Inhibitory Effects of the Bioactive Thermorubin Isolated from the Fungus Thermoactinomyces Antibioticus on Melanogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
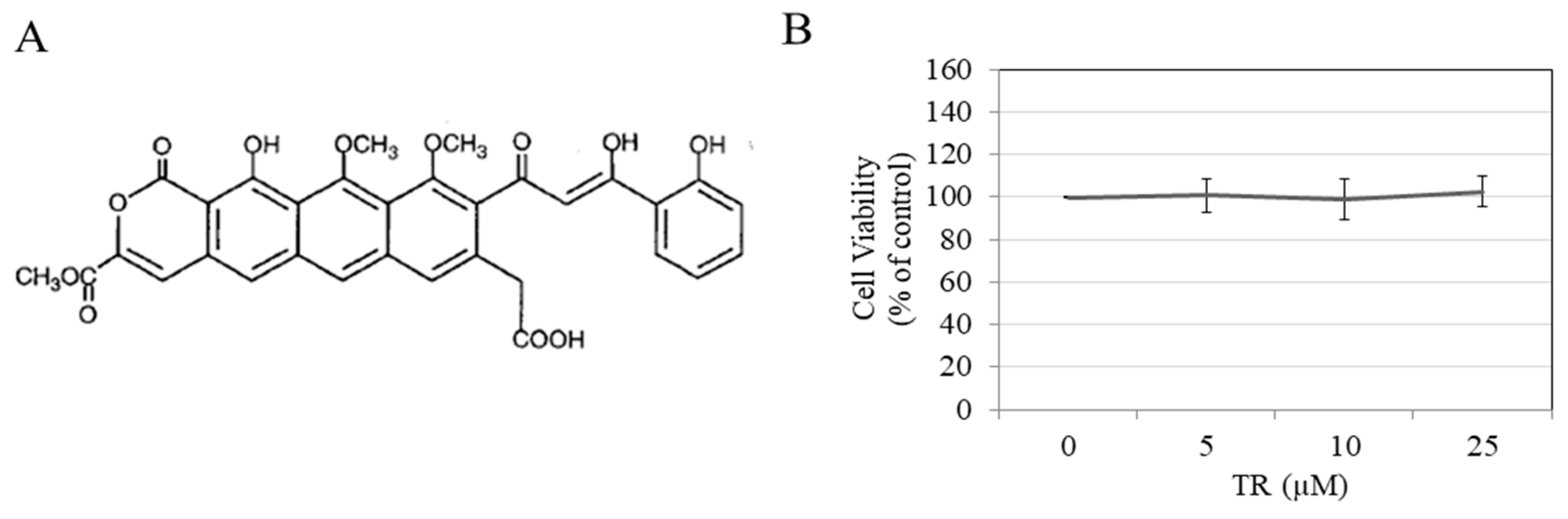
2.3. MTS Cytotoxicity Assay in B16F10 Cells
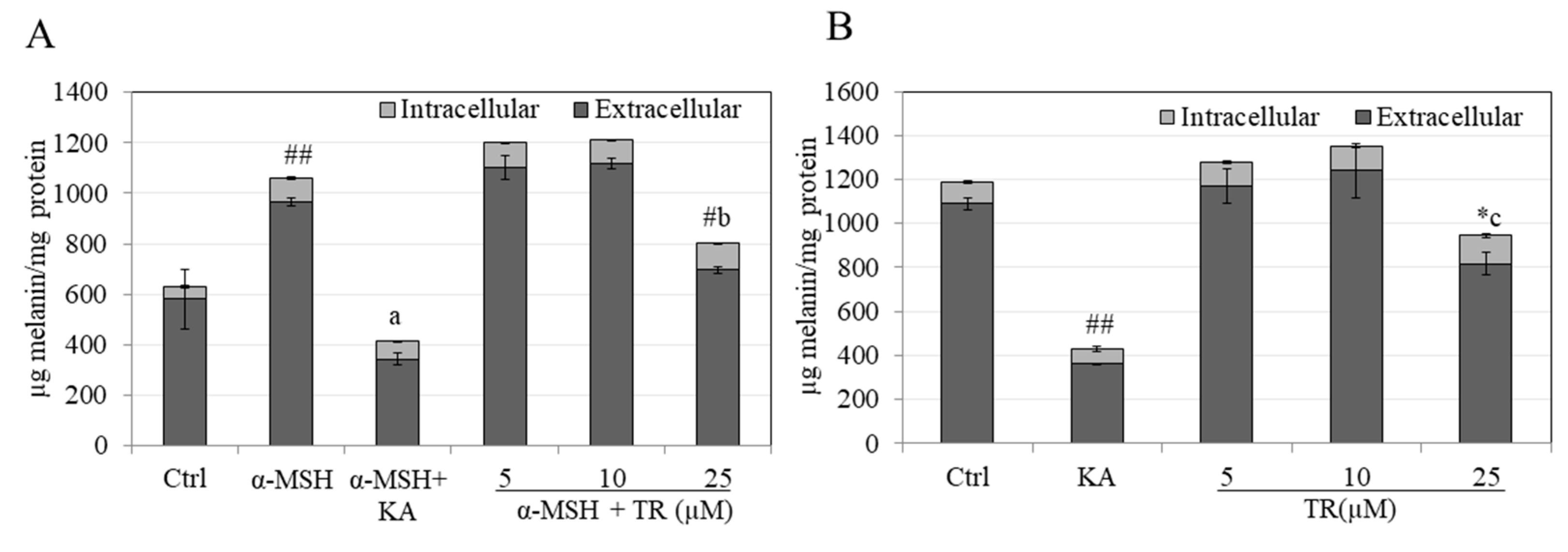
2.4. Extracellular and Intracellular Melanin Quantitation in B16F10 Cells
2.5. Cellular Tyrosinase Activity in B16F10 Cells
2.6. Mushroom Tyrosinase Activity
2.7. Copper Chelating Activity
2.8. DPPH Radical Scavenging Assay
2.9. Cellular Reactive Oxygen Species (ROS) Estimation in B16F10 Cells
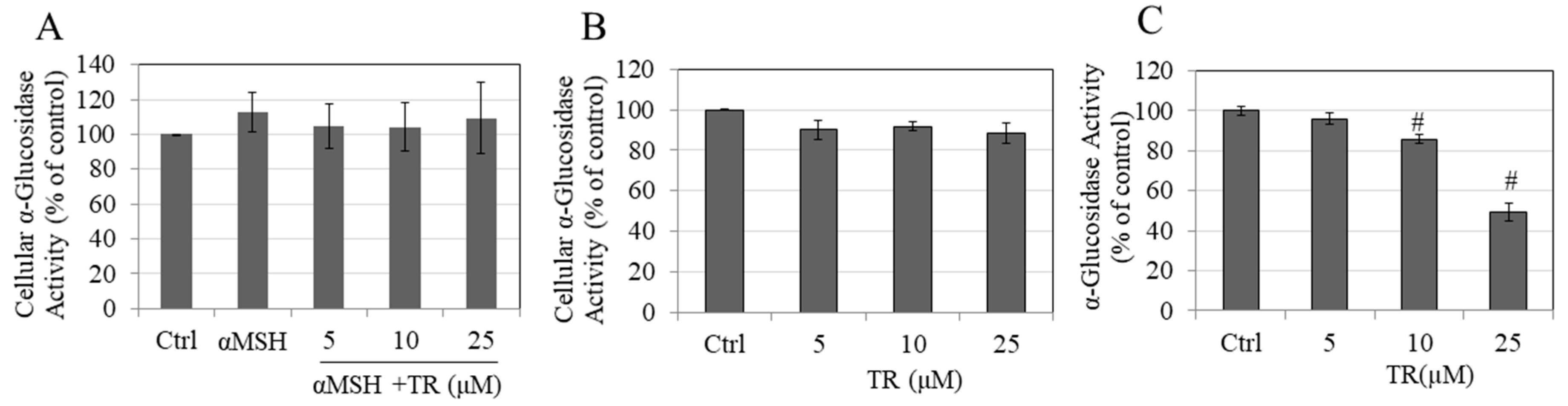
2.10. α-Glucosidase Activity in Cellular Lysates and in Cell-Free System
2.11. Testing in Primary Human Melanocytes from Darkly Pigmented Skin (HEM-DP)
2.11.1. MTS Cytotoxicity Assay
2.11.2. Melanin Content Measurement in HEM-DP Cells
2.11.3. Cellular Tyrosinase Activity in HEM-DP Cells
2.11.4. Dendricity Quantitation in HEM-DP Cells
2.12. Statistical Analysis
3. Results
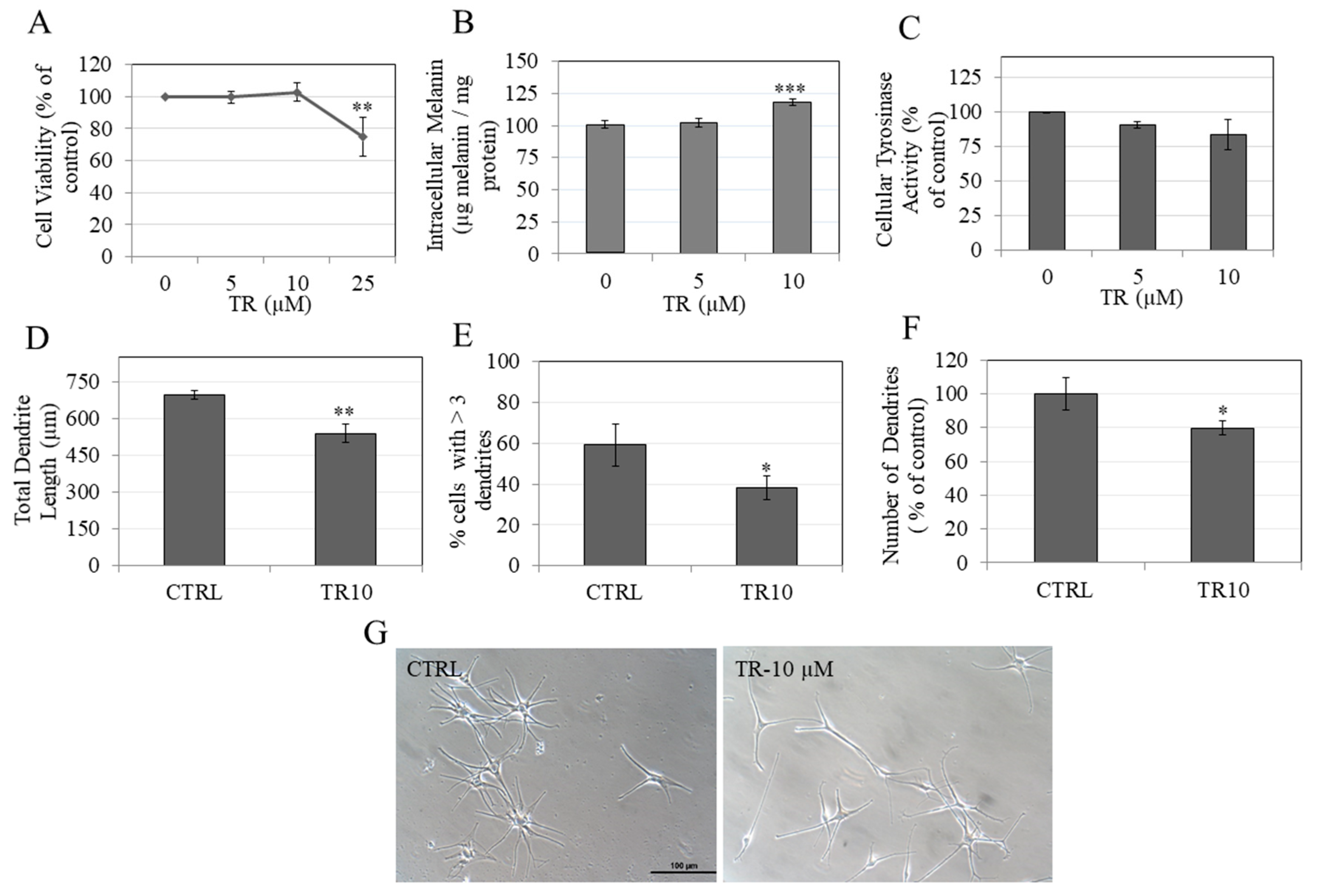
3.1. TR is Nontoxic to B16F10 Cells
3.2. TR Reduces Total Melanin in B16F10 Cells under Hormone-Stimulated and Basal Conditions
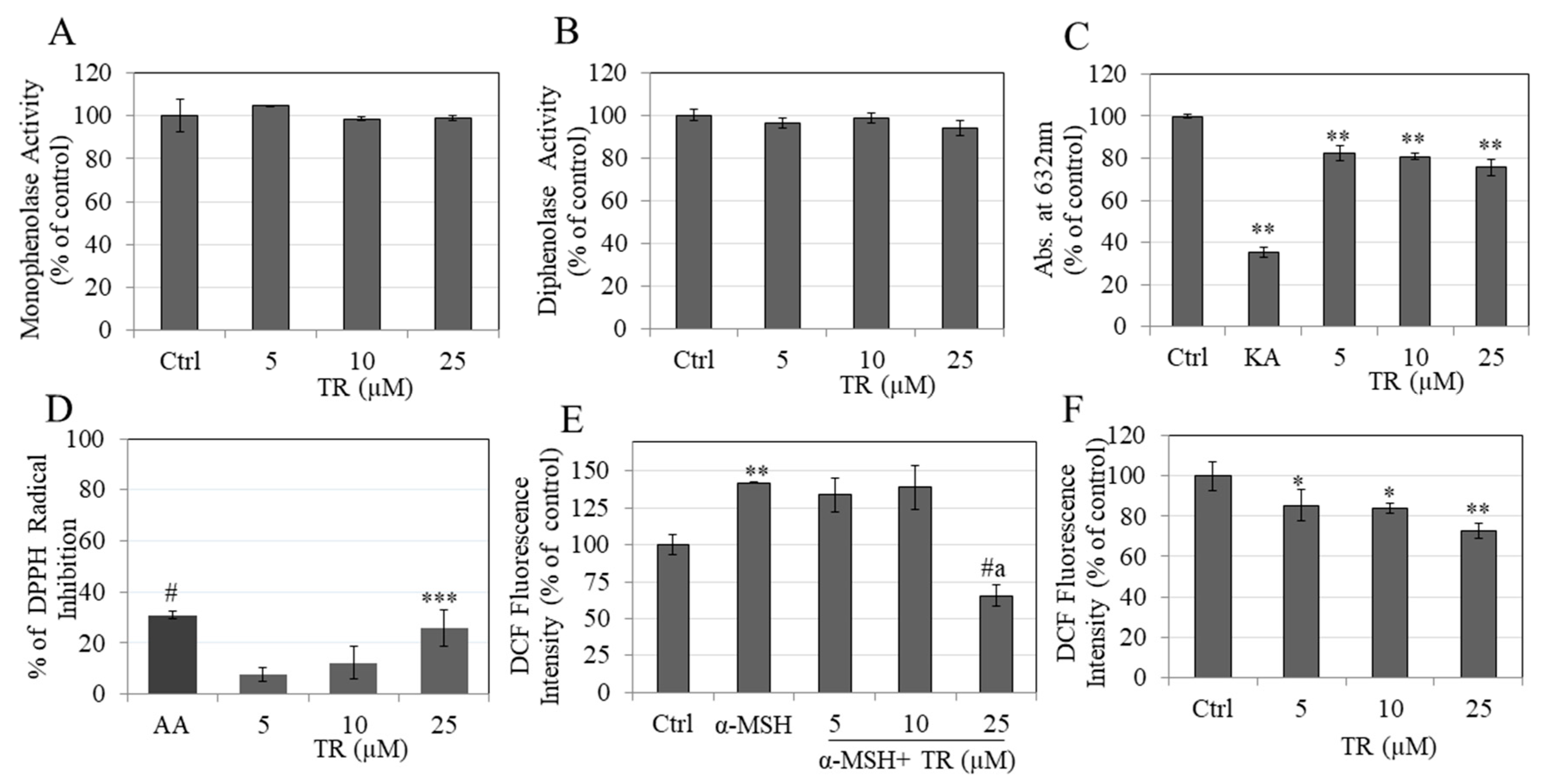
3.3. TR Does Not Inhibit Intracellular Tyrosinase Activity
3.4. TR Does Not Inhibit Mushroom Tyrosinase Activity
3.5. TR Shows Copper Chelation
3.6. TR Shows Antioxidant Activity by Scavenging DPPH Radical
3.7. TR Reduced Intracellular ROS Levels
3.8. TR Does Not Inhibit Cellular α-Glucosidase Activity but Potently Inhibits Direct α-Glucosidase Activity
3.9. TR Enhanced Melanin Accumulation in Primary Human Melanocytes without Affecting Intracellular Tyrosinase Activity
3.10. TR Inhibited Melanosome Export by Inhibiting Dendricity in Primary Human Melanocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ando, H.; Niki, Y.; Ito, M.; Akiyama, K.; Matsui, M.S.; Yarosh, D.B.; Ichihashi, M. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J. Investig. Dermatol. 2012, 132, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Mosby, N.; Yang, J.; Xu, A.; Abdel-Malek, Z.; Kadekaro, A.L. Alpha-MSH activates immediate defense responses to UV-induced oxidative stress in human melanocytes. Pigment Cell Melanoma Res. 2009, 22, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Malviya, N.; Pandya, A. Disorders of Hyperpigmentation. In Dermatoanthropology of Ethnic Skin and Hair; Springer: Berlin/Heidelberg, Germany, 2017; pp. 197–214. [Google Scholar]
- Silpa-Archa, N.; Kohli, I.; Chaowattanapanit, S.; Lim, H.W.; Hamzavi, I. Postinflammatory hyperpigmentation: A comprehensive overview: Epidemiology, pathogenesis, clinical presentation, and noninvasive assessment technique. J. Am. Acad. Dermatol. 2017, 77, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Hebert, D.N. Tyrosinase maturation through the mammalian secretory pathway: Bringing color to life. Pigment Cell Res. 2006, 19, 3–18. [Google Scholar] [CrossRef]
- Smit, N.; Vicanova, J.; Pavel, S. The hunt for natural skin whitening agents. Int. J. Mol. Sci. 2009, 10, 5326–5349. [Google Scholar] [CrossRef]
- Chang, T.-S. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- Mata, T.L.; Sanchez, J.P.; de la Cuadra Oyanguren, J. Allergic contact dermatitis due to kojic acid. Dermatitis 2005, 16, 89. [Google Scholar]
- Hirose, M.; Imai, T.; Mitsumori, K. Carcinogenicity of kojic acid in rodents. JSM Mycotoxins 2004, 2003, 59–67. [Google Scholar] [CrossRef]
- Luo, L.; Jiang, L.; Geng, C.; Cao, J.; Zhong, L. Hydroquinone-induced genotoxicity and oxidative DNA damage in HepG2 cells. Chem. Biol. Interact. 2008, 173, 1–8. [Google Scholar] [CrossRef]
- Ando, H.; Kondoh, H.; Ichihashi, M.; Hearing, V.J. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J. Investig. Dermatol. 2007, 127, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Ahn, S.; Chang, H.; Cho, N.S.; Joo, K.; Lee, B.G.; Chang, I.; Hwang, J.S. Influence of N-glycan processing disruption on tyrosinase and melanin synthesis in HM3KO melanoma cells. Exp. Dermatol. 2007, 16, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hammer, J.A. Melanosome transfer: It is best to give and receive. Curr. Opin. Cell Biol. 2014, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Lorenz, P.; Petersen, R.; Heldermann, M.; Borchert, S. Skin-lightening agent with different pathways of action on melanogenesis. Sofw. J. 2005, 131, 40. [Google Scholar]
- Fitzpatrick, T.B.; Breathnach, A.S. The epidermal melanin unit system. Dermatol. Wochenschr. 1963, 147, 481–489. [Google Scholar] [PubMed]
- Jimbow, K.; Quevedo, W.C., Jr.; Fitzpatrick, T.B.; Szabo, G. Some aspects of melanin biology: 1950–1975. J. Investig. Dermatol. 1976, 67, 72–89. [Google Scholar] [CrossRef]
- Lv, J.; Fu, Y.; Cao, Y.; Jiang, S.; Yang, Y.; Song, G.; Yun, C.; Gao, R. Isoliquiritigenin inhibits melanogenesis, melanocyte dendricity and melanosome transport by regulating ERK-mediated MITF degradation. Exp. Dermatol. 2020, 29, 149–157. [Google Scholar] [CrossRef]
- Regnier, M.; Tremblaye, C.; Schmidt, R. Vitamin C affects melanocyte dendricity via keratinocytes. Pigment Cell Res. 2005, 18, 389–390. [Google Scholar] [CrossRef]
- Jeon, S.; Kim, N.H.; Koo, B.S.; Lee, H.J.; Lee, A.Y. Bee venom stimulates human melanocyte proliferation, melanogenesis, dendricity and migration. Exp. Mol. Med. 2007, 39, 603–613. [Google Scholar] [CrossRef]
- Lv, J.; Fu, Y.; Gao, R.; Li, J.; Kang, M.; Song, G.; Yun, C. Diazepam enhances melanogenesis, melanocyte dendricity and melanosome transport via the PBR/cAMP/PKA pathway. Int. J. Biochem. Cell Biol. 2019, 116, 105620. [Google Scholar] [CrossRef]
- Renato, C.; Carolina, C.; Piero, S. Antibiotic Thermorubin and Methods of Preparing the Same. US.. Patent 3,300,379A, 24 January 1967. [Google Scholar]
- Craveri, R.; Coronelli, C.; Pagani, H.; Sensi, P. Thermorubin, a New Antibiotic from a Thermoactinomycete. Clin. Med. 1964, 71, 511–521. [Google Scholar] [PubMed]
- Cavalleri, B.; Turconi, M.; Pallanza, R. Synthesis and antibacterial activity of some derivatives of the antibiotic thermorubin. J. Antibiot. 1985, 38, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Johnson, F.; Chandra, B.; Iden, C.R.; Naiksatam, P.; Kahen, R.; Okaya, Y.; Lin, S.-Y. Thermorubin 1. Structure studies. J. Am. Chem. Soc. 1980, 102, 5580–5585. [Google Scholar] [CrossRef]
- Moppett, C.E.; Dix, D.T.; Johnson, F.; Coronelli, C. Structure of thermorubin A, the major orange-red antibiotic of Thermoactinomyces antibioticus. J. Am. Chem. Soc. 1972, 94, 3269–3272. [Google Scholar] [CrossRef] [PubMed]
- Bulkley, D.; Johnson, F.; Steitz, T.A. The antibiotic thermorubin inhibits protein synthesis by binding to inter-subunit bridge B2a of the ribosome. J. Mol. Biol. 2012, 416, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Dombou, M.; Sekiya, M.; Nakajima, H.; Fujita, T.; Nakayama, M. Thermorubin and 2-hydroxyphenyl acetic acid, aldose reductase inhibitors. J. Antibiot. 1995, 48, 1345–1346. [Google Scholar] [CrossRef]
- Said, R. Inhibitors of Serine Proteinases, Matrix Metalloproteinases and Histone Deacetylases: Thermorubin, COL-308, Myricetin, and Tellimagrandin; Stony Brook: New York, NY, USA, 2011; pp. 41–46. [Google Scholar]
- Arenz, S.; Wilson, D.N. Blast from the past: Reassessing forgotten translation inhibitors, antibiotic selectivity, and resistance mechanisms to aid drug development. Mol. Cell 2016, 61, 3–14. [Google Scholar] [CrossRef]
- Sim, M.-O.; Choi, I.-Y.; Cho, J.-H.; Shin, H.-M.; Cho, H.-W. Anti-melanogenesis and anti-oxidant of Salix pseudo-lasiogyne water extract in α-MSH-induced B16F10 melanoma cells. Food Agric. Immunol. 2017, 28, 1003–1016. [Google Scholar] [CrossRef]
- Goenka, S.; Ceccoli, J.; Simon, S.R. Anti-melanogenic activity of ellagitannin casuarictin in B16F10 mouse melanoma cells. Nat. Prod. Res. 2019, 27, 1–6. [Google Scholar] [CrossRef]
- Goenka, S.; Nagabhushanam, K.; Majeed, M.; Simon, S.R. Calebin-A, a curcuminoid analog inhibits α-MSH-induced melanogenesis in B16F10 mouse melanoma cells. Cosmetics 2019, 6, 51. [Google Scholar] [CrossRef]
- Chang, C.H.; Tsai, R.K.; Tsai, M.H.; Lin, Y.H.; Hirobe, T. The roles of Frizzled-3 and Wnt3a on melanocyte development: In vitro studies on neural crest cells and melanocyte precursor cell lines. J. Dermatol. Sci. 2014, 75, 100–108. [Google Scholar] [CrossRef]
- Ohbayashi, N.; Yatsu, A.; Tamura, K.; Fukuda, M. The Rab21-GEF activity of Varp, but not its Rab32/38 effector function, is required for dendrite formation in melanocytes. Mol. Biol. Cell 2012, 23, 669–678. [Google Scholar] [CrossRef]
- Jian, Q.; An, Q.; Zhu, D.; Hui, K.; Liu, Y.; Chi, S.; Li, C. MicroRNA 340 is involved in UVB-induced dendrite formation through the regulation of RhoA expression in melanocytes. Mol. Cell. Biol. 2014, 34, 3407–3420. [Google Scholar] [CrossRef]
- Kim, J.H.; Sohn, K.C.; Choi, T.Y.; Kim, M.Y.; Ando, H.; Choi, S.J.; Kim, S.; Lee, Y.H.; Lee, J.H.; Kim, C.D. β-Catenin regulates melanocyte dendricity through the modulation of PKCζ and PKCδ. Pigment Cell Melanoma Res. 2010, 23, 385–393. [Google Scholar] [CrossRef]
- Kim, M.Y.; Choi, T.-Y.; Kim, J.-H.; Lee, J.H.; Kim, J.G.; Sohn, K.-C.; Yoon, K.-S.; Kim, C.D.; Lee, J.-H.; Yoon, T.-J. MKK6 increases the melanocyte dendricity through the regulation of Rho family GTPases. J. Dermatol. Sci. 2010, 60, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Mann, T.; Gerwat, W.; Batzer, J.; Eggers, K.; Scherner, C.; Wenck, H.; Stab, F.; Hearing, V.J.; Rohm, K.H.; Kolbe, L. Inhibition of human tyrosinase requires molecular motifs distinctively different from mushroom tyrosinase. J. Investig. Dermatol. 2018, 138, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.W.; Jeong, S.H.; Curtis-Long, M.J.; Jung, S.; Lee, J.W.; Woo, H.S.; Cho, J.K.; Park, K.H. Inhibition effects of mangosenone F from Garcinia mangostana on melanin formation in B16F10 cells. J. Agric. Food Chem. 2012, 60, 8372–8378. [Google Scholar] [CrossRef] [PubMed]
- Chiaverini, C.; Beuret, L.; Flori, E.; Busca, R.; Abbe, P.; Bille, K.; Bahadoran, P.; Ortonne, J.P.; Bertolotto, C.; Ballotti, R. Microphthalmia-associated transcription factor regulates RAB27A gene expression and controls melanosome transport. J. Biol. Chem. 2008, 283, 12635–12642. [Google Scholar] [CrossRef] [PubMed]
- Hunt, G.; Todd, C.; Thody, A.J. Unresponsiveness of human epidermal melanocytes to melanocyte-stimulating hormone and its association with red hair. Mol. Cell. Endocrinol. 1996, 116, 131–136. [Google Scholar] [CrossRef]
- Lee, C.-S.; Nam, G.; Bae, I.-H.; Park, J. Whitening efficacy of ginsenoside F1 through inhibition of melanin transfer in cocultured human melanocytes–keratinocytes and three-dimensional human skin equivalent. J. Ginseng Res. 2019, 43, 300. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Mitsunaga, T.; Itakura, Y.; Batubara, I. Extracellular melanogenesis inhibitory activity and the structure-activity relationships of ugonins from Helminthostachys zeylanica roots. Fitoterapia 2015, 104, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Azumi, J.; Takeda, T.; Shimada, Y.; Aso, H.; Nakamura, T. The Organogermanium Compound THGP Suppresses Melanin Synthesis via Complex Formation with L-DOPA on Mushroom Tyrosinase and in B16 4A5 Melanoma Cells. Int. J. Mol. Sci. 2019, 20, 4785. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goenka, S.; R. Simon, S. Inhibitory Effects of the Bioactive Thermorubin Isolated from the Fungus Thermoactinomyces Antibioticus on Melanogenesis. Cosmetics 2020, 7, 61. https://doi.org/10.3390/cosmetics7030061
Goenka S, R. Simon S. Inhibitory Effects of the Bioactive Thermorubin Isolated from the Fungus Thermoactinomyces Antibioticus on Melanogenesis. Cosmetics. 2020; 7(3):61. https://doi.org/10.3390/cosmetics7030061
Chicago/Turabian StyleGoenka, Shilpi, and Sanford R. Simon. 2020. "Inhibitory Effects of the Bioactive Thermorubin Isolated from the Fungus Thermoactinomyces Antibioticus on Melanogenesis" Cosmetics 7, no. 3: 61. https://doi.org/10.3390/cosmetics7030061
APA StyleGoenka, S., & R. Simon, S. (2020). Inhibitory Effects of the Bioactive Thermorubin Isolated from the Fungus Thermoactinomyces Antibioticus on Melanogenesis. Cosmetics, 7(3), 61. https://doi.org/10.3390/cosmetics7030061




