Abstract
The indiscriminate use of non-regulated skin lighteners among African populations has raised health concerns due to the negative effects associated with skin lightener toxicity. For this reason, there is a growing interest in the cosmetic development of plants and their metabolites as alternatives to available chemical-derived skin lightening formulations. Approximately 90% of Africa’s population depends on traditional medicine, and the continent’s biodiversity holds plant material with various biological activities, thus attracting considerable research interest. This study aimed to review existing evidence and document indigenous African plant species capable of inhibiting the enzyme tyrosinase and melanogenesis for potential incorporation into skin lightening products. Literature search on melanin biosynthesis, skin lightening, and tyrosinase inhibitors resulted in the identification of 35 plant species were distributed among 31 genera and 21 families across 15 African countries and 9 South African provinces. All plants identified in this study showed competent tyrosinase and melanogenesis inhibitory capabilities. These results indicate that African plants have the potential to serve as alternatives to current chemically-derived skin lighteners.
1. Introduction
Melanin is a widespread natural pigment that is responsible for color in hair, skin, and eyes. It provides protection against the deleterious effects of ultraviolet (UV) irradiation [1]. Melanogenesis is the physiological process of melanin formation in which TYR, a copper-dependent enzyme, initiates the first step. Tyrosinase catalyzes the rate-limiting step where L-tyrosine is converted to L-3,4,-dihydroxyphenylalanine (L-DOPA), leading to the eventual formation of the pigment (Illustrated by Scheme 1) [2,3,4,5]. Abnormal TYR activity leads to pigmentary disorders, such as the abnormal accumulation of melanin (hyperpigmentation) that accounts for most dermatology visits [6,7,8]. Skin lighteners can be divided by their mechanisms of action, such as inhibition of tyrosinase transcription, inhibition of melanosome transfer, and accelerated epidermal turnover, with the most common target being tyrosinase (TYR) inhibition [9,10]. By decreasing the activity and/or expression of TYR, melanogenesis can be inhibited, leading to reduced melanin production [11].
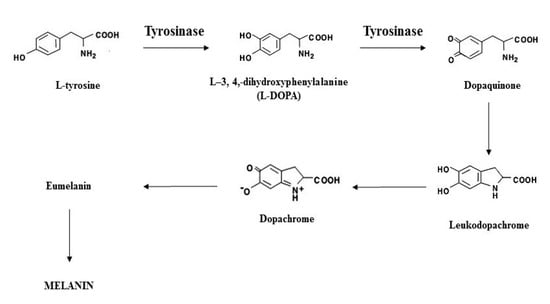
Scheme 1.
Illustration of the melanogenesis pathway.
The skin lightening industry is one of the fastest-growing segments of the global beauty industry. Global industry analysts (GIA) have predicted that by 2020, the universal skin lightening market will reach $23 billion [12]. A recent meta-analysis provided evidence of the global prevalence of skin lightening use by reporting an estimate of 27.7%, with Africa at a current estimated prevalence of 27.1% [13]. Previous epidemiological studies have also reported a high prevalence of skin lightener use among African populations. This is evident among South African, Senegalese, and Nigerian study populations that revealed between 32 to 75% skin lightener use [14,15,16].
This practice is motivated by a long-standing history of social divisions, including societal pressures and stigmas, leading to the demand for lighter skin tones [17,18]. Creams, lotions, soaps, and injections indicated as a treatment for hyperpigmentation disorders are exceedingly abused as self-medication to achieve a lighter skin complexion [19,20]. In many African countries, a variety of these skin lightening preparations are easily obtained over-the-counter without a medical prescription, despite this being a requirement by law [15,21]. The most frequently used ingredients include steroids, mercury, hydroquinone (HQ) (considered the gold standard), and its derivatives [7]. Health concerns associated with the long-term use of these skin lightener ingredients include exogenous ochronosis and infectious dermatosis [22,23]. Furthermore, heavy metal exposure can lead to damage to the circulatory and urinary systems [24]. Due to their toxicity, these compounds have been prohibited as skin lightening compounds in several African countries, including South Africa, Nigeria, Kenya, and the Ivory Coast [25,26]. Despite this ban, these damaging chemicals are often illegally introduced into cosmetic formulations and, the public continues to gain access via informal channels such as street vendors, markets, and non-pharmaceutical shops [27,28]. In contrast, botanicals and natural ingredients offer safer alternatives as they may not exhibit the same kind of toxicity as synthetic compounds and could exhibit much less harmful side effects [29]. Despite this, consumers are not generally aware that natural products are composed of a variety of chemical compounds that could lead to the development of some adverse reactions. These potential effects could be overcome by researchers chemically characterizing extracts with respect to its composition [30].
Botanicals and natural ingredients provide abundant sources of treatment for various diseases such as cancer, diabetes, and dermatological conditions [31,32]. The use of plants is a common practice in traditional medicines of many cultures using several plant extracts as cosmetics to improve skin health [33,34]. This could be attributed to plant extracts being a rich source of vitamins, antioxidants, oils/essential oils, and other bioactive compounds, which provide the body with nutrients necessary for healthy skin [30]. Plants also constitute a variety of chemical compounds that elicit various pharmacological activities with the possibility that these compounds act synergistically to produce a net pharmacological effect [35]. Examples of such compounds include polyphenols and flavonoids. Polyphenols are widely distributed in plants, and several polyphenol types have been reported as being responsible for melanogenesis inhibition [36,37]. Flavonoids and chalcones are a group of polyphenols with flavonoids being one of the most explored and most numerous groups of polyphenols [38]. Flavonoids are found within the leaves, seeds, bark, and flowers of most plants, and have been studied for its oxidation of L-DOPA and have shown good antityrosinase. Furthermore, chalcones exhibit a wide array of biological activities with a number of chalcones eliciting antityrosinase activity [39,40,41].
The significant advancement of research using plant extracts in cosmetics demonstrates the growing interest of researchers and pharmaceutical companies in developing natural skin lightening products [42]. The objective of this review was to examine existing literature to identify and document indigenous African plant species capable of inhibiting the enzyme TYR and melanogenesis for possible use as alternatives to current skin lightening formulations.
2. Materials and Methods
A computerized literature search was performed using the following databases: MEDLINE, SCOPUS, GOOGLE SCHOLAR, MEDLINE EBSCOHOST, and SCIENCE DIRECT databases. In addition, the South African National Electronic Thesis Database (ETD) was searched for grey literature, which included Masters and Doctoral theses. The following key terms were used for the retrieval of articles in the databases: “skin lightening”, “tyrosinase”, “melanin”, “antimelanogenesis”, “antityrosinase”, “melanogenesis”, “tyrosinase inhibition”, “melanin inhibition”. For an article to be considered eligible, the following criteria needed to be met: (1) The use of indigenous African plant extracts (alone or in combination with other African plants); (2) performed in vivo and in vitro studies only; (3) investigated tyrosinase and melanogenesis inhibition. This literature search also had no restrictions on the following: Language; date of publication, and publication status (inclusive of published, unpublished, in the press and in progress). Studies that reported on both non-African (not indigenous to Africa and imported plants) and African plants were isolated, and only the African plants were included in this study. In addition, studies that included tests other than tyrosinase and melanin assays were isolated, and only the tyrosinase and melanin assays were reported on. All qualitative studies were excluded. Three independent reviewers completed the above-described methods independently. Any disagreements between the reviewers were discussed and resolved.
The articles that had been retrieved through the computerized literature searches were combined, giving a total of 128 articles. A preliminary analysis of the titles and abstracts of each article was performed, and all duplicates were excluded. After the screening of abstracts by at least 2 authors, the articles classified as ineligible based on the previously described criteria were excluded, and a total of 49 articles underwent a full-text review.
After further application of the exclusion and inclusion criteria, a total of 36 articles were classified as eligible for discussion in this review. Table 1 and Table 2 summarizes the plant species identified along with their melanin and tyrosinase results, respectively. In both tables, the plant names are arranged according to their family, along with the region the plants are found in Africa and plant part used.

Table 1.
Summary of the plant species identified and their melanin results.

Table 2.
Summary of the plant species identified and their tyrosinase results.
3. Results and Discussion
In this study, 35 plant species distributed across 31 genera and 21 families were identified as being effective as TYR and melanogenesis inhibitors. In addition, the plants identified in this study were distributed among 15 African countries and 9 South African provinces. 17 (47.2%) were found in South Africa, with 19 (52.7%) found within other African countries. The most represented families were Fabaceae (5 plant species), Melianthaceae (3 plant species), Sapotaceae (3 plant species), Chenopodiaceae (2 plant species), Proteaceae (2 plant species), Clusiaceae (2 plant species), Rhizophoraceae (2 plant species), and Lamiaceae (2 plant species). The rest of the families were represented with only 1 plant species—Anacardiaceae, Apiaceae, Asteraceae, Brassiaceae, Capparaceae, Euphorbiaceae, Myrsinaceae, Pedaliaceae, Picrodendraceae, Poaceae, Podocarpaceae, Rubiaceae, and Thymelaeaceae.
African forests are the world’s second-largest tropical reservoir holding very promising plant materials with various biological activities, which has attracted considerable research interest [50]. Up to 90% of Africa’s human population depends directly on traditional medicine. Plants form a central component of the African traditional healthcare system and is probably the oldest of all therapeutic systems [71,72]. The importance of this resource can be illustrated by the comprehensive list of African medicinal plants in which more than 5400 plant taxa and over 16,300 medicinal uses for the plants have been identified. The use of plant extracts as topical treatments has been practiced for many generations with extracts being used for the treatment of various skin ailments, including wounds, skin infections, and inflammation [73,74]. The demand for cosmetic skin-lightening products is growing, with predictions particular to Asia and Africa forecasting the beauty industry to be worth an estimated $US 31.2 billion by 2024 [12,75]. This significant increase can also be accounted for by the pleasant aromatics and the general consensus that plant extracts are safer than synthetic products available. Thus, there is an ever-growing endeavour to explore plant-based melanogenesis inhibitors [76,77].
Various plant extracts and compounds have been investigated for their anti-tyrosinase and antimelanogenic effects [78]. Three methods are extensively used to study tyrosinase activity, which includes 2 radiometric assays (tyrosinase hydroxylase and melanin formation activities) and one spectrophotometric assay (dopa oxidase activity). Tyrosinase hydroxylase assay estimates the tyrosinase hydroxylase activity of tyrosinase by measuring tritiated water released from L-[3,5-3H]-tyrosine. The melanin formation activity assay estimates the radioactive melanin synthesized from L-[U-14C]-tyrosine while the dopa oxidase activity measures the rate of dopachrome formation, of which all three are in vitro assays [79]. These assays also include the use of positive controls whose potencies are well-known, such as kojic acid (KA), to which the substance of interest can be compared [80]. Results obtained from these assays are often presented in IC50 values, which refers to the concentration of plant extract at which half the original TYR activity is inhibited [40].
As shown in the results described in Table 2, plants reported from the Fabaceae family were only tested for their ability as TYR inhibitors, and all proved to be strong inhibitors. Further results obtained by Lall et al. [65], supported the findings for Ormocarpum and Acacia nilotica, which demonstrated the lowest IC50 value of 2.95 μg/mL and showed to have the highest TYR inhibition of 98.3% (IC50 8.61 μg/mL), respectively [36,63]. Cassipourea congoensis demonstrated significant effects of both crude extract and isolated compounds on melanin and TYR activity, respectively [49]. Rorippa nasturtium-aquaticum (Brassiaceae) showed in studies conducted by both Thibane et al. [59,60], that the extract is an effective TYR inhibitor (IC50 values of 22.24 and 1.513 μg/mL respectively) when compared to the kojic acid (KA) control (19.38 and 1.421 μg/mL, respectively). It is also noted that KA is the most prominent (91.7%, 33 articles) positive control used in the studies identified, due to its well-established potency in literature [80]. Arbutin, a HQ derivative, was the second most common (35.3%, 12 articles) used positive control as it is generally used in cosmetics as a hypopigmenting agent [81].
Studies on Thymelaea hirsuta (Thymelaeaceae) reported that this extract inhibited more than 50% of melanin at 1 μg/mL [53]. Furthermore, isolated compounds of this extract indicated that melanin production was reduced by 37% at 0.1 μg/mL, in comparison to its arbutin control, which only inhibited 33% of melanin at a higher concentration—100 μg/mL [54]. These results are further supported by Villareal et al. [55], who concluded that isolated compounds of Thymelaea hirsuta at 0.1 μg/mL (33% reduction of MC) is as effective as arbutin, a common depigmenting agent, at 100 μg/mL. Rhizophoraceae extracts (Cassipourea flanaganii and Cassipourea congoensis) exhibited compelling skin lightening properties with IC50 values obtained from studies conducted on Cassipourea flanaganii, which indicated values (1.425 μg/mL and 22.24 μg/mL, respectively) comparable to their KA controls (1.421 μg/mL and 19.38 μg/mL, respectively) [48,49]. Argania spinosa (Sapotaceae) effectively inhibited melanogenesis at 55% after 72 hours’ exposure [50]. These findings are supported by Villareal et al. [23], showing that there is a greater than 50% reduction in melanin content after 72 hours of exposure. Although significant results were obtained from separate studies, the difference in the result could be attributed to the researchers investigating different parts of the same plant (Table 1) as well as differences in plant preparation and assay protocols.
The plants in this study were distributed among 15 African countries with studies, including data from 9 South African provinces. Twenty plants species were investigated using aerial parts/leaves with these plants being collected in different regions of their respective countries and/or provinces. Thus, in natural ecosystems, factors affecting the plant’s performance include climate, soil, and geographic locations yielding various molecular complexes, thus, emphasizing the environment’s crucial role in the metabolism of plants [82,83].
The results obtained from the TYR and melanin assays of Harpephyllum caffrum showed the bark extract to have the highest inhibitory effect on TYR and melanin production in comparison to the leaf extract of the same species [32]. These results are further corroborated by a review conducted by Lall and Kishore. [84], where it was noted that Harpephyllum caffrum and Greyia flanaganii, among other listed plants, showed promising pharmacological activities, a finding that warrants further scientific investigation. Similar comparisons can be seen with Ceratonia siliqua concerning TYR activity where its isolated compounds (90% inhibition) were shown to be a more potent TYR inhibitor than its crude extract (50% inhibition) at the same concentrations (200 μg/mL).
Further comparisons can also be observed by the contrast in results obtained for TYR assays from the use of substrates L-DOPA and L-tyrosine. Here, several plant extracts have proven to be more effective in targeting the inhibition of the oxidation of either L-DOPA or L-tyrosine. This is illustrated by the TYR assay results obtained for Haloxylon articulatum, Greyia radlkoferi, Pituranthos scoparius, Myrsine africana, Hyaenanche globose, and Cleome Arabica [40,45,57]. Additional studies also included extracts of Dolichopentas longiflora, where preparations exhibited a stimulatory response on melanogenesis, whereas the IC50 value for TYR activity (26 ± μg/mL) showed contrasting results. This included Sesamum angolense of the same study, where pellets of the cells that were treated with the extract indicated no significant inhibition. However, the IC50 value (24 μg/mL) obtained indicated that the plant extract can illicit an inhibitory response [64]. Due to the complexity of pigment production, melanogenesis regulation takes place at different levels and various means of interference are possible—providing a possible explanation for the above-described contrasting results [85,86]. Mechanisms of depigmenting include; (1) tyrosinase inhibition, (2) decrease in DOPA polymerase, (3) induction of anti-inflammatory, and (4) anti-oxidant effects [87].
Extracts from the Lamiaceae family also proved to be effective inhibitors with Plectranthus ecklonii showing an IC50 value of 21.58 μg/mL with more than 70% TYR inhibition and Salvia officinalis decreasing MC to 27% at various concentrations [44,66,67]. In addition, other plant extracts elicited a significant inhibitory response on both melanin and TYR activities. These include Garcinia livingstonei and Garcinia kola (Clustiaceae), Myrsine africana (Myrsinaceae), Protea madiensis, and Serruria furcellata—both from the Proteaceae family and Sideroxylon inerme (Sapotaceae). Species from other families such as Clustiaceae (Garcinia livingstonei and Garcinia kola) exhibited significant activities with G. livingstonei exhibiting a large decrease of melanin concentration at 25 μg/mL and the seeds of G. kola inhibiting 79% of tyrosinase at 500 μg/mL [22,61].
4. Conclusions
Several studies have been conducted to identify inhibitors from both natural and synthetic sources, and a number of research papers have been published and regularly updated in this aspect. This study was conducted as a means of identifying plant-based skin lightening alternatives to the current toxic substances. Despite the serious and life-threatening complications associated with the chronic use of these products, the use of skin lighteners is still a widespread and common practice in several African countries [88,89].
All plants identified in this study showed competent antimelanogenesis and antityrosinase capabilities, with the most effective of the extracts being the following: Acacia nilotica, Cassipourea congoensis, Cassipourea flanaganii, Garcinia kola, Greyia flanaganii, Greyia radlkoferi, Hyaenanche globosa, Myrsine africana, Ormocarpum trichocarpum, Plectranthus ecklonii, Protea madiensis, Rorippa nasturtium-aquaticum, Serruria furcellata, Sesamum angolense, and Vachellia karroo. The reproducibility of the identified studies and interpretation of the results is limited by the inconsistencies in methodologies and means of plant extraction in these studies. Other variables also include geographical location and varied climate regions.
This review shows that plants of the African continent have the potential to act as melanin and TYR inhibitors and can be used to replace synthetic and other derived chemicals. Although many of these plants have been effective in their pigment reduction properties, plants are still known to cause allergic reactions and elicit phototoxic effects [87]. This is due to natural products being a complex mixture of chemical compounds, a fact often unknown to consumers. To combat this, extracts should be chemically characterized with respect to the product composition [30]. In addition, it is imperative that toxicity studies be conducted to establish a safe dose range. These findings could aid in the production and commercialization of these plants in natural-based remedies for cosmetic and skincare product industries.
Author Contributions
All authors listed made substantial contribution to the manuscript and qualify for authorship. No authors have been omitted. Concept and design: F.R.; data collection and interpretation of data: L.O., F.R., M.D.K., J.K.; writing—review and editing: L.O., F.R., M.D.K., J.K.; supervision: F.R.; project administration: L.O. All authors have read and agreed to the published version of the manuscript.
Funding
The authors greatly appreciate funding received by the National Research Foundation (NRF) South Africa for this study.
Acknowledgments
The authors would like to thank research assistants Jade Harris (JH) and Pedro Abrantes (PA) for assisting in the data collection.
Disclaimer
Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors and, therefore, the NRF does not accept any liability in regard thereto.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Masum, M.N.; Yamauchi, K.; Mitsunaga, T. Tyrosinase Inhibitors from Natural and Synthetic Sources as Skin-lightening Agents. Rev. Agric. Sci. 2019, 7, 41–58. [Google Scholar] [CrossRef]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, S.; Khan, T.H.; Osborn, H.M.; Williams, N.A.O. Tyrosinase activated melanoma prodrugs. Anticancer. Agents Med. Chem. 2009, 9, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Mapunya, M.B.; Lall, N. Melanin and Its Role in Hyper-Pigmentation–Current Knowledge and Future Trends in Research. Breakthr. Melanoma Res. 2011. [Google Scholar] [CrossRef][Green Version]
- Videira, I.F.D.S.; Moura, D.F.L.; Magina, S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef]
- Campos, P.M.; da Silva Horinouchi, C.D.; da Silveira Prudente, A.; Cechinel-Filho, V.; de Almeida Cabrini, D.; Otuki, M.F. Effect of a Garcinia gardneriana (Planchon and Triana) Zappi hydroalcoholic extract on melanogenesis in B16F10 melanoma cells. J. Ethnopharmacol. 2013, 148, 199–204. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F.J.I.C. Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crops Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Hollinger, J.C.; Angra, K.; Halder, R.M. Are natural ingredients effective in the management of hyperpigmentation? A systematic review. J. Clin. Aesthet Dermatol. 2018, 11, 28–37. [Google Scholar]
- Gillbro, J.M.; Olsson, M.J. The melanogenesis and mechanisms of skin-lightening agents–existing and new approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Overview of skin whitening agents: Drugs and cosmetic products. Cosmetic 2016, 3, 27. [Google Scholar] [CrossRef]
- Sari, D.M.; Anwar, E.; Arifianti, A.E. Antioxidant and tyrosinase inhibitor activities of ethanol extracts of brown seaweed (Turbinaria conoides) as lightening ingredient. Phcog. J. 2019, 11, 379–382. [Google Scholar] [CrossRef]
- Mohiuddin, A.K. Skin Lightening & Management of Hyperpigmentation. Pharma Sci. Anal. Res. J. 2019, 2. [Google Scholar] [CrossRef]
- Sagoe, D.; Pallesen, S.; Dlova, N.C.; Lartey, M.; Ezzedine, K.; Dadzie, O. The global prevalence and correlates of skin bleaching: A meta-analysis and meta-regression analysis. Int. J. Dermatol. 2019, 58, 24–44. [Google Scholar] [CrossRef]
- Adebajo, S.B. An epidemiological survey of the use of cosmetic skin lightening cosmetics among traders in Lagos, Nigeria. Mercury 2002, 5, 434–438. [Google Scholar]
- Dlova, N.C.; Hamed, S.H.; Tsoka-Gwegweni, J.; Grobler, A. Skin lightening practices: An epidemiological study of South African women of African and Indian ancestries. Br. J. Dermatol. 2015, 173, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Wone, I.; Tal-Dia, A.; Diallo, O.F.; Badiane, M.; Touré, K.; Diallo, I. Prevalence of the use of skin bleaching cosmetics in two areas in Dakar (Sénégal). Dakar Med. 2000, 45, 154–157. [Google Scholar]
- Charles, C. Skin bleaching and the deconstruction of blackness. Ideaz 2003, 2, 42–54. [Google Scholar]
- Charles, C.A. Skin bleachers’ representations of skin color in Jamaica. J. Black Stud. 2009, 40, 153–170. [Google Scholar] [CrossRef]
- Arbab, A.; Eltahir, M.M. Review on skin whitening agents. Khartoum. Pharm. J. 2010, 13, 5–9. [Google Scholar]
- Dlova, N.; Hamed, S.H.; Tsoka-Gwegweni, J.; Grobler, A.; Hift, R. Women’s perceptions of the benefits and risks of skin-lightening creams in two South African communities. J. Cosmet. Dermatol. 2014, 13, 236–241. [Google Scholar] [CrossRef]
- Nnoruka, E.; Okoye, O. Topical steroid abuse: Its use as a depigmenting agent. J. Natl. Med. Assoc. 2006, 98, 934. [Google Scholar] [PubMed]
- Mulholland, D.A.; Mwangi, E.M.; Dlova, N.C.; Plant, N.; Crouch, N.R.; Coombes, P.H. Non-toxic melanin production inhibitors from Garcinia livingstonei (Clusiaceae). J. Ethnopharmacol. 2013, 149, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Villareal, M.O.; Kume, S.; Bourhim, T.; Bakhtaoui, F.Z.; Kashiwagi, K.; Han, J.; Gadhi, C.; Isoda, H. Activation of MITF by argan oil leads to the inhibition of the tyrosinase and dopachrome tautomerase expressions in B16 murine melanoma cells. Evid. Based Complement. Altern. Med. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Michalek, I.M.; Benn, E.K.; Dos Santos, F.L.C.; Gordon, S.; Wen, C.; Liu, B. A systematic review of global legal regulations on the permissible level of heavy metals in cosmetics with particular emphasis on skin lightening products. Environ. Res. 2019, 170, 187–193. [Google Scholar] [CrossRef]
- Chan, T.Y. Inorganic mercury poisoning associated with skin-lightening cosmetic products. Clin. Toxicol. 2011, 49, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Davids, L.M.; Van Wyk, J.; Khumalo, N.P.; Jablonski, N.G. The phenomenon of skin lightening: Is it right to be light? S. Afr. J. Sci. 2016, 112, 1–5. [Google Scholar] [CrossRef]
- Kamagaju, L.; Morandini, R.; Gahongayire, F.; Stévigny, C.; Ghanem, G.; Pirotte, G.; Duez, P. Survey on skin-lightening practices and cosmetics in Kigali, Rwanda. Int. J. Dermatol. 2016, 55, 45–51. [Google Scholar] [CrossRef]
- Gbetoh, M.H.; Amyot, M. Mercury, hydroquinone and clobetasol propionate in skin lightening products in West Africa and Canada. Environ. Res. 2016, 150, 403–410. [Google Scholar] [CrossRef]
- Di Petrillo, A.; González-Paramás, A.M.; Era, B.; Medda, R.; Pintus, F.; Santos-Buelga, C.; Fais, A. Tyrosinase inhibition and antioxidant properties of Asphodelus microcarpus extracts. BMC Complement. Altern. Med. 2016, 16, 453. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Sousa Lobo, J.M. Main benefits and applicability of plant extracts in skin care products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Kumari, S.; Elancheran, R.; Devi, R. Phytochemical screening, antioxidant, antityrosinase, and antigenotoxic potential of Amaranthus viridis extract. Indian J. Pharmacol. 2018, 50, 130. [Google Scholar] [PubMed]
- Mapunya, M.B.; Nikolova, R.V.; Lall, N. Melanogenesis and antityrosinase activity of selected South African plants. Evid. Based Complement. Altern. Med. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Momtaz, S.; Mapunya, B.M.; Houghton, P.J.; Edgerly, C.; Hussein, A.; Naidoo, S.; Lall, N. Tyrosinase inhibition by extracts and constituents of Sideroxylon inerme L. stem bark, used in South Africa for skin lightening. J. Ethnopharmacol. 2008, 119, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Twilley, D.; Lall, N. African plants with dermatological and ocular relevance. Toxicol. Surv. Afr. Med. Plants 2014, 493–512. [Google Scholar] [CrossRef]
- Abdillahi, H.S.; Finnie, J.F.; Van Staden, J. Anti-inflammatory, antioxidant, anti-tyrosinase and phenolic contents of four Podocarpus species used in traditional medicine in South Africa. J. Ethnopharmacol. 2011, 136, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Muddathir, A.M.; Yamauchi, K.; Batubara, I.; Mohieldin, E.A.M.; Mitsunaga, T. Anti-tyrosinase, total phenolic content and antioxidant activity of selected Sudanese medicinal plants. S. Afr. J. Bot. 2017, 109, 9–15. [Google Scholar] [CrossRef]
- Zhu, W.; Gao, J. The use of botanical extracts as topical skin-lightening agents for the improvement of skin pigmentation disorders. J. Investig. Dermatol. Symp. Proc. 2008, 13, 20–24. [Google Scholar] [CrossRef]
- Mapunya, M.B.; Hussein, A.A.; Rodriguez, B.; Lall, N. Tyrosinase activity of Greyia flanaganii (Bolus) constituents. Phytomedicine 2011, 18, 1006–1012. [Google Scholar] [CrossRef]
- Lee, S.Y.; Baek, N.; Nam, T.G. Natural, semisynthetic and synthetic tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2016, 31, 1–13. [Google Scholar] [CrossRef]
- Momtaz, S.; Lall, N.; Basson, A. Inhibitory activities of mushroom tyrosine and DOPA oxidation by plant extracts. S. Afr. J. Bot. 2008, 74, 577–582. [Google Scholar] [CrossRef]
- Ali, S.A. Recent advances in treatment of skin disorders using herbal products. J. Skin 2017, 1, 6–7. [Google Scholar]
- Huang, Z.; Hashida, K.; Makino, R.; Kawamura, F.; Shimizu, K.; Kondo, R.; Ohara, S. Evaluation of biological activities of extracts from 22 African tropical wood species. J. Wood Sci. 2009, 55, 225–229. [Google Scholar] [CrossRef]
- Chao, H.C.; Najjaa, H.; Villareal, M.O.; Ksouri, R.; Han, J.; Neffati, M.; Isoda, H. Arthrophytum scoparium inhibits melanogenesis through the down-regulation of tyrosinase and melanogenic gene expressions in B 16 melanoma cells. Exp. Dermatol. 2013, 22, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.; Mira, A.; Ashour, A.; Shimizu, K. Acetylcholine esterase inhibitors and melanin synthesis inhibitors from Salvia officinalis. Phytomedicine 2016, 23, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Lall, N.; Mogapi, E.; De Canha, M.N.; Crampton, B.; Nqephe, M.; Hussein, A.A.; Kumar, V. Insights into tyrosinase inhibition by compounds isolated from Greyia radlkoferi Szyszyl using biological activity, molecular docking and gene expression analysis. Bioorg. Med. Chem. 2016, 24, 5953–5959. [Google Scholar] [CrossRef]
- Kishore, N.; Twilley, D.; Blom van Staden, A.; Verma, P.; Singh, B.; Cardinali, G.; Kovacs, D.; Picardo, M.; Kumar, V.; Lall, N. Isolation of flavonoids and flavonoid glycosides from Myrsine africana and their inhibitory activities against mushroom tyrosinase. J. Nat. Prod. 2018, 81, 49–56. [Google Scholar] [CrossRef]
- Kamagaju, L.; Morandini, R.; Bizuru, E.; Nyetera, P.; Nduwayezu, J.B.; Stévigny, C.; Ghanem, G.; Duez, P. Tyrosinase modulation by five Rwandese herbal medicines traditionally used for skin treatment. J. Ethnopharmacol. 2013, 146, 824–834. [Google Scholar] [CrossRef]
- Sonka, L. Exploring Anti-Tyrosinase Bioactive Compounds from the Cape Flora. Master’s Thesis, University of the Western Cape, Cape Town, South Africa, 2018. [Google Scholar]
- Takou, D.M.; Waffo, A.F.K.; Langat, M.K.; Wansi, J.D.; Mulcahy-Ryan, L.E.; Schwikkard, S.L.; Opara, E.I.; Mas-Claret, E.; Mulholland, D.A. Melanin Production Inhibitors from the West African Cassipourea Congoensis. Planta Med. 2019, 6, 50–56. [Google Scholar] [CrossRef]
- Bourhim, T.; Villareal, M.O.; Gadhi, C.; Hafidi, A.; Isoda, H. Depigmenting effect of argan press-cake extract through the down-regulation of Mitf and melanogenic enzymes expression in B16 murine melanoma cells. Cytotechnology 2018, 70, 1389–1397. [Google Scholar] [CrossRef]
- Momtaz, S. Tyrosinase Inhibitors Isolated from Ceratonia Siliqua (L.) and Sideroxylon Inerme (L.). Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2007. [Google Scholar]
- Zhang, J.; Li, D.; Lv, Q.; Ye, F.; Jing, X.; Masters, E.T.; Shimizu, N.; Abe, M.; Akihisa, T.; Feng, F. Compositions and melanogenesis-inhibitory activities of the extracts of defatted shea (Vitellaria paradoxa) kernels from seven African countries. J. Food Compos. Anal. 2018, 70, 89–97. [Google Scholar] [CrossRef]
- Kawano, M.; Matsuyama, K.; Miyamae, Y.; Shinmoto, H.; Kchouk, M.E.; Morio, T.; Shigemori, H.; Isoda, H. Antimelanogenesis effect of Tunisian herb Thymelaea hirsuta extract on B16 murine melanoma cells. Exp. Dermatol. 2007, 16, 977–984. [Google Scholar] [CrossRef]
- Miyamae, Y.; Villareal, M.O.; Abdrabbah, M.B.; Isoda, H.; Shigemori, H. Hirseins A and B, daphnane diterpenoids from Thymelaea hirsuta that inhibit melanogenesis in B16 melanoma cells. J. Nat. Prod. 2009, 72, 938–941. [Google Scholar] [CrossRef] [PubMed]
- Villareal, M.O.; Han, J.; Yamada, P.; Shigemori, H.; Isoda, H. Hirseins inhibit melanogenesis by regulating the gene expressions of Mitf and melanogenesis enzymes. Exp. Dermatol. 2010, 19, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Momtaz, S.; Lall, N.; Hussein, A.; Ostad, S.N.; Abdollahi, M. Investigation of the possible biological activities of a poisonous South African plant; Hyaenanche globosa (Euphorbiaceae). Pharmacogn. Mag. 2010, 6, 34. [Google Scholar]
- Jdey, A.; Falleh, H.; Jannet, S.B.; Hammi, K.M.; Dauvergne, X.; Magné, C.; Ksouri, R. Anti-aging activities of extracts from Tunisian medicinal halophytes and their aromatic constituents. EXCLI J. 2017, 16, 755. [Google Scholar]
- Popoola, O.K.; Marnewick, J.L.; Rautenbach, F.; Iwuoha, E.I.; Hussein, A.A. Acylphloroglucinol derivatives from the South African Helichrysum niveum and their biological activities. Molecules 2015, 20, 17309–17324. [Google Scholar] [CrossRef]
- Thibane, V.S.; Ndhlala, A.R.; Abdelgadir, H.A.; Finnie, J.F.; Van Staden, J. The cosmetic potential of plants from the Eastern Cape Province traditionally used for skincare and beauty. S. Afr. J. Bot. 2019, 122, 475–483. [Google Scholar] [CrossRef]
- Thibane, V.S.; Ndhlala, A.R.; Finnie, J.F.; Van Staden, J. Cosmeceutical efficiency by some plant extracts used traditionally for skin care in inhibiting tyrosinase activity in a human epidermal melanocyte (HEM) cell line. S. Afr. J. Bot. 2019, 126, 256–260. [Google Scholar] [CrossRef]
- Okunji, C.; Komarnytsky, S.; Fear, G.; Poulev, A.; Ribnicky, D.M.; Awachie, P.I.; Ito, Y.; Raskin, I. Preparative isolation and identification of tyrosinase inhibitors from the seeds of Garcinia kola by high-speed counter-current chromatography. J. Chromatogr. A 2007, 1151, 45–50. [Google Scholar] [CrossRef]
- Sadeer, N.B.; Llorent-Martínez, E.J.; Bene, K.; Mahomoodally, M.F.; Mollica, A.; Sinan, K.I.; Stefanucci, A.; Ruiz-Riaguas, A.; Fernández-de Córdova, M.L.; Zengin, G. Chemical profiling, antioxidant, enzyme inhibitory and molecular modelling studies on the leaves and stem bark extracts of three African medicinal plants. J. Pharm. Biomed. Anal. 2019, 174, 19–33. [Google Scholar] [CrossRef]
- Stapelberg, J.; Nqephe, M.; Lambrechts, I.; Crampton, B.; Lall, N. Selected South African plants with tyrosinase enzyme inhibition and their effect on gene expression. S. Afr. J. Bot. 2019, 120, 280–285. [Google Scholar] [CrossRef]
- Rondo, M. Phytochemical and Biological Studies on Some South African Plants Used in Traditional Medicine for Skin Hyperpigmentation. Master’s Thesis, University of the Western Cape, Cape Town, South Africa, 2017. [Google Scholar]
- Lall, N.; Van Staden, A.B.; Rademan, S.; Lambrechts, I.; De Canha, M.N.; Mahore, J.; Winterboer, S.; Twilley, D. Antityrosinase and anti-acne potential of plants traditionally used in the Jongilanga community in Mpumalanga. S. Afr. J. Bot. 2019, 126, 241–249. [Google Scholar] [CrossRef]
- Nyila, M. Antilisterial Bioactivity and/or Biofilm-Formation by Compounds from Plectranthus Ecklonii Benth. and Acacia Karroo Hayne. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2011. [Google Scholar]
- Ronauld, N.G.E. Detection of Selective Tyrosinase Inhibitors from Some South African Plant Extracts of Lamiaceae Family. Master’s Thesis, University of the Western Cape, Cape Town, South Africa, 2016. [Google Scholar]
- Lehbili, M.; Alabdul Magid, A.; Kabouche, A.; Voutquenne-Nazabadioko, L.; Abedini, A.; Morjani, H.; Gangloff, S.C.; Kabouche, Z. Antibacterial, antioxidant and cytotoxic activities of triterpenes and flavonoids from the aerial parts of Salvia barrelieri Etl. Nat. Prod. Res. 2018, 32, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Sinan, K.I.; Bene, K.; Zengin, G.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Picot-Allain, C.M.N.; Mollica, A.; Rengasamy, K.R.; Mahomoodally, M.F. A comparative study of the HPLC-MS profiles and biological efficiency of different solvent leaf extracts of two African plants: Bersama abyssinica and Scoparia dulcis. Int. J. Environ. 2019, 1–13. [Google Scholar] [CrossRef]
- Lee, D.E.; Kwon, J.E.; Choung, E.S.; Lee, S.R.; Kang, S.C. The antiwrinkle and antimelanogenic effects of the nonedible part of Sorghum bicolor (L.) Moench and their augmentation by fermentation. J. Cosmet. Sci. 2017, 68, 271–283. [Google Scholar] [PubMed]
- Bene, K.; Sinan, K.I.; Zengin, G.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Aumeeruddy, M.Z.; Xiao, J.; Mahomoodally, M.F. A multidirectional investigation of stem bark extracts of four African plants: HPLC-MS/MS profiling and biological potentials. J. Pharm. Biomed. Anal. 2019, 168, 217–224. [Google Scholar] [CrossRef]
- Elansary, H.O.; Mahmoud, E.A.; Shokralla, S.; Yessoufou, K. Diversity of plants, traditional knowledge, and practices in local cosmetics: A case study from Alexandria, Egypt. Econ. Bot. 2015, 69, 114–126. [Google Scholar] [CrossRef]
- Van Wyk, B.E. The potential of South African plants in the development of new medicinal products. S. Afr. J. Bot. 2011, 77, 812–829. [Google Scholar] [CrossRef]
- Leyden, J.J.; Shergill, B.; Micali, G.; Downie, J.; Wallo, W. Natural options for the management of hyperpigmentation. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1140–1145. [Google Scholar] [CrossRef]
- Kim, J.S.; Seo, Y.C.; No, R.H.; Lee, H.Y. Improved cosmetic activity by optimizing the Lithospermum erythrorhizon extraction process. Cytotechnology 2015, 67, 51–65. [Google Scholar] [CrossRef]
- Jennifer, C.; Stephie, C.M.; Abhishri, S.B.; Shalini, B.U. A review on skin whitening property of plant extracts. Int. J. Pharma Bio Sci. 2012, 3, 332–347. [Google Scholar]
- Parvez, S.; Kang, M.; Chung, H.S.; Bae, H. Naturally occurring tyrosinase inhibitors: Mechanism and applications in skin health, cosmetics and agriculture industries. Phytother. Res. 2007, 21, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kang, S.M.; Sok, C.H.; Hong, J.T.; Oh, J.Y.; Jeon, Y.J. Cellular activities and docking studies of eckol isolated from Ecklonia cava (Laminariales, Phaeophyceae) as potential tyrosinase inhibitor. Algae 2015, 30, 163–170. [Google Scholar]
- Jara, J.R.; Solano, F.; Lozano, J.A. Assays for mammalian tyrosinase: A comparative study. Pigment Cell Res. 1988, 1, 332–339. [Google Scholar] [CrossRef]
- Faig, J.J.; Moretti, A.; Joseph, L.B.; Zhang, Y.; Nova, M.J.; Smith, K.; Uhrich, K.E. Biodegradable kojic acid-based polymers: Controlled delivery of bioactives for melanogenesis inhibition. Biomacromolecules 2017, 18, 363–373. [Google Scholar] [CrossRef]
- Chang, T.S. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- Correia, A.F.; Segovia, J.F.O.; Gonçalves, M.C.A.; De Oliveira, V.L.; Silveira, D.; Carvalho, J.C.T.; Kanzaki, L.I.B. Amazonian plant crude extract screening for activity against multidrug-resistant bacteria. Eur. Rev. Med. 2008, 12, 369–380. [Google Scholar]
- Zargoosh, Z.; Ghavam, M.; Bacchetta, G.; Tavili, A. Effects of ecological factors on the antioxidant potential and total phenol content of Scrophularia striata Boiss. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Lall, N.; Kishore, N. Are plants used for skin care in South Africa fully explored? J. Ethnopharmacol. 2014, 153, 61–84. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Hearing, V.J. Modifying skin pigmentation–approaches through intrinsic biochemistry and exogenous agents. Drug Discov. Today Dis. Mech. 2008, 5, 189–199. [Google Scholar] [CrossRef]
- Smit, N.; Vicanova, J.; Pavel, S. The hunt for natural skin whitening agents. Int. J. Mol. Sci. 2009, 10, 5326–5349. [Google Scholar] [CrossRef] [PubMed]
- Fisk, W.A.; Agbai, O.; Lev-Tov, H.A.; Sivamani, R.K. The use of botanically derived agents for hyperpigmentation: A systematic review. J. Am. Acad. Dermatol. 2014, 70, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Dlova, N.C.; Hendricks, N.E.; Martincgh, B.S. Skin-lightening creams used in Durban, South Africa. Int. J. Dermatol. 2012, 51, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Olumide, Y.M. Use of skin lightening cream. Br. Med. J. 2010, 342, 345–346. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).