Rosmarinus officinalis L. (Rosemary): An Ancient Plant with Uses in Personal Healthcare and Cosmetics
Abstract
1. Introduction
2. Methodology
3. Results and Discussion
3.1. Historical Fragments Concerning the Use of Rosemary in Medicine and Cosmetics
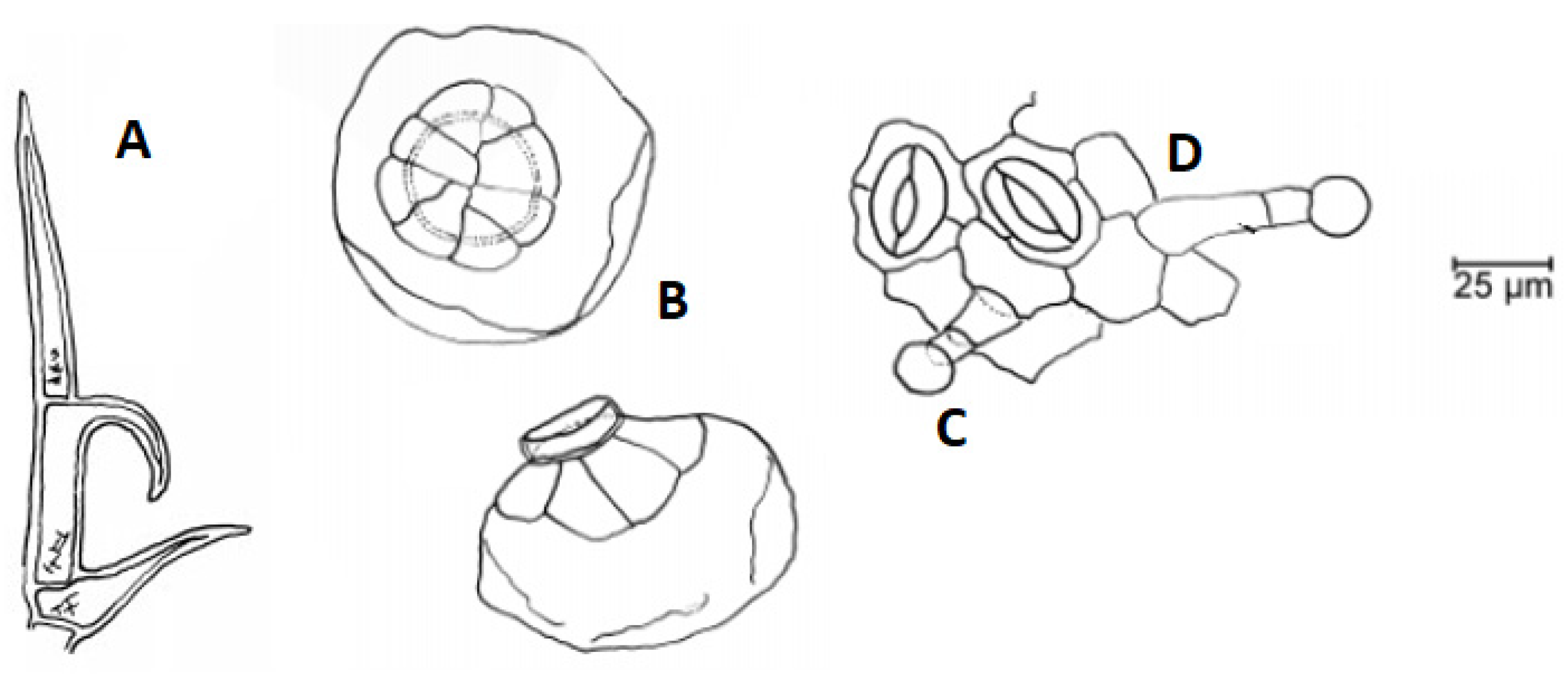
3.2. Botany
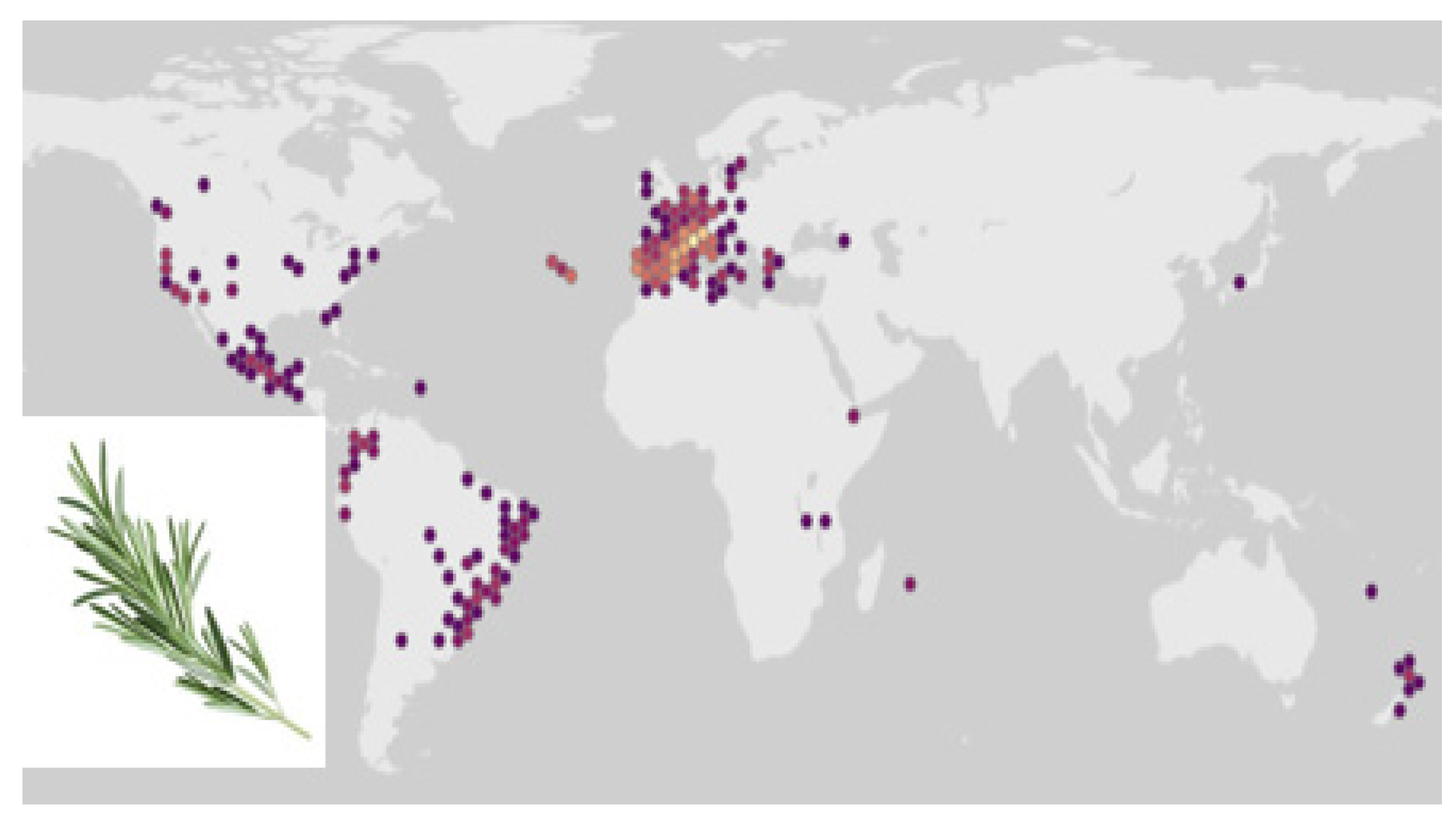
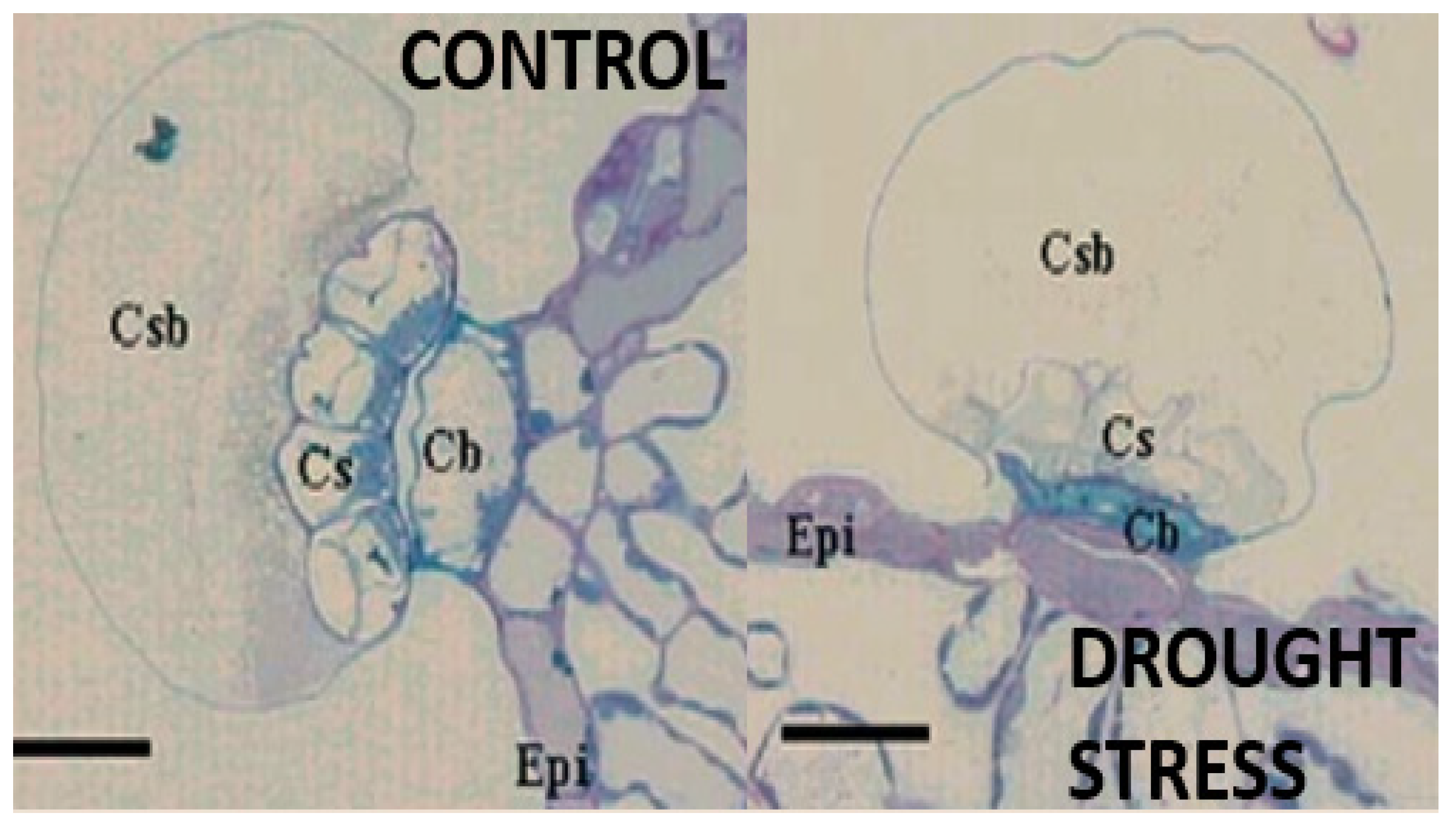
3.3. Ecology of Rosemary and Other Labiates in Mediterranean Forests
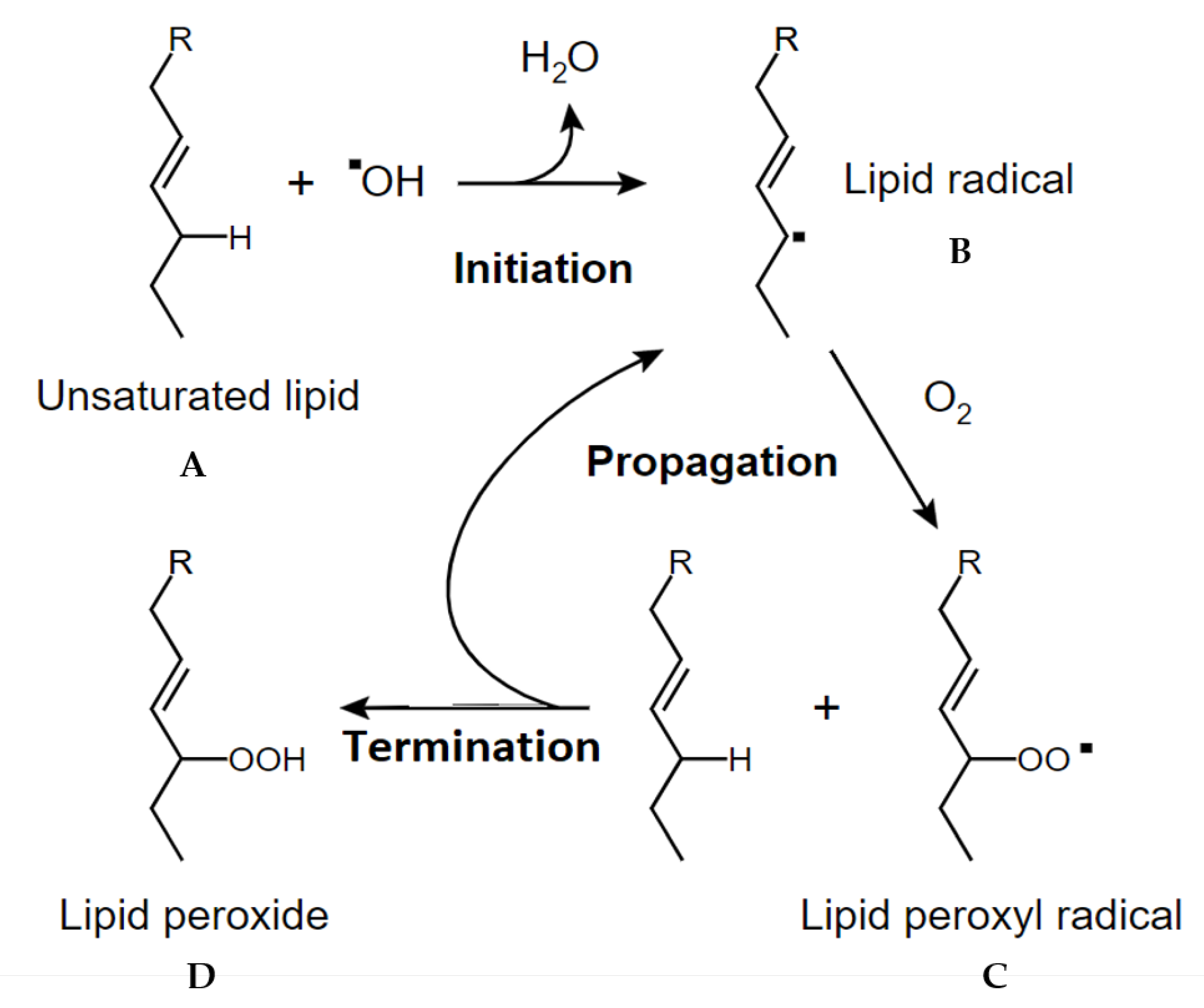
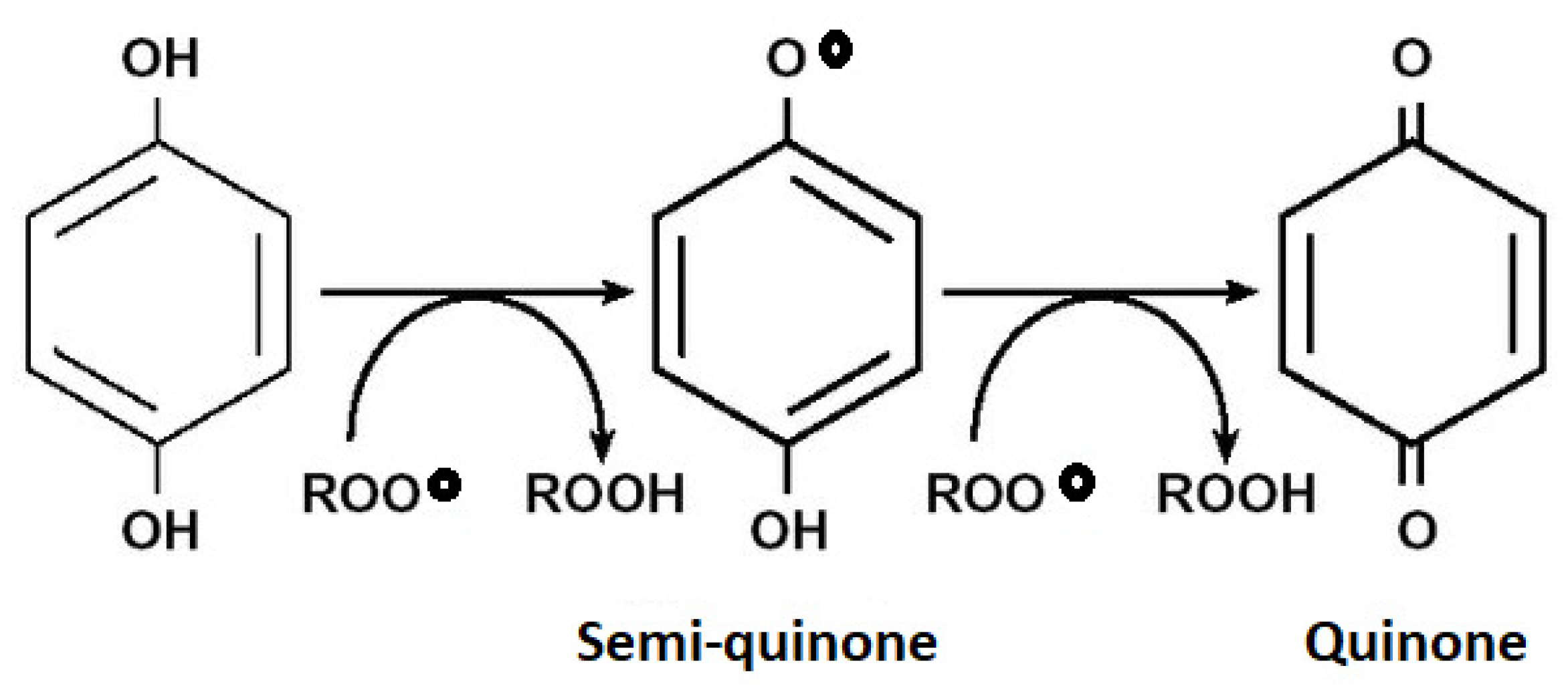
3.4. Rosemary Phytochemistry and Biological Activity
3.5. Use of Rosemary in Food and Cosmetics, and Examples of Other Topical Actions
- Acetic acid 2–5 g
- Rosemary tincture 25 g
- Jarobardi tincture 25 g
- Quinine tincture 25 g
- Water 50–60 g
4. Conclusions
Funding
Conflicts of Interest
References
- Definición de Salud. Available online: https://www.who.int/es/about/who-we-are/frequently-asked-questions#:~:text=%C2%BFC%C3%B3mo%20define%20la%20OMS%20la,ausencia%20de%20afecciones%20o%20enfe (accessed on 23 August 2020).
- González-Minero, F.J.; Bravo-Díaz, L. Historia y actualidad de los productos para la piel, cosméticos y fragancias. Ars Pharm. 2017, 58, 5–12. [Google Scholar]
- Zhang, l.; Adique, A.; Sarkar, P.; Shenai, V.; Sampath, M.; Lai, R.; Qi, J.; Wang, M.; Miranda, A.; Farage, M.A. The Impact of Routine Skin Care on the Qualityof Life. Cosmetics 2020, 7, 59. [Google Scholar] [CrossRef]
- Oumeish, Y. The Cultural and Philosophical Concepts of Cosmetics in Beauty and Art through the Medical History and Mankind. Clin. Derm. 2001, 19, 375–386. [Google Scholar] [CrossRef]
- Aburjai, T.; Natsheh, F.M. Plants used in cosmetics. Phytother. Res. 2003, 17, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Cronin, H.; Draelos, Z.D. Top 10 botanical ingredients in 2010 anti-aging creams. Cosmet. Derm. 2010, 9, 218–225. [Google Scholar] [CrossRef]
- Kumar, S. Exploratory analysis of global cosmetic industry: Major players, technology and market trends. Technovation 2005, 25, 1263–1272. [Google Scholar] [CrossRef]
- Real Farmacopea Española. Available online: https://extranet.boe.es/farmacopea/doc.php?id=1560 (accessed on 23 August 2020).
- Andrade, J.M.; Faustino, C.; García, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. Future Sci. OA 2018, 4, 283. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Carvalho-Costa, D.; Cavaleiro, C.; Costa, H.S.; Gonçalves-Albuquerque, T.; Castilho, M.C.; Ramos, F.; Melo, N.R.; Sanches-Silva, A. A novel insight on an ancient aromatic plant: The rosemary (Rosmarinus officinalis L). Trends Food Sci. Technol. 2015, 45, 355–368. [Google Scholar] [CrossRef]
- Sousa-Borges, R.; Sánchez-Ortiz, B.L.; Matías-Pereira, A.C.; Keita, H.; Tavares-Carvalho, J.C. Rosmarinus officinalis essential oil: A rewiew of the phytochemistry, anti-inflammatory activty, and mechanims of action involves. J. Etnopharmacol. 2019, 229, 29–45. [Google Scholar] [CrossRef]
- De Oliveira, J.R.; Alfonso-Camargo, A.E.; Dias de Oliveira, L. Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. Biomed. Sci. 2019, 26, 5. [Google Scholar] [CrossRef]
- Malvezzi de Macedo, L.; Mendes dos Santos, E.; Militão, L.; Tundisi, L.; Ataide, J.A.; Barbosa-Souto, E.; Gava Mazzola, P. Rosemary (Rosmarinus officinalis L., syn Salvia rosmarinus Spenn.) and Its Topical Applications: A Review. Plants 2020, 9, 651. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, P.T. Ancient Egyptian Materials and Technology; Cambridge University Press: Cambridge, UK, 2000; p. 175. [Google Scholar]
- Muñoz-Centeno, L.M. Spanish Medicinal Plants. Rosmarinus officinalis L. (Lamiaceae) (Rosemary). Available online: http://revistas.usal.es/index.php/0211-9714/article/view/6111/6131 (accessed on 9 March 2020).
- Flora of China. Available online: http://www.efloras.org/flora_page.aspx?flora_id=2 (accessed on 7 March 2020).
- González-Bueno, A. Un Dioscórides para el Profano, Atribución, Significado y Utilidad de un Herbario Renacentista Castellano: El “Libro de las Yerbas” de Juan de Jarava; Colegio Oficial de Farmacéuticos: Burgos, Spain, 2006; p. 272. [Google Scholar]
- Font-Quer, P. Plantas Medicinales. El Dioscórides Renovado; Península: Barcelona, Spain, 2007; p. 562. [Google Scholar]
- Al-Sereitia, M.; Abu-Amerb, K.; Sena, P. Pharmacology of Rosemary (Rosmarinus officinialis Linn.) and Its Therapeutic Potentials. Ind. J. Exp. Biol. 1999, 37, 124–131. [Google Scholar]
- Heinrich, M.; Kufer, J.; Leonti, M.; Pardo-de-Santayana, M. Ethnobotany and ethnopharmacology—Interdisciplinary links with the historical sciences. J. Ethnopharmcol. 2006, 107, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Mahmoud, N.A.; Shokralla, N.; Yessoufou, K. Diversity of Plants, Traditional Knowledge, and Practices in Local Cosmetics: A Case Study from Alexandria, Egypt. Econ. Bot. 2015, 30, 1–13. [Google Scholar] [CrossRef]
- Theplanlist, Royal Botánical Gardens, Kew. Available online: http://www.theplantlist.org/ (accessed on 7 March 2020).
- Begum, A.; Sandhya, S.; Shaff ath Ali, S.; Vinod, K.R.; Reddy, S.; Banji, D. An in-depth review on the medicinal flora Rosmarinus officinalis (Lamiaceae). Acta Sci. Pol. Technol. Aliment. 2013, 12, 61–73. [Google Scholar]
- Morales, R. Flora Ibérica; CSIC: Madrid, Spain, 2010; Volume 12, pp. 321–327. [Google Scholar]
- González-Minero, F.J.; González-García, A.; Venegas-Fito, C. Miscelánea Botánica. Apuntes Sobre los Herbarios y su Relación con la Farmacia; Ende: Sevilla, Spain, 2020; p. 43. [Google Scholar]
- Drew, B.T.; González-Gallegos, J.G.; Xiang, C.L.; Kriebel, R.; Drummond, C.P.; Walker, J.V.; Kenneth, J. Salvia united: The greatest good for the greatest number. Taxon 2017, 66, 133–145. [Google Scholar] [CrossRef]
- Global Biodiversity Information Facility—GBIF. Available online: https://www.gbif.org/ (accessed on 23 August 2020).
- Tawfik, A.A.; Read, P.E.; Cuppett, S.L. Rosmarinus officinalis L. (Rosemary): In Vitro Culture, Regeneration of Plants, and the Level of Essential Oil and Monoterpenoid Constituents. Medicinal and Aromatic Plants; Springer-Verlag: Berlin/Heidelberg, Germany, 1998; pp. 349–365. [Google Scholar]
- FAO/WHO. Comisión del Codex Alimentarius. Roma. 2013. Available online: http://www.fao.org/tempref/codex/Meetings/CAC/cac36/cac36_10_add2s.pdf (accessed on 23 August 2020).
- Christenhusz, M.J.M.; Fay, M.F.; Chase, M.W. Plants of the World. An Illustrated Encyclopedia of—Vascular Plants; Kew Publishing Royal Botanic Gardens: Kew, UK, 2017. [Google Scholar]
- Castro de, M.; Martín-Vide, J.; Alonso, S. El Clima en España: Pasado, Presente y Escenario del Clima Para el Siglo XXI; Ministerio de Medio Ambiente: Madrid, Spain, 2005; p. 8. [Google Scholar]
- Di Ferdinando, M.; Brunetti, C.; Agati, G.; Tattini, M. Multiple functions of polyphenols in plants inhabiting unfavorable Mediterranean areas. Environ. Exp. Bot. 2014, 103, 107–116. [Google Scholar] [CrossRef]
- Thomson, J.D. Plant Evolution in the Mediterranean; Oxford University Press: New York, NY, USA, 2005; pp. 114, 144, 147. [Google Scholar]
- Olmos, E.; Sánchez-Blanco, M.J.; Ferrández, T.; Alarcón, J.J. Subcellular Effects of Drought Stress in Rosmarinus officinalis. Plant Biol. 2007, 9, 77–84. [Google Scholar] [CrossRef]
- Boix, Y.F.; Pimentel-Victório, C.; Antunes-Defaveri, A.C.; Arruda, R.D.; Sato, A.; Salgueiro Lage, C.L. Glandular trichomes of Rosmarinus officinalis L.: Anatomical and phytochemical analyses of leaf volátiles. Plant Biosyst. 2011, 145, 848–856. [Google Scholar] [CrossRef]
- Staudta, M.; Bourgeoisa, I.; Al Halabia, R.; Song, W.; Williams, J. New insights into the parametrization of temperature and lightresponses of mono-and sesquiterpene emissions from Aleppo pineand Rosemary. Atmos. Environ. 2017, 152, 212–221. [Google Scholar] [CrossRef]
- Diniz do Nascimento, L.; Moraes, A.; Barbosa de Costa, A.; Santana da, K.; Pereira Galucio, J.M.; Taube, P.S.; Costa, C.M.; Neves, C.; Jorddy de Aguiar, A.; Eloisa, H.; et al. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 7, 988. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Chen, L.; Chang, T.; Ke, W.M.; Lo, Y.F.; Wang, C.C. The Correlation between Skin-Care Effects and Phytochemical Contents in Lamiaceae Plants. Food Chem. 2011, 124, 833–841. [Google Scholar] [CrossRef]
- Base de Datos de Biodiversidad en España; CSIC: Madrid, Spain; Available online: http://www.anthos.es/ (accessed on 23 August 2020).
- Gawel-Beben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M.; Strzepek-Gomolka, M.; Antosiewicz, B. Characterization of Cistus x incanus L. and Cistus ladanifer L. Extracts as Potential Multifunctional Antioxidant Ingredients for Skin Protecting Cometics. Antioxidants 2020, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Velamuri, R.; Fagan, J.; Schaefer, J.; Streicher, C.; Stimson, J. Identification and characterization of polyphenols and volatile terpenoid compounds in different extracts of garden sage (Salvia officinalis L.). Pharmacog. Res. 2020, 12, 149–157. [Google Scholar] [CrossRef]
- Shahbazian, D.; Karami, A.; Eshghi, S. Comparative Analysis of Essential Oils from Myrtus communis Berry Color Morphs from Southern Iran. Nat. Prod. J. 2018, 8, 317–322. [Google Scholar] [CrossRef]
- El Hassouni, A.; El Bachiri, A.; Belbachir, C. Lavnadula dentata Solid Residue from Essential Oil Industry. J. Essent. Oil Bear Plants 2019, 22, 1601–1613. [Google Scholar] [CrossRef]
- Pachi, V.; Mikropoulou, E.V.; Gkiouvetidis, P.; Siafakas, K.; Argyropoulou, A.; Angelis, A.; Mitakou, S.; Halabalaki, M. Traditional uses, phytochemistry and pharmacology of Chios mastic gum (Pistacia lentiscus var. Chia, Anacardiaceae): A review. J. Ethnophamacol. 2020, 254, 112485. [Google Scholar] [CrossRef]
- Hernández, M.D.; Sotomayor, J.A.; Hernández, A.; Jordán, M.J. Rosemary (Rosmarinus officinalis L.) oils. In Esential Olis in Food Preservation, Flavor and Safety; Preedy, V., Ed.; Academic Press: London, UK, 2016; pp. 677–688. [Google Scholar]
- Melito, S.; Petretto, G.L.; Chanhine, S.; Pintore, G.; Chessa, M. Seasonal variation of essential oil in Rosmarinus officinalis leaves in Sardinia. Nat. Prod. Comm. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Hcini, K.; Sotomay, M.A.; Jordan, M.J.; Bouzid, S. Chemical composition of the essential oil of Rosemary (Rosmarinus officinalis L.) of Tunisian origin. As. J. Chem. 2013, 25, 2601–2603. [Google Scholar] [CrossRef]
- Tahri, M.; Imelouane, B.; Aouinti, F.; Amhamdi, H.; Elbachiri, A. The organic and mineral compounds of the medicinal aromatics, Rosmarinus tournefortii and Rosmarinus officinalis, growing in eastern Morocco. Res. Chem. Int. 2013, 40, 2651–2658. [Google Scholar] [CrossRef]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.I.; Snyder, P.W.; et al. Safety Assessment of Rosmarinus officinalis (Rosemary)-Derived Ingredients as Used in Cosmetics. Int. J. Toxicol. 2018, 37, 125–150. [Google Scholar] [CrossRef]
- Wang, B.; Ma, L.; Yin, L.; Chen, J.; Zhang, Y.; Dong, L.; Zhang, X.; Fu, X. Regional variation in the chemical composition and antioxidant activity of Rosmarinus officinalis L. from China and the Mediterranean region. Pak. J. Pharm. Sci. 2018, 31, 221–229. [Google Scholar] [PubMed]
- Lakusic, D.; Ristic, M.; Slavkovska, V.; Lakusic, B. Seasonal variations in the composition of the essential oil of Rosemary (Rosmarinus officinalis, Lamiaceae). Nat. Prod. Comm. 2013, 8, 131–134. [Google Scholar]
- González-Minero, F.J. Estudio transversal de Moringa oleifera Lam. (Moringaceae) Revisión. Dominguezia 2018, 34, 5–25. [Google Scholar]
- Khan, B.A.; Akhtar, N.; Menaa, B.; Menaa, A.; Braga, V.A.; Menaa, F. Relative Free Radicals Scavenging and Enzymatic Activities of Hippophae rhamnoides and Cassia fistula Extracts: Importance for Cosmetic, Food and Medicinal Applications. Cosmetics 2017, 4, 3. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; Rodríguez-Herrera, R.; Aguilar, R. Essential oils: As natural alternative to combat antibiotics resistence. In Antibiotic Resistance Mechanisms and New Antimicrobial Approaches; Kon, K., Rai, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 227–238. [Google Scholar]
- Wang, W.; Li, N.; Luo, M.; Zu, Y.; Efferht, T. Anbibacterial activity abd anticancer activity of Rosmarinus officinalis L. essential oil compared to that of its main components. Molecules 2012, 17, 2704–2713. [Google Scholar] [CrossRef]
- Genena, K.A.; Hense, J.A.; Smania, J.; Souza, M.A.S. Rosemary (Rosmarinus officinalis L.) a study of the composition, antioxidant and antimicrobial activities of extracts obtained with supercritical carbon dioxide. Cienc. Tecnol. Aliment. 2008, 28, 463–469. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Chatha, S.A.S.; Jabbar, A.; Mahboob, M.; Nigam, P.S. Rosmarinus officinalis essential oil: Antiproliferative, antioxidant and antibacterial activities. Braz. J. Microbiol. 2010, 41, 10170–11078. [Google Scholar] [CrossRef]
- Birtic, S.; Dussort, P.; Pierre, F.X.; Bily, A.C.; Roller, M. Carnosic acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef]
- Menaa, F.; Menaa, A.; Tréton, J. Polyphenols against Skin Aging. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: London, UK, 2014; pp. 819–830. [Google Scholar]
- Calabrese, V.; Scapagnini, G.; Catalano, C.; Dinotta, F.; Geraci, D.; Morganti, P. Biochemical studies of a natural antioxidant isolated from rosemary and its application in cosmetic dermatology. Int. J. Tissue React. 2000, 22, 5–13. [Google Scholar]
- EFSA. Use of rosemary extracts as a food additive. Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food. EFSA J. 2008, 721, 1–29. [Google Scholar]
- EFSA Panel on Food Additives; Nutrient Sources added to Food (EFSA ANS Panel); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; et al. Refined exposure assessment of extracts of rosemary (E 392) from its use as food additive Panel on Food Additives and Nutrient Sources added to Food. EFSA J. 2018, 5373. [Google Scholar] [CrossRef]
- Damianova, S.; Tasheva, S.; Stoyanova, A.; Damianov, D. Investigation of Extracts from Rosemary (Rosmarinus officinalis L.) for Application in Cosmetics. J. Essent. Oil Bear. Plants 2010, 13, 1–11. [Google Scholar] [CrossRef]
- Muyima, N.Y.O.; Zulu, G.; Bhengu, T.; Popplewell, D. The potential application of some novel essential oils as natural cosmetic preservatives in an aqueous cream formulation. Flavour Fragr. J. 2002, 174, 258–266. [Google Scholar] [CrossRef]
- Satyal, P.; Jones, T.H.; Lopez, E.M.; McFeeters, R.L.; Awadh Ali, N.A.; Mansi, I.; Al-kaf, A.G.; Setzer, W.N. Chemotypic Characterization and Biological Activity of Rosmarinus officinalis. Foods 2017, 6, 20. [Google Scholar] [CrossRef]
- Gauch, L.D.R.; Soares-Pedrosa, S.; Antunes-Esteves, R.; Silveira-Gomes, F.; Cajueiro-Gurgel, E.S.; Marques-da-Silva, S.H. Antifungal activity of Rosmarinus officinalis Linn. essential oil against Candida albicans, Candida dubliniensis, Candida parapsilosis and Candida krusei. Rev. Pan-Amaz. Saude 2014, 5, 61–66. [Google Scholar] [CrossRef]
- Varvaresou, A.; Papageorgiou, S.; Tsirivas, E.; Protopapa, E.; Kintziou, H.; Kefala, V.; Demetzos, C. Self-preserving cosmetics. Int. J. Cosmet. Sci. 2009, 31, 163–175. [Google Scholar] [CrossRef]
- Khan, B.A.; Mahmood, T.; Menaa, F.; Shahzad, Y.; Yousaf, A.M.; Hussain, T.; Ray, S.D. New Perspectives on the Efficacy of Gallic Acid in Cosmetics & Nanocosmeceuticals. Curr. Pharm. Des. 2018, 24, 5181–5187. [Google Scholar]
- Khan, B.A.; Akhtar, N.; Menaa, A.; Menaa, F. A Novel Cassia fistula (L.)-Based Emulsion Elicits Skin Anti-Aging Benefits in Humans. Cosmetics 2015, 2, 368–383. [Google Scholar] [CrossRef]
- Sharif, A.; Akhtar, N.; Khan, M.S.; Menaa, A.; Menaa, B.; Khan, B.A.; Menaa, F. Formulation and evaluation on human skin of a water-in-oil emulsion containing Muscat hamburg black grape seed extract. Int. J. Cosmet. Sci. 2015, 7, 253–258. [Google Scholar] [CrossRef]
- Cizauskaite, U.; Ivanauskas, L.; Jakštas, V.; Marksiene, R.; Jonaitiene, L.; Bernatoniene, J. Rosmarinus officinalis L. extract and some of its active ingredients as potential emulsion stabilizers: A new approach to the formation of multiple (W/O/W) emulsion. Pharm. Dev. Technol. 2016, 21, 716–724. [Google Scholar] [PubMed]
- Carvalho, I.T.; Estevinho, B.N.; Santos, L. Application of microencapulated essential oils in cosmetic and personal healthcare products—A review. Int. J. Cosm. Sci. 2016, 3, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Silva-Neves, J.; Lopes-da-Silva, Z.; de Sousa Brito Neta, M.; Braun-Chaves, S.; de Medeiros- Nobrega, I.K.; de Lira-Machado, A.H.; Machado, F. Preparation of terpolymer capsules containing Rosmarinus officinalis essential oil and evaluation of its antifungal activity. RSC Adv. 2019, 9, 22586. [Google Scholar] [CrossRef]
- Reglamento Europeo. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:342:0059:0209:es:PDF (accessed on 23 August 2020).
- Avila-Sosa, R.; Navarro-Cruz, A.R.; Vera-López, O.; Dávila-Márquez, R.M.; Melgoza-Palma, N.; Meza-Pluma, R. Romero (Rosmarinus officinalis L.): Una revisión de sus usos no culinarios. Cienc. Mar. 2011, 16, 923–936. [Google Scholar]
- Guin, J.D. Rosemary cheilitis: One to remember. Contact Dermat. 2001, 45, 63. [Google Scholar] [CrossRef]
- Inui, S.; Katayama, S. Allergic Contact Dermatitis Induced by Rosemary Leaf Extract in a Cleansing Gel. J. Dermatol. 2005, 32, 667–669. [Google Scholar] [CrossRef]
- Miroddi, M.; Calapai, G.; Isola, S.; Minciullo, P.L.; Gangemi, S. Rosmarinus officinalis L. as cause of contact dermatitis. Allergol. Inmonpathol. 2014, 42, 616–619. [Google Scholar] [CrossRef]
- Murata, K.; Noguch, K.; Kondo, M.; Onishi, M.; Watanabe, N. Promotion of Hair Growth by Rosmarinus officinalis Leaf Extract. Phytother. Res. 2013, 27, 212–217. [Google Scholar] [CrossRef]
- Medicamenta. Guía Teórico-Práctica Para Farmacéuticos, Médicos y Veterinarios; Labor: Barcelona, Spain, 1917; p. 969. [Google Scholar]
- Panahi, Y.; Taghizadeh, M.; Tahmasbpour-Marzony, E.; Sahebkar, A. Rosemary oil vs minoxidil 2% for the treatment of androgenetic alopecia: A randomized comparative trial. Skinmed 2015, 13, 15–21. [Google Scholar]
- Dhariwala, M.J.; Ravikumar, P. An overview of herbal alternatives in androgenetic alopecia. J. Cosmet. Dermatol. 2019, 18, 966–975. [Google Scholar] [CrossRef]
- Cattaneo, L.; Cicconi, R.; Mignogna, G.; Giorgi, A.; Mattei, M.; Graziani, G.; Ferracane, R.; Grosso, A.; Aducci, P.; Schininà, M.E.; et al. Anti-Proliferative Effect of Rosmarinus officinalis L. Extract on Human Melanoma A375 Cells. PLoS ONE 2015, 10, e0132439. [Google Scholar] [CrossRef] [PubMed]
- Offord, E.A.; Gautier, J.C.; Avanti, O.; Scaletta, C.; Runge, F.; Krämer, K.; Applegate, L.A. Photoprotective potential of lycopene, beta-carotene, vitamin E, vitamin C and carnosic acid in UVA-irradiated human skin fibroblasts. Free Radic. Biol. Med. 2002, 32, 1293–1303. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Caturla, N.; Castillo, J.; Benavente-García, O.; Alcaraz, M.; Mico, V. Protective effects of citrus and rosemary extracts on UV-induced damage in skin cell model and human volunteers. J. Photochem. Photobiol. 2014, 136, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Nolkemper, S.; Reichling, J.; Stintzing, F.C.; Carle, R. Antiviral effect of aqueous extracts from species of the Lamiaceae family against Herpes simplex virus type 1 and type 2 in vitro. Plant Med. 2006, 72, 1378–1382. [Google Scholar] [CrossRef]
- Mikaeili, A.; Modaresi, M.; Sozani, S.; Karimi, I. The Antifungal Activities of Rosemary against Trichophyton Tonsurans and Microsporum Canis. Int. J. Pharm. Res. Allied Sci. 2016, 5, 472–483. [Google Scholar]
- Sepahvand, A.; Elliasy, H.; Mohammadi, M.; Safarzadeh, A.; Azarbaijani, K.; Shahsavari, S.; Alizabeh, M.; Beyranvaand, F. A review of the most effective medicinal plants for dermatophytosis in traditional medicine. Biomed. Res. Ther. 2018, 5, 2378–2388. [Google Scholar] [CrossRef]
- Yimam, M.; Lee, Y.; Giao, P.; Hong, M.; Brownell, L.; Jia, Q. A Standardized Composition Comprised of Extracts from Rosmarinus officinalis, Annona squamosa and Zanthoxylum clava-herculis for Cellulite. Pharmacog. Res. 2017, 9, 319–324. [Google Scholar] [CrossRef]
- Akbari1, J.; Saeedi1, M.; Farzin, D.; Morteza-Semnani, K.; Esmaili, Z. Transdermal absorption enhancing effect of the essential oil of Rosmarinus officinalison percutaneous absorption of Na diclofenac from topical gel. Pharm. Biol. 2015, 53, 1442–1447. [Google Scholar] [CrossRef]
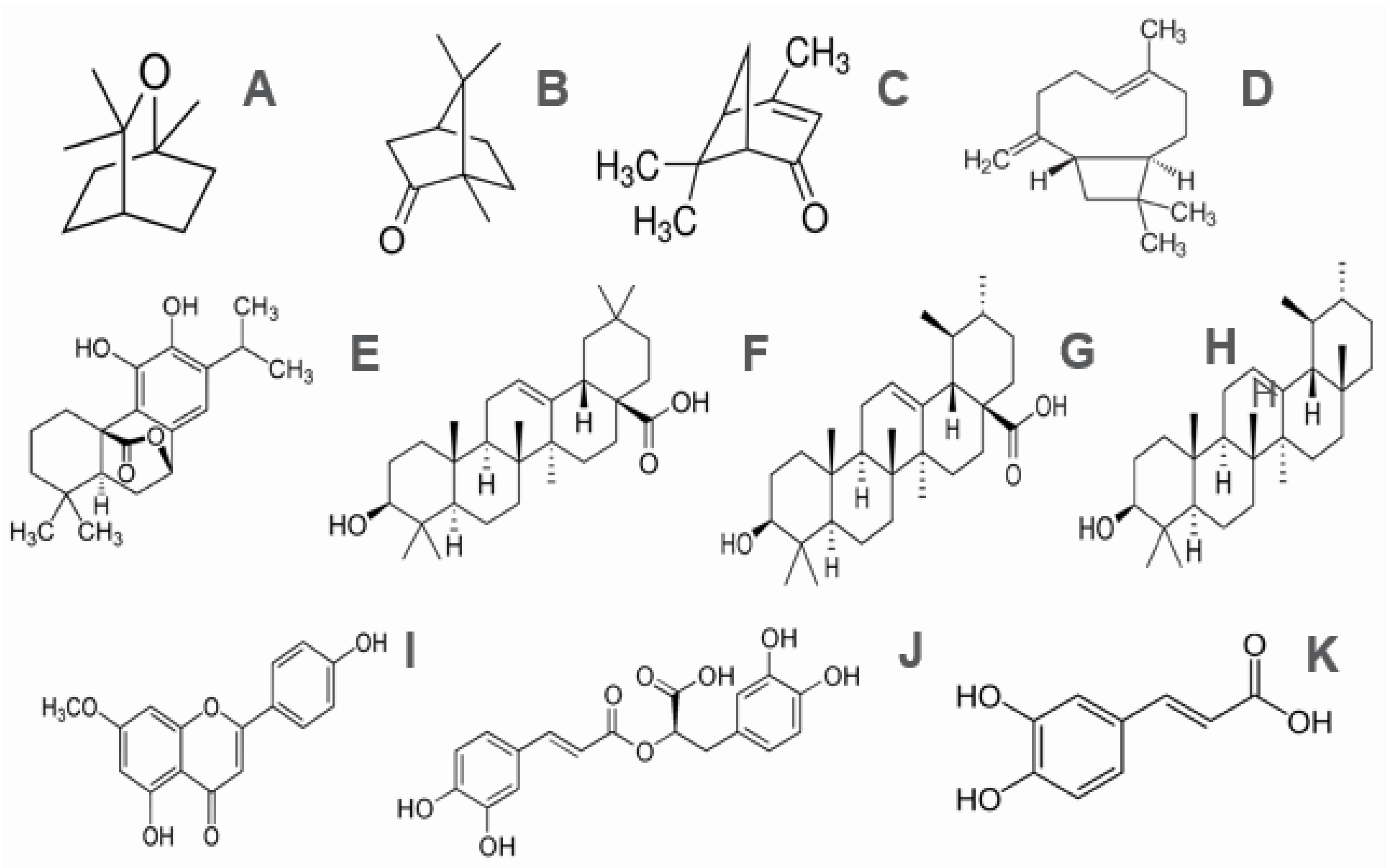
| Main Monoterpenes | 1,8-Cineole (eucalyptol), Camphor (ketone), α-pinene, Borneol, β-pinene, Limonene y p-cymene, Verbenone (ketona), and Sesquiterpenes (β-caryophyllene). |
| Main diterpenes | Carnosic acid, carnosol, rosmarol, epirosmanol, isorosmanol, and rosmaridifenol. |
| Main triterpenes | Oleanolic acid, ursolic acid, betulin, α-amyrin, and β-amyrin. |
| Flavonoids | Luteolin, apigenin, genkwanin, diosmetin, hispidulin, 5-hidroxi-7, 4′-dimetoxi-flavone, and cirsimaritin. |
| Phenolic acids | Caffeic acid, chlorogenic acid, and rosmarinic acid. |
| Chemical Constituents | Spanish Rosemary Oil % | Moroccan and Tunisian Rosemary Oil % |
|---|---|---|
| α-pinene | 18–26 | 9–14 |
| camphene | 8–12 | 2.5–6 |
| β-pinene | 2–6 | 4–9 |
| β-myricene | 1.5–5 | 1–2 |
| limonene | 2.5–5 | 1.5–4 |
| cineole | 16–20 | 38–55 |
| p-cymene | 1–2.2 | 0.8–2.5 |
| camphor | 13–21 | 5–15 |
| bornil acetate | 0.5–2.5 | 0.1–2.6 |
| α-terpineol | 1–3.5 | 5 |
| borneol | 2–4 | 1.5–15 |
| verbenone | 0.7–2.5 | Max 0.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Minero, F.J.; Bravo-Díaz, L.; Ayala-Gómez, A. Rosmarinus officinalis L. (Rosemary): An Ancient Plant with Uses in Personal Healthcare and Cosmetics. Cosmetics 2020, 7, 77. https://doi.org/10.3390/cosmetics7040077
González-Minero FJ, Bravo-Díaz L, Ayala-Gómez A. Rosmarinus officinalis L. (Rosemary): An Ancient Plant with Uses in Personal Healthcare and Cosmetics. Cosmetics. 2020; 7(4):77. https://doi.org/10.3390/cosmetics7040077
Chicago/Turabian StyleGonzález-Minero, Francisco José, Luis Bravo-Díaz, and Antonio Ayala-Gómez. 2020. "Rosmarinus officinalis L. (Rosemary): An Ancient Plant with Uses in Personal Healthcare and Cosmetics" Cosmetics 7, no. 4: 77. https://doi.org/10.3390/cosmetics7040077
APA StyleGonzález-Minero, F. J., Bravo-Díaz, L., & Ayala-Gómez, A. (2020). Rosmarinus officinalis L. (Rosemary): An Ancient Plant with Uses in Personal Healthcare and Cosmetics. Cosmetics, 7(4), 77. https://doi.org/10.3390/cosmetics7040077





