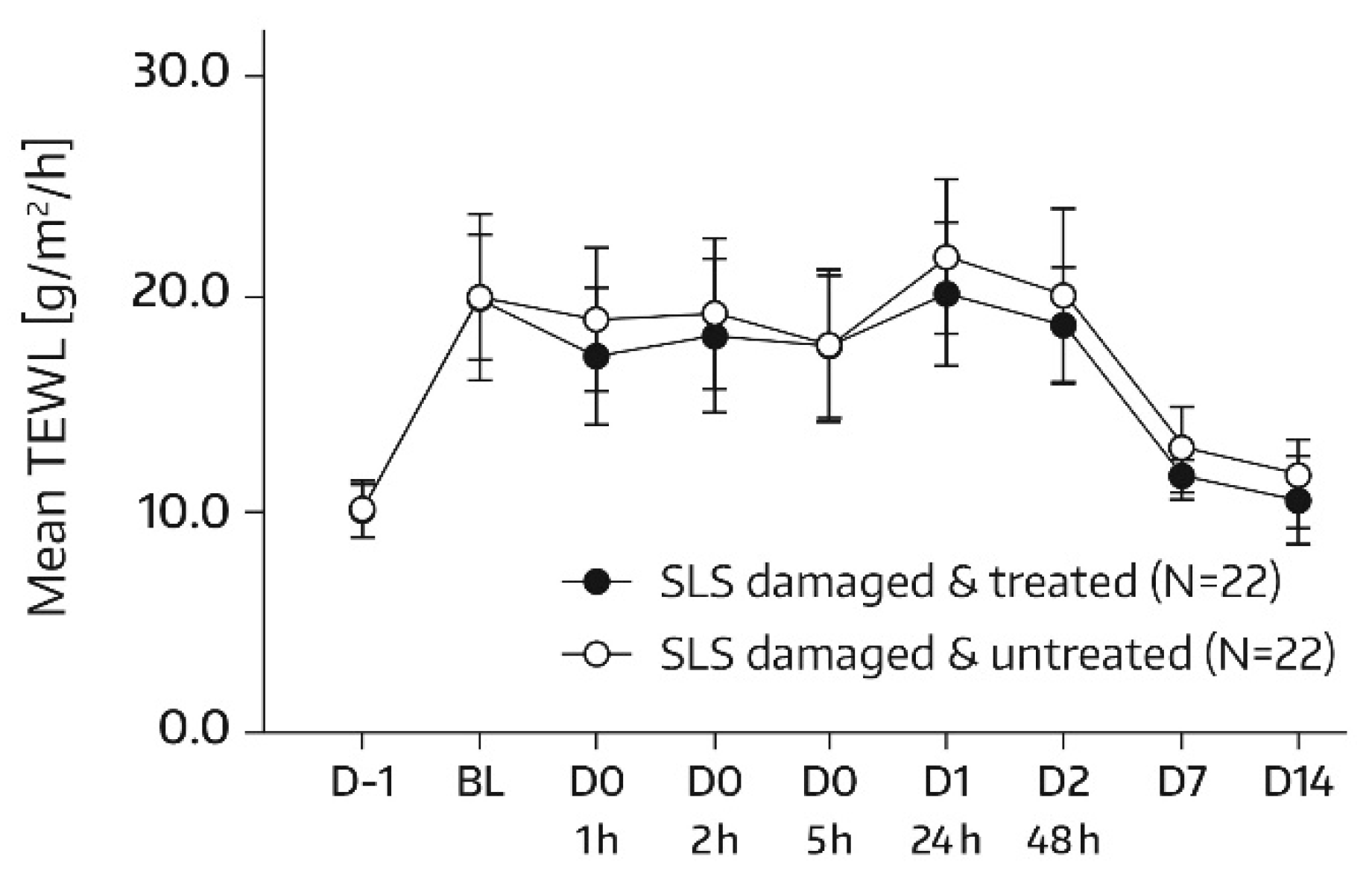
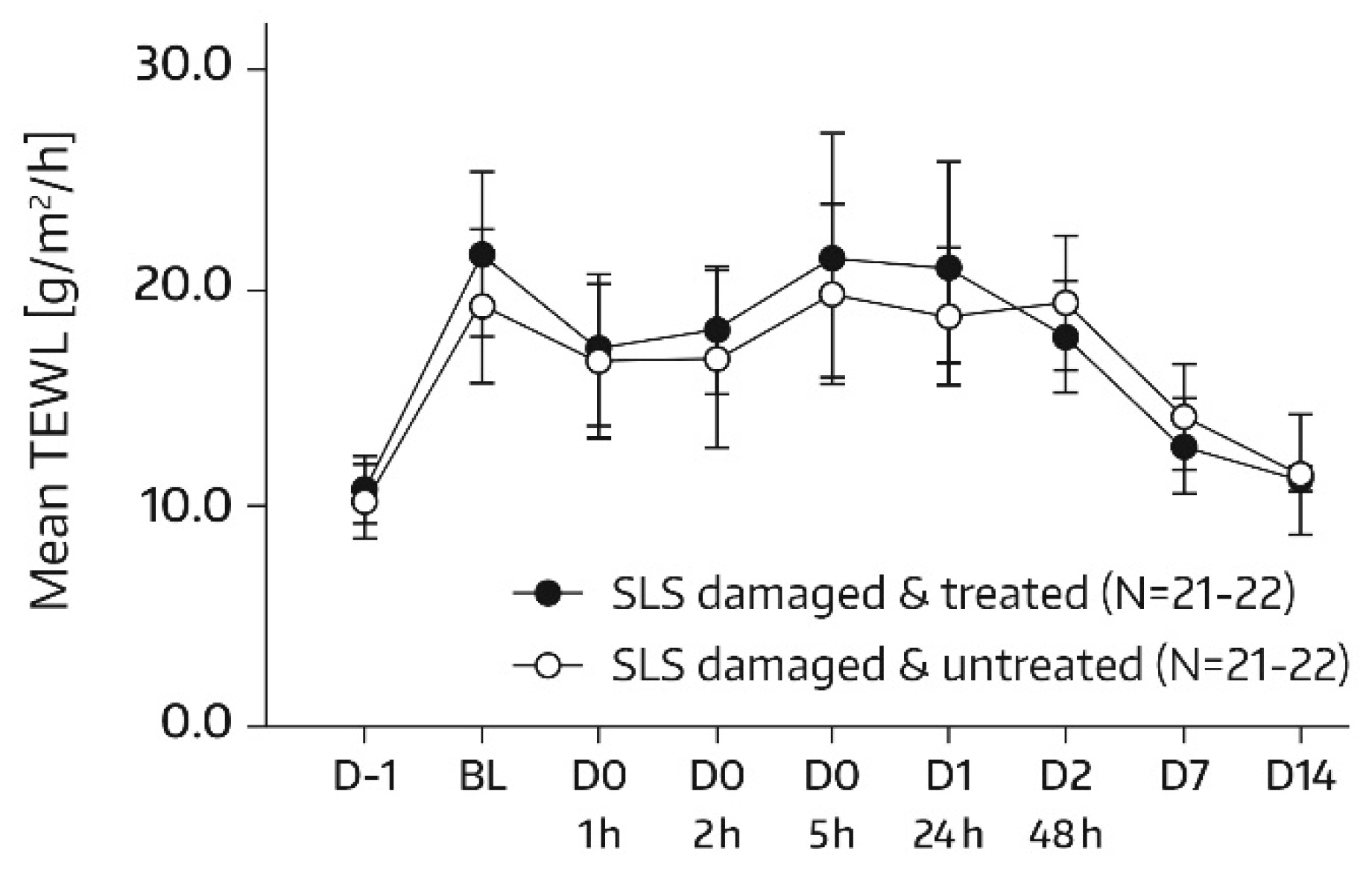
TEWL (MPA Tewameter
® TM300, Courage & Khazaka, Cologne, Germany) measurements were performed on the allocated skin areas (A1 and A2) according to the same time schedule as SC hydration assessments. In subjects of the subgroup, no TEWL measurement took place on days 21 and 28, but TEWL was additionally quantified in skin areas A3 and A4 on day -1 of SLS application. There was one measurement per test area and assessment time. TEWL represents a sensitive and noninvasive method to quantify SC barrier function; a decrease in TEWL corresponds to an improvement in this function [
18,
19].
The Cutometer
® MPA580 (Courage & Khazaka, Cologne, Germany) was used to evaluate biomechanical properties of the skin. It is a noninvasive, objective suction method to evaluate the effect of dermatological and cosmetic products on skin mechanics [
20]. Details on the methodology and measured parameters have been reported previously [
20,
21,
22,
23]. In brief, the Cutometer
® creates a negative pressure by suction which draws the skin into the aperture of the probe (i.e., upper layers of the skin are stretched vertically). The resistance of the skin to being sucked up (firmness) and its ability to return to its initial position (elasticity) are graphically shown [
24]. In our study, total skin distensibility (Uf), immediate distensibility (Ue), and immediate recovery (Ur) were derived from the deformation by time curve [
22]. The relative parameters Ur/Ue (net elasticity) and Ur/Uf (ratio of elastic recovery to distensibility) were calculated. Data were generated using the device software. Both Ur/Ue and Ur/Uf are elasticity parameters which decrease with aging [
22,
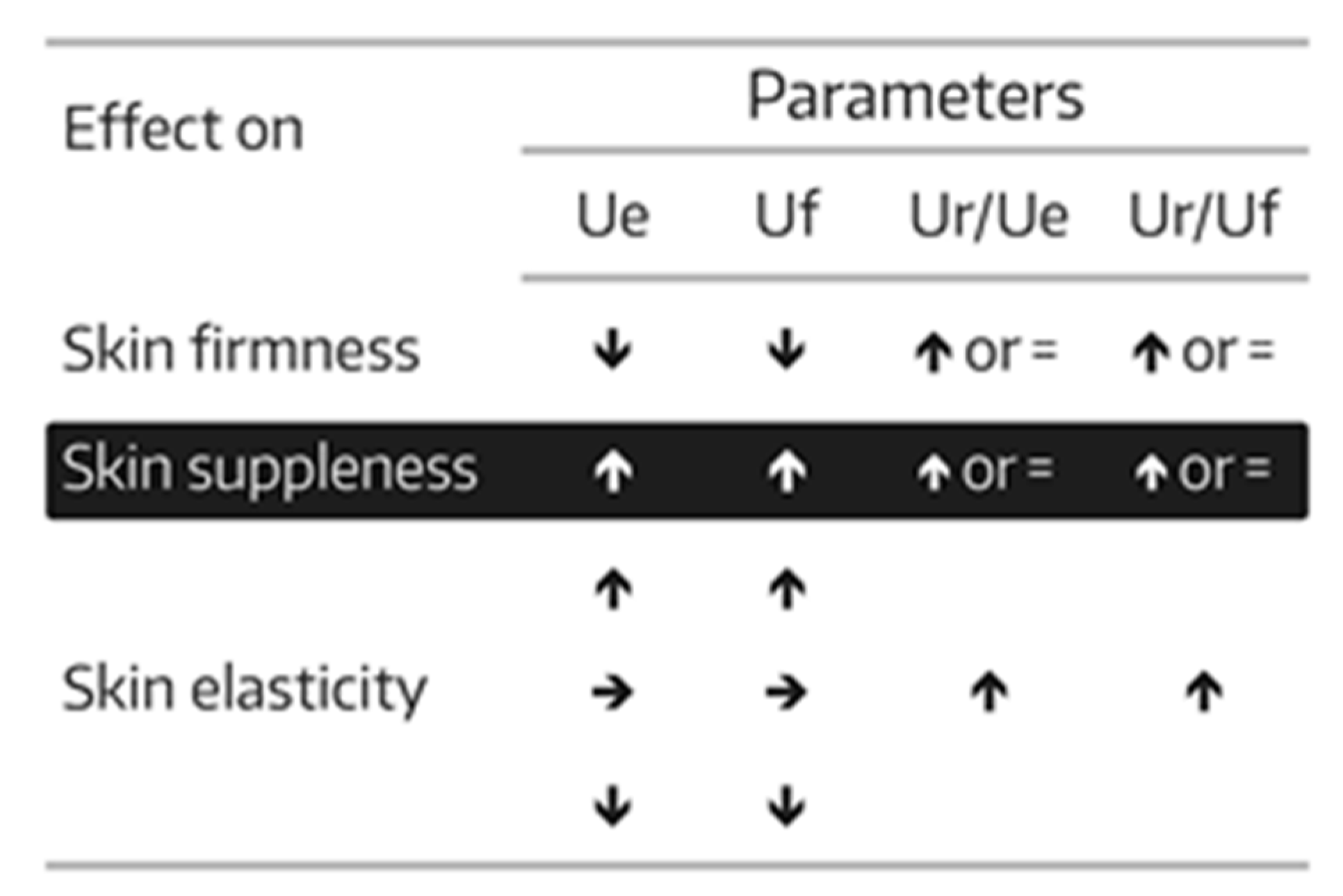
23]. The proportion of subjects for whom there was an improvement of skin firmness, skin suppleness, and skin elasticity— as defined in
Figure 1—were calculated and entitled “positive subjects”. Biomechanical skin measurements were conducted on each of the two test areas (A1 and A2) at baseline and on days 7, 14, 21, and 28. One measurement was performed in the allocated skin areas per assessment time.
Skin surface samples (swab samplings) for quantification of SC lipids involved in barrier function (i.e., ceramides, cholesterol, and free fatty acids) were taken at baseline and on days 7 and 28. Two swab samplings were collected per assessment time by rubbing them on the skin of area A0. The swabs were previously soaked in an aqueous nonionic surfactant solution (Synelvia, Labège, France—proprietary method). Subsequently, the swab heads were placed in Eppendorf tubes and frozen at −20 °C until analysis by liquid or gas chromatography both coupled with a mass spectrometer [
25].
Before instrumental measurements (corneometry, TEWL, skin biochemical parameters), subjects remained in a climatized room (20 ± 2 °C, 45 ± 15% relative humidity) for 15–20 min. The skin areas treated with Emollient 1 were to be wiped with a paper towel before measurements to remove the excess product. If different instrumental measurements were performed in the same skin area at a given time-point, different skin sites have been used. Monitoring of adverse events (AEs) took place over the entire study period.