Rhodomyrtus tomentosa Fruit Extract and Skin Microbiota: A Focus on C. acnes Phylotypes in Acne Subjects
Abstract
1. Introduction
2. Material and Methods
2.1. Participants
2.2. Procedure
2.3. Study Product
2.4. Preparation of Extract
2.5. Microbiota Sampling
2.6. Clinical Evaluations
2.7. Statistical Analysis
3. Results
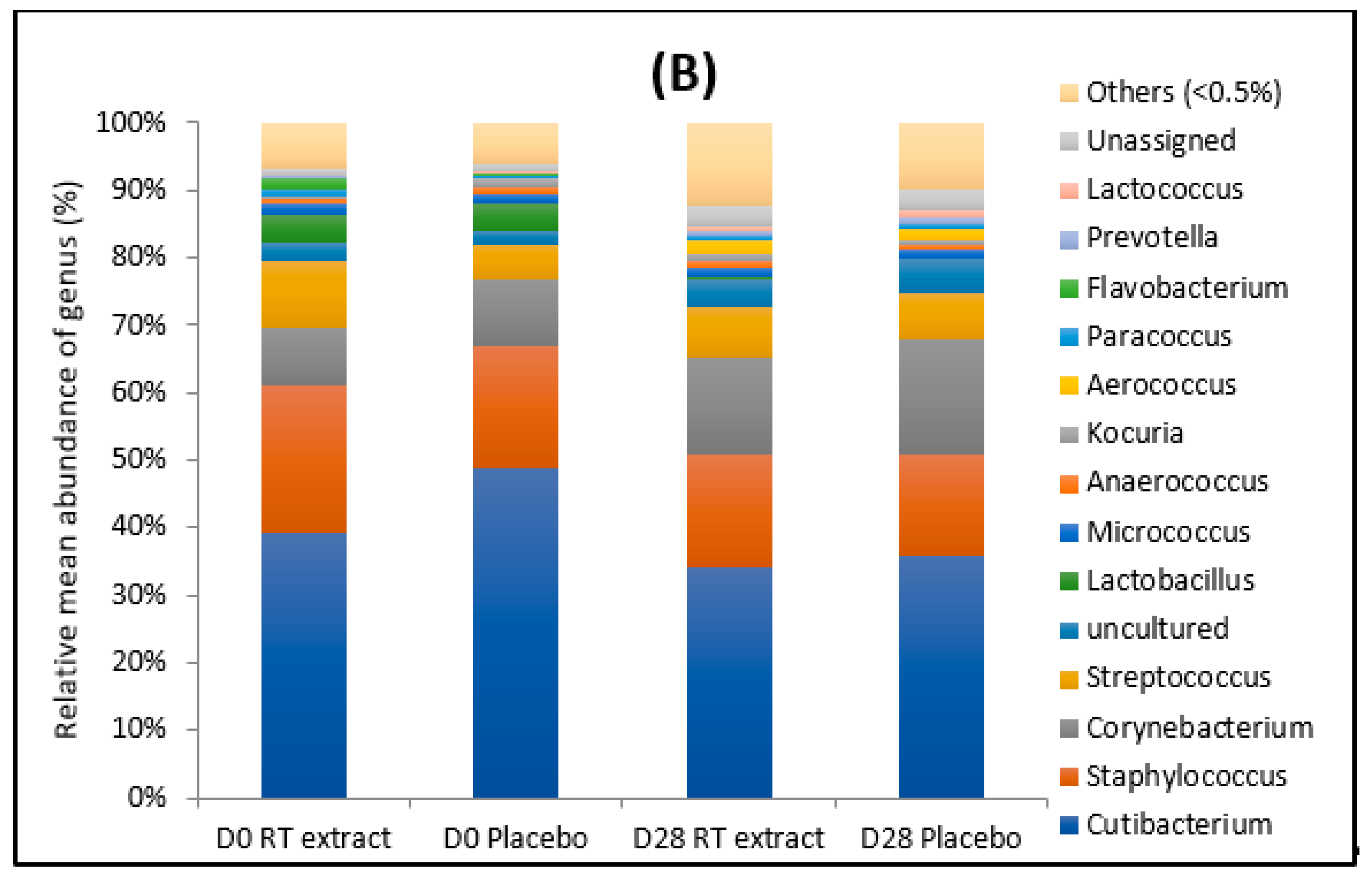
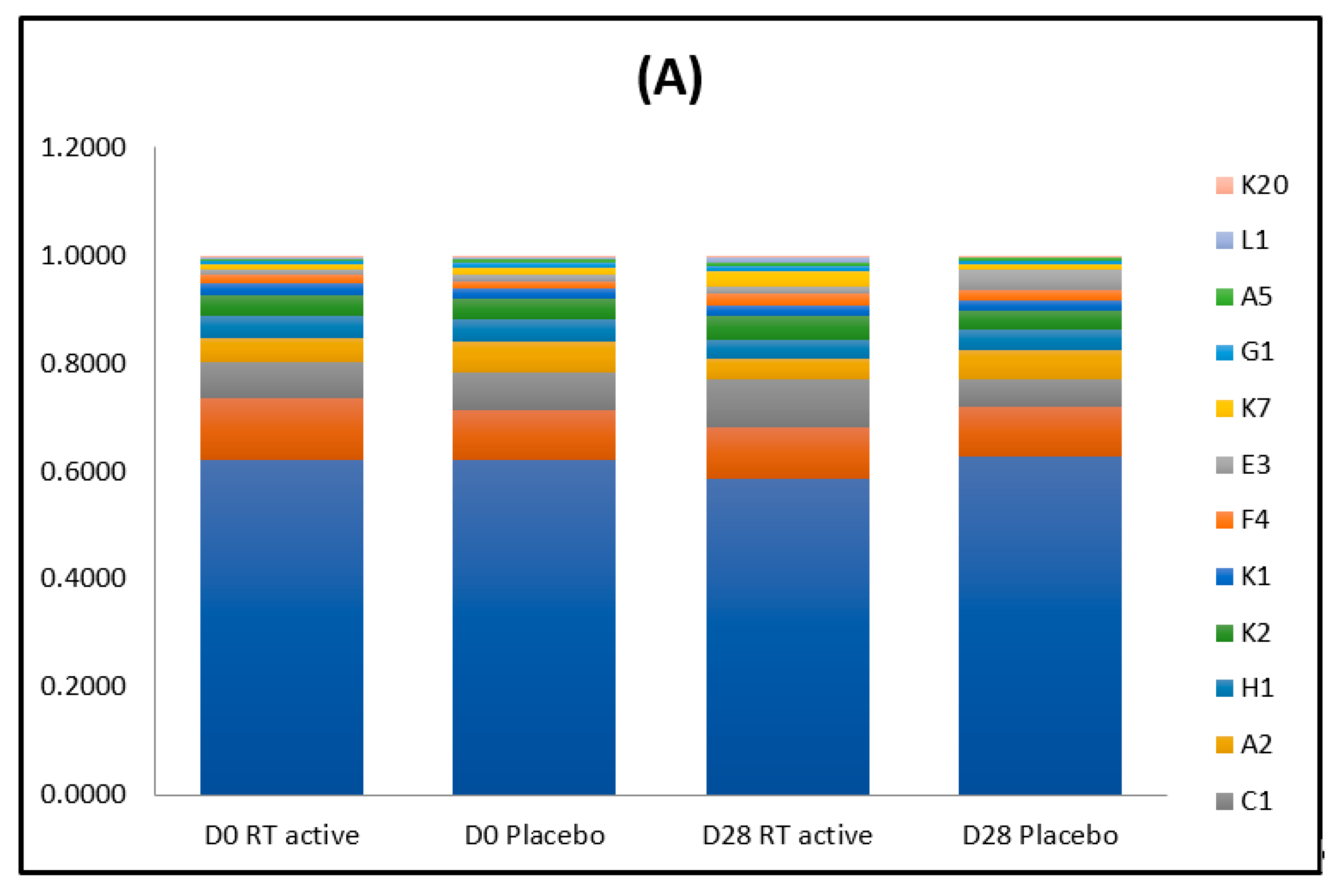
3.1. Microbiota
3.1.1. Before the Treatment
3.1.2. After the Treatment
3.2. Clinical Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rocha, M.A.; Bagatin, E. Skin barrier and microbiome in acne. Arch. Dermat. Res. 2017, 310, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Byun, E.J.; Kim, H.S. Potential role of the microbiome in acne: A comprehensive review. J. Clin. Med. 2019, 8, 987. [Google Scholar] [CrossRef] [PubMed]
- Dagnelie, M.A.; Corvec, S.; Saint-Jean, M.; Bourdès, V.; Nguyen, J.M.; Khammari, A.; Dréno, B. Decrease in Diversity of Propionibacterium acnes Phylotypes in Patients with Severe Acne on the Back. Acta Dermat. Vener. 2018, 7, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Fitz-Gibbon, S.; Tomida, B.H.; Chiu, L.; Nguyen, C.; Du, M.; Liu, D.; Elashoff, M.C.; Erfe, A.; Loncaric, J.; Kim, L.; et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J. Investig. Dermatol. 2013, 133, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.J.; Bruggemann, H. Bacterial skin commensals and their role as host guardians. Benef. Microbes. 2014, 5, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Erdei, L.; Bolla, B.S.; Tax, G.; Biro, T.; Kemeny, L. Factors shaping the composition of the cutaneous microbiota. Br. J. Dermat. 2017, 176, 344–351. [Google Scholar] [CrossRef]
- Xu, H.; Li, H. Acne, the Skin Microbiome, and Antibiotic Treatment. Am. J. Clin. Dermat. 2019, 20, 335–344. [Google Scholar] [CrossRef]
- Lomholt, H.B.; Scholz, C.F.P.; Brüggemann, H.; Tettelin, H.; Kilian, M. A comparative study of Cutibacterium (Propionibacterium) acnes clones from acne patients and healthy controls. Anaerobe 2017, 47, 57–63. [Google Scholar] [CrossRef]
- McDowell, A.; Perry, A.L.; Lambert, P.A.; Patrick, S. A new phylogenetic group of Propionibacterium acnes. J. Med. Microbiol. 2008, 57, 218–524. [Google Scholar] [CrossRef]
- Yu, Y.; Champer, J.; Garbán, H.; Kim, J. Typing of Propionibacterium acnes: A review of methods and comparative analysis. Br. J. Dermatol. 2015, 172, 1204–1209. [Google Scholar] [CrossRef]
- McLaughlin, J.; Watterson, S.; Layton, A.M.; Bjourson, A.J.; Barnard, E.; McDowell, A. Propionibacterium acnes and Acne Vulgaris: New Insights from the Integration of Population Genetic, Multi-Omic, Biochemical and Host-Microbe Studies. Microorganisms 2019, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Leyden, J.J. New understandings of the pathogenesis of acne. J. Am. Acad. Dermatol. 1995, 32, 15–25. [Google Scholar] [CrossRef]
- Barnard, E.; Shi, B.; Kang, D.; Craft, N.; Li, H. The balance of metagenomic elements shapes the skin microbiome in acne and health. Sci. Report. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Gehse, M.; Hoffler, U.; Gloor, M.; Pulverer, G. Propionibacteria in patients with acne vulgaris and in healthy persons. Arch. Dermat. Res. 1983, 275, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, J.A.; Voss, J.G. Incidence and lipolytic activity of Propionibacterium acnes (Corynebacterium acnes group I) and P. granulosum (C. acnes group II) in acne and in normal skin. J. Investig. Dermatol. 1973, 60, 94–97. [Google Scholar] [CrossRef][Green Version]
- Rajiv, P.; Nitesh, K.; Raj, K.; Hemant, G. Staphylococcus epidermidis in Human Skin Microbiome associated with Acne: A Cause of Disease or Defence? Res. J. Biotech. 2013, 8, 78–82. [Google Scholar]
- Wang, Y.; Kuo, S.; Shu, M.; Yu, J.; Huang, S.; Dai, A.; Two, A.; Gallo, R.L.; Huang, C.M. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: Implications of probiotics in acne vulgaris. Appl. Microbiol. Biotechnol. 2014, 98, 411–424. [Google Scholar] [CrossRef]
- Christensen, G.J.; Scholz, C.F.; Enghild, J.; Rohde, H.; Kilian, M.; Thurmer, A.; Brzuszkiewicz, E.; Lomholt, H.B. Antagonism between Staphylococcus epidermidis and Propionibacterium acnes and its genomic basis. BMC Med. Genom. 2016, 17, 152. [Google Scholar] [CrossRef]
- Xia, X.; Li, Z.; Liu, K.; Wu, Y.; Jiang, D.; Lai, Y. Staphylococcal LTA-Induced miR-143 Inhibits Propionibacterium acnes-Mediated Inflammatory Response in Skin. J. Investig. Dermatol. 2016, 136, 621–630. [Google Scholar] [CrossRef]
- Fisk, W.A.; Lev-Tov, H.A.; Sivamani, R.K. Botanical and phytochemical therapy of acne: A systematic review. Phyto. Res. 2014, 28, 1137–1152. [Google Scholar] [CrossRef]
- Pineau, R.M.; Hanson, S.E.; Lyles, J.T.; Quave, C.L. Growth inhibitory activity of callicarpa americana leaf extracts against cutibacterium acnes. Front. Pharm. 2019, 15, 1206. [Google Scholar] [CrossRef] [PubMed]
- Dursun, R.; Daye, M.; Durmaz, K. Acne and rosacea: What’s new for treatment? Dermatol. Ther. 2019, 32, e13020. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, S. Antibiotic resistance in acne treatment. Skin Ther. Lett. 2012, 9, 1–3. [Google Scholar]
- Vo, T.S.; Ngo, D.H. The Health Beneficial Properties of Rhodomyrtus tomentosa as Potential Functional Food. Biomelecules 2019, 9, 76. [Google Scholar] [CrossRef]
- Lai, T.N.H.; André, C.; Rogez, H.; Mignolet, E.; Nguyen, T.B.T.; Larondelle, Y. Nutritional composition and antioxidant properties of the sim fruit (Rhodomyrtus tomentosa). Food Chem. 2015, 168, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhang, R.; Liu, L.; Deng, Y.; Wei, Z.; Zhang, Y.; Ma, Y.; Zhang, M. Different thermal drying methods affect the phenolic profiles, their bioaccessibility and antioxidant activity in Rhodomyrtus tomentosa. Food Sci. Technol. 2017, 79, 260–266. [Google Scholar] [CrossRef]
- Kukreja, A.; Wadhwa, N.; Tiwari, A. Therapeutic Role of Resveratrol and Piceatannol in Disease Prevention. J. Blood Dis. Transfus. 2014, 5, 240. [Google Scholar] [CrossRef]
- Amrutha, B.; Sundar, K.; Shetty, P.H. Effect of organic acids on biofilm formation and quorum signaling of pathogens from fresh fruits and vegetables. Microb. Pathog. 2017, 111, 156–162. [Google Scholar] [CrossRef]
- Wunnoo, S.; Saising, J.; Voravuthikunchai, S.P. Rhodomyrtone inhibits lipase production, biofilm formation, and disorganizes established biofilm in Propionibacterium acnes. Anaerobe 2017, 43, 61–68. [Google Scholar] [CrossRef]
- Dreno, B.; Poli, F.; Pawin, H.; Beylot, C.; Faure, M.; Chivot, M.; Auffret, N.; Moyse, D.; Ballanger, F.; Revuz, J. Development and evaluation of a Global Acne Severity Scale (GEA Scale) suitable for France and Europe. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 43–48. [Google Scholar] [CrossRef]
- Scholtz, C.; Jensen, A.; Lomholt, H.B.; Brüggemann, H.; Kilian, M. A Novel High-Resolution Single Locus Sequence Typing Scheme for Mixed Populations of Propionibacterium acnes in vivo. PLoS ONE 2014, 9, e104199. [Google Scholar]
- Ridaura, V.K.; Bouladoux, N.; Claesen, J.; Chen, Y.E.; Byrd, A.L.; Constantinides, M.G.; Merrill, E.D.; Tamoutounour, S.; Fischbach, M.A. Contextual control of skin immunity and inflammation by Corynebacterium. J. Exp. Med. 2018, 215, 785. [Google Scholar] [CrossRef]
- Moosbrugger-Martinz, V.; Hackl, H.; Gruber, R.; Pilecki, M.; Knabl, L.; Orth-Höller, D.; Dubrac, S. First evidences of distinguishable bacterial and fungal dysbiosis in the skin of patients with atopic dermatitis of netherton Syndrome. J. Invest. Dermatol. 2020, S0022-202X, 31668. [Google Scholar] [CrossRef] [PubMed]
- McDowell, A.; Barnard, E.; Liu, J.; Li, H.; Patrick, S. Corrigendum: Proposal to reclassify Propionibacterium acnes type I as Propionibacterium acnes subsp. acnes subsp. nov. and Propionibacterium acnes type II as Propionibacterium acnes subsp. defendens subsp. nov. Int. J. Syst. Evol. Microbiol. 2017, 67, 4880. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Omer, H.; McDowell, A.; Alexeyev, O.A. Understanding the role of Propionibacterium acnes in acne vulgaris: The critical importance of skin sampling methodologies. Clin. Dermatol. 2017, 35, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.; Kang, D.; Barnard, E.; Li, H. Strain-level differences in porphyrin production and regulation in Propionibacterium acnes elucidate disease associations. mSphere 2016, 1, 1–12. [Google Scholar] [CrossRef]
- Nakase, K.; Hayashi, N.; Akiyama, Y.; Aoki, S.; Noguchi, N. Antimicrobial susceptibility and phylogenetic analysis of Propionibacterium acnes isolated from acne patients in Japan between 2013 and 2015. J. Dermat. 2017, 44, 1248–1254. [Google Scholar] [CrossRef]
- McDowell, A.; Nagy, I.; Magyari, M.; Barnard, E.; Patrick, S. The opportunistic pathogen Propionibacterium acnes: Insights into typing, human disease, clonal diversification and CAMP factor evolution. PLoS ONE 2013, 8, e70897. [Google Scholar] [CrossRef]
- Romano-Bertrand, S.; Beretta, M.; Jean-Pierre, H. Propionibacterium acnes populations involved in deep pathological samples and their dynamics along the cardiac surgical pathway. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 287–301. [Google Scholar] [CrossRef]
- Paugam, C.; Corvec, S.; Saint Jean, M.; Le Moigne, M.; Khammari, A.; Boisrobert, A.; Nguyen, J.M.; Gaultier, A.; Dreno, B. Propionibacterium acnes phylotypes and acne severity: An observational prospective study. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e398–e399. [Google Scholar] [CrossRef]
- Pécastaings, S.; Roques, C.; Nocera, T.; Peraud, C.; Mengeaud, V.; Khammari, A.; Dreno, B. Characterisation of Cutibacterium acnes phylotypes in acne and in vivo exploratory evaluation of Myrtacine®. J. Eur. Dermatol. Venereol. 2018, 32, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Herent, M.F.; Quetin-Leclercq, J.; Nguyen, T.B.T.; Rogez, H.; Larondelle, Y.; Andre, C.M. Piceatannol, a potent bioactive stilbene, as major phenolic component in Rhodomyrtus tomentosa. Food Chem. 2013, 138, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Hamid, H.A.; Mutazah, S.S.; Yusoff, M.M. Rhodomyrtus tomentosa: A phytochemical and pharmacological review. Am. J. Pharm. Clin. Res. 2017, 10, 10–16. [Google Scholar] [CrossRef]
- Abu-Qatouseh, L.; Mallah, E.; Mansour, K. Evaluation of anti-Propionibacterium acnes and anti-inflammatory effects of polyphenolic extracts of medicinal herbs in Jordan. Biomed. Pharm. J. 2019, 12, 211–217. [Google Scholar] [CrossRef]
- Wang, L.; Yang, X.; Qin, P.; Shan, F.; Ren, G. Flavonoid composition, antibacterial and antioxidant properties of tartary buckwheat bran extract. Ind. Crops Prod. 2013, 49, 312–317. [Google Scholar] [CrossRef]
- Sivasankar, C.; Maruthupadiyan, S.; Balamurugan, K.; James, P.B.; Krishnan, V.; Pandian, S.K. A combinaison of ellagic acid and tetracycline inhibits biofilm formation and the associated virulence of Propionibacterium acnes in vitro and in vivo. Biofouling 2016, 32, 397–410. [Google Scholar] [CrossRef]
- Docherty, J.J.; McEwen, H.A.; Sweet, T.J.; Bailey, E.; Booth, T.D.J. Resveratrol inhibition of Propionibacterium acnes. J. Antimicrob. Chemother. 2007, 59, 1182–1184. [Google Scholar] [CrossRef]
- Zhu, T.; Fang, F.; Sun, S.; Yang, X.; Zhang, X.; Yu, L.; Yang, L. Piceatannol Inhibits P. acnes-Induced Keratinocyte Proliferation and Migration by Downregulating Oxidative Stress and the Inflammatory Response. Inflammation 2020, 43, 347–357. [Google Scholar] [CrossRef]
- Saising, J.; Voravuthikunchai, S.P. Anti Propionibacterium acnes activity of rhodomyrtone, an effective compound from Rhodomyrtus tomentosa (Aiton) Hassk. leaves. Anaerobe 2012, 18, 400–404. [Google Scholar] [CrossRef]
- Kapuscinska, A.; Nowak, I. Use of organic acids in acne and skin discolorations therapy. Postepy Higieny I Medycyny Doswiadczalnej 2015, 69, 374–383. [Google Scholar] [CrossRef]
- Kusuma, I.W.; Ainiyati, N.; Suwinarti, W. Search for biological activities from an invasive shrub species rose myrtle (Rhodomyrtus tomentosa). Nusantara Biosci. 2016, 8, 55–59. [Google Scholar] [CrossRef]


| Compounds | Concentration (mg·L−1) in RT Extract |
|---|---|
| Phenolic Compounds | |
| Polyphenols (catechin) | 5000 |
| Stilbenes | |
| Piceatannol | 500 |
| Flavonoids (rutin) | 250 |
| Phenolic acids | |
| Gallic acid | 50 |
| Ellagic acid | 50 |
| Acylphloroglucinol | |
| Rhodomyrtone | 5 |
| Acid Compounds | |
| Organic acids | 1000 |
| Malic Acid | 300 |
| Citric Acid | 700 |
| Shikimic acid | 150 |
| Quinic acid | 1250 |
| Sugars | |
| Monosaccharides | |
| Glucose | 15,000 |
| Fructose | 17,500 |
| Polysaccharides | 3750 |
| Parameters | Variation Placebo Group | Variation RT Extract Group | p Value Placebo Versus RT Extract Group |
|---|---|---|---|
| Blackheads | −0.3 ± 1.4 | −3.5 ± 1.1 ** | 0.01 |
| Papules | −0.4 ± 0.9 | −1.6 ± 0.4 *** | 0.05 |
| Global NIL | −1.9 ± 0.2 | −4.1 ± 1.4 ** | 0.01 |
| GIL | −0.6 ± 0.5 | −2.2 ± 0.6 *** | 0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gervason, S.; Metton, I.; Gemrot, E.; Ranouille, E.; Skorski, G.; Cabannes, M.; Berthon, J.-Y.; Filaire, E. Rhodomyrtus tomentosa Fruit Extract and Skin Microbiota: A Focus on C. acnes Phylotypes in Acne Subjects. Cosmetics 2020, 7, 53. https://doi.org/10.3390/cosmetics7030053
Gervason S, Metton I, Gemrot E, Ranouille E, Skorski G, Cabannes M, Berthon J-Y, Filaire E. Rhodomyrtus tomentosa Fruit Extract and Skin Microbiota: A Focus on C. acnes Phylotypes in Acne Subjects. Cosmetics. 2020; 7(3):53. https://doi.org/10.3390/cosmetics7030053
Chicago/Turabian StyleGervason, Sandie, Isabelle Metton, Elodie Gemrot, Edwige Ranouille, Gilbert Skorski, Magalie Cabannes, Jean-Yves Berthon, and Edith Filaire. 2020. "Rhodomyrtus tomentosa Fruit Extract and Skin Microbiota: A Focus on C. acnes Phylotypes in Acne Subjects" Cosmetics 7, no. 3: 53. https://doi.org/10.3390/cosmetics7030053
APA StyleGervason, S., Metton, I., Gemrot, E., Ranouille, E., Skorski, G., Cabannes, M., Berthon, J.-Y., & Filaire, E. (2020). Rhodomyrtus tomentosa Fruit Extract and Skin Microbiota: A Focus on C. acnes Phylotypes in Acne Subjects. Cosmetics, 7(3), 53. https://doi.org/10.3390/cosmetics7030053






