Effects of Fermented Oils on Alpha-Biodiversity and Relative Abundance of Cheek Resident Skin Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Study Design
2.3. Swab Sample Collection
2.4. DNA Extraction and 16S Amplicon Generation, Sequencing and Analysis
2.5. Statistical Analysis
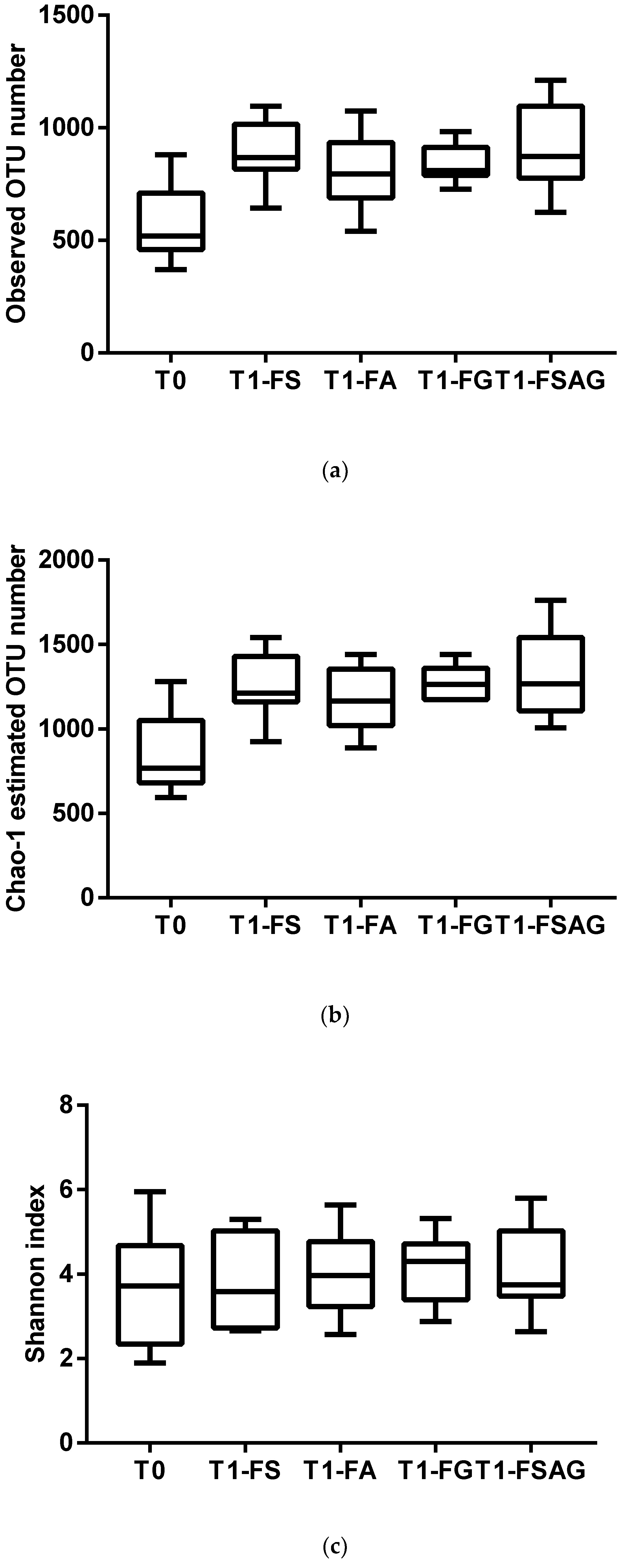
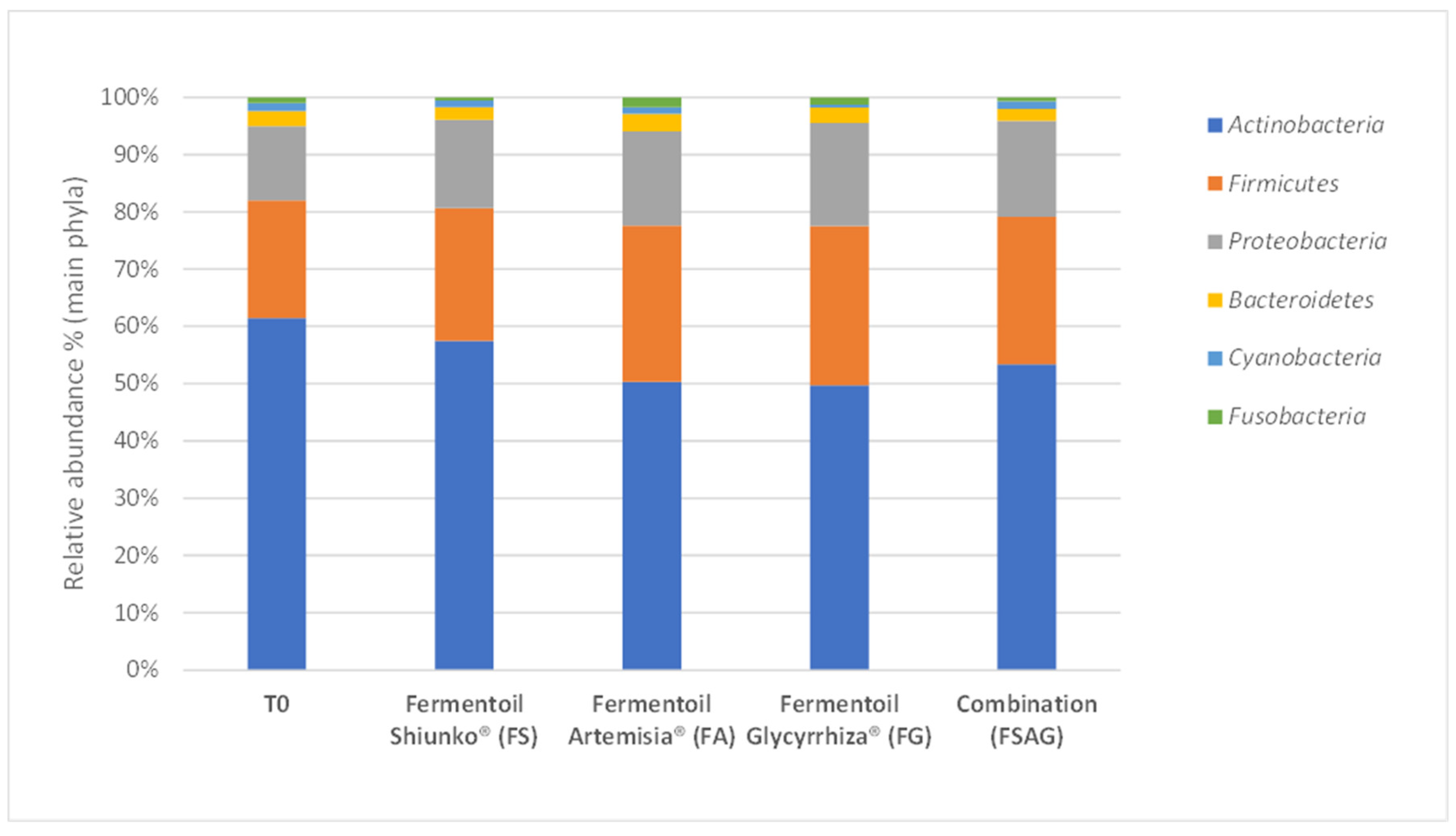
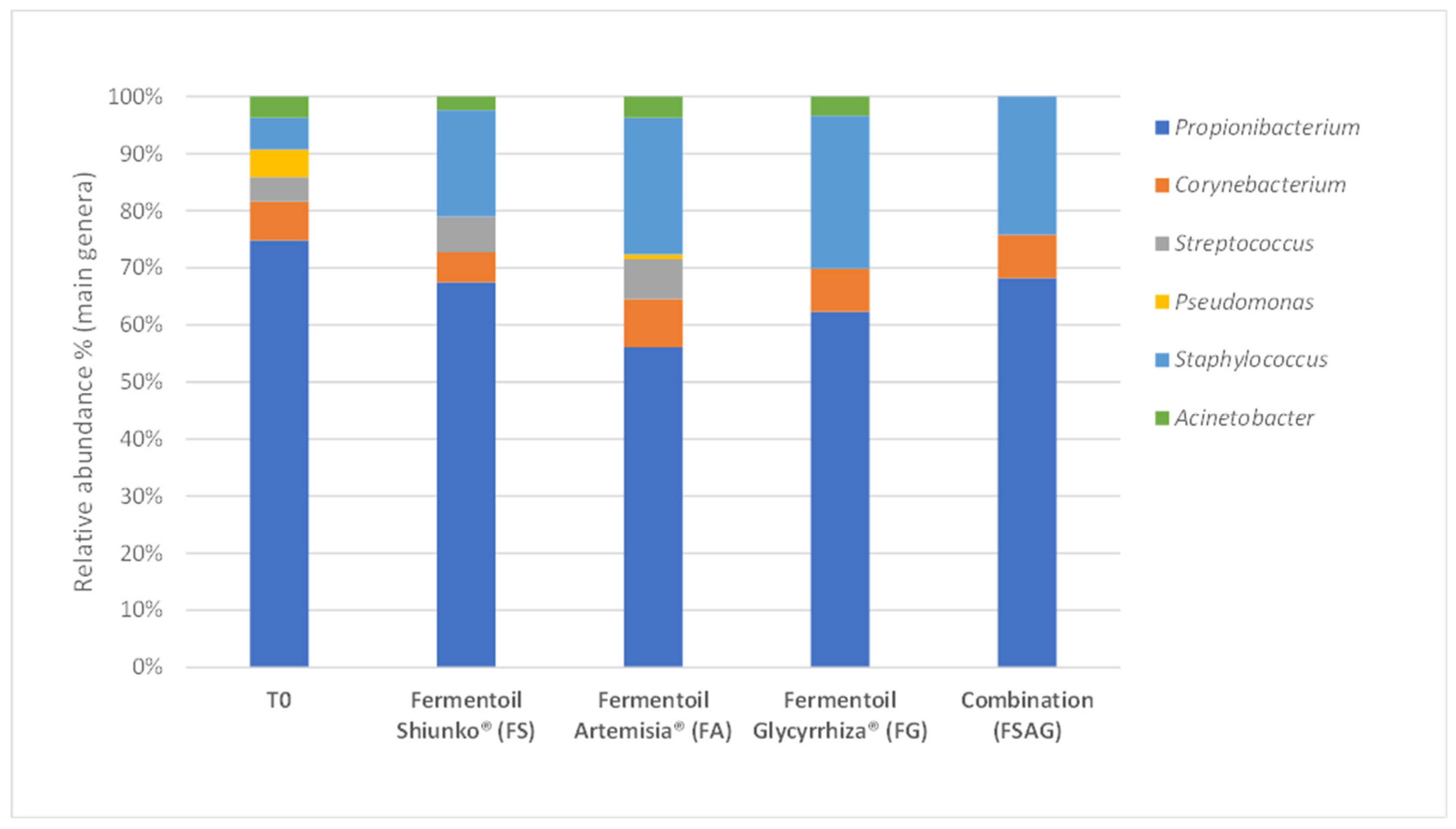
3. Results
Semi-Quantitative Bacterial Composition: Alpha-Biodiversity and Relative Abundance
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin microbiota-host interactions. Nature 2018, 24, 427–436. [Google Scholar] [CrossRef]
- Belkaid, Y.; Segre, J.A. Dialogue between skin microbiota and immunity. Science 2014, 346, 954–959. [Google Scholar] [CrossRef]
- Strauss, J.S.; Pochi, P.E.; Downing, D.T. The sebaceous glands: Twenty-five years of progress. J. Invest. Dermatol. 1976, 67, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, N. Skin lipids: Their biochemical uniqueness. Science 1974, 186, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Scholz, C.F.P.; Kilian, M. The natural history of cutaneous propionibacteria, and reclassification of Bselected species within the genus Propionibacterium to the proposed novel genera Acidipropionibacterium gen. nov., Cutibacterium gen. nov. and Pseudopropionibacterium gen. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 4422–4432. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; Green, E.D.; et al. Topographical and temporal diversity of the human skin microbiome. Science 2010, 324, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Program, N.C.S.; Byrd, A.L.; Deming, C.; Conlan, S.; Kong, H.H.; A Segre, J. Biogeography and individuality shape function in the human skin metagenome. Nature 2014, 514, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.L.; Deming, C.; Cassidy, S.K.B.; Harrison, O.; Ng, W.-I.; Conlan, S.; Belkaid, Y.; A Segre, J.; Kong, H.H.; Program, N.C.S. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci. Transl. Med. 2017, 9, eaal4651. [Google Scholar] [CrossRef]
- Pinto, D.; Calabrese, F.M.; De Angelis, M.; Celano, G.; Giuliani, G.; Gobbetti, M.; Rinaldi, F. Predictive metagenomic profiling, urine metabolomics, and human marker gene expression as an integrated approach to study alopecia areata. Front. Cell. Inf. Microbiol. 2020, in press. [Google Scholar] [CrossRef]
- Pinto, D.; Sorbellini, E.; Marzani, B.; Rucco, M.; Giuliani, G.; Rinaldi, F. Scalp bacterial shift in Alopecia areata. PLoS ONE 2019, 14, e0215206. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, F.; Pinto, D.; Marzani, B.; Rucco, M.; Giuliani, G.; Sorbellini, E. Human microbiome: What’s new in scalp diseases. J. Transl. Sci. 2018, 4, 1–4. [Google Scholar]
- Egert, M.; Simmering, R.; Riedel, C.U. The Association of the Skin Microbiota with Health, Immunity, and Disease. Clin. Pharmacol. Ther. 2017, 102, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.C. Cosmetology and Pharmacology of the Skin; PZWL Publishing: Warszawa, Poland, 2007; pp. 37–44, 52, 105–112, 122. [Google Scholar]
- Wallen-Russel, C. The Role of Every-Day Cosmetics in Altering the Skin Microbiome: A Study Using Biodiversity. Cosmetics 2018, 6, 2. [Google Scholar] [CrossRef]
- Blaser, M.J.; Falkow, S. What are the consequences of the disappearing human microbiota? Nat. Rev. Microbiol. 2009, 7, 887–894. [Google Scholar] [CrossRef]
- Goossens, A. Contact-allergic reactions to cosmetics. J. Allergy 2011, 2011, 467071. [Google Scholar] [CrossRef]
- Salverda, J.G.W.; Bragt, P.J.C.; de Wit-Bos, L.; Rustemeyer, T.; Coenraads, P.J.; Tupker, R.A.; Kunkeler, L.C.M.; Laheij-de Boer, A.-M.; Stenveld, H.J.; van Ginkel, C.J.W.; et al. Results of a cosmetovigilance survey in The Netherlands. Contact Dermat. 2013, 68, 139–148. [Google Scholar] [CrossRef]
- Heisterberg, M.V.; Menné, T.; Johansen, J.D. Contact allergy to the 26 specific fragrance ingredients to bedeclared on cosmetic products in accordance with the EU cosmetics directive. Contact Dermat. 2011, 65, 266–275. [Google Scholar] [CrossRef]
- Warshaw, E.M.; Buchholz, H.J.; Belsito, D.V.; Maibach, H.I.; Fowler, J.F.; Rietschel, R.L.; Zug, K.A.; Mathias, C.G.T.; Pratt, M.D.; Sasseville, D.; et al. Allergic patch test reactions associated with cosmetics: Retrospective analysis of cross-sectional data from the North American Contact Dermatitis Group, 2001–2004. J. Am. Acad. Dermatol. 2009, 60, 23–38. [Google Scholar] [CrossRef]
- Berne, B.; Tammela, M.; Färm, G.; Inerot, A.; Lindberg, M. Can the reporting of adverse skin reactions to cosmetics be improved? A prospective clinical study using a structured protocol. Contact Dermat. 2008, 58, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Berne, B.; Boström, A.; Grahnén, A.F.; Tammela, M. Adverse effects of cosmetics and toiletries reported tothe Swedish Medical Products Agency 1989–1994. Contact Dermat. 1996, 34, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, F.; Pinto, D.; Giuliani, G. Postbiotic Evolution in Dermatology. EC Microbiol. 2020, 16, 1–4. [Google Scholar]
- Aguilar-Toalá, J.; Garcia-Varela, R.; Garcia, H.; Mata-Haro, V.; González-Córdova, A.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Tech. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Tsilingiri, K.; Rescigno, M. Postbiotics: What else? Benef. Microbes 2013, 4, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Cao, H.; Cover, T.L.; Whitehead, R.; Washington, M.K.; Polk, D.B. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterol 2007, 132, 562–575. [Google Scholar] [CrossRef]
- Yan, F.; Liu, L.; Dempsey, P.J.; Tsai, Y.-H.; Raines, E.W.; Wilson, C.L.; Cao, H.; Cao, Z.; Liu, L.; Polk, D.B. A Lactobacillus rhamnosus GG-derived soluble protein, p40, stimulates ligand release from intestinal epithelial cells to transactivate EGF receptor. J. Biol. Chem. 2013, 42, 30742–30751. [Google Scholar] [CrossRef]
- Sanchez, B.; Urdaci, M.C.; Margolles, A. Extracellular proteins secreted by probiotic bacteria as mediators of effects that promote mucosa–bacteria interactions. Microbiol 2010, 156, 3232–3242. [Google Scholar] [CrossRef]
- Lew, L.C.; Liong, M.T. Bioactives from probiotics for dermal health: Functions and benefits. J. Appl. Microbiol. 2013, 114, 1241–1253. [Google Scholar] [CrossRef]
- Menaa, F. Emulsions Systems for Skin Care: From Macro to Nano-Formulations. J. Pharma. Care Health Syst. 2014, 1, 2. [Google Scholar] [CrossRef]
- Puhvel, S.M.; Reisner, R.M.; Sakamoto, M. Analysis of lipid composition of isolated human sebaceous gland homogenates after incubation with cutaneous bacteria Thin-layer chromatography. J. Invest. Dermatol. 1975, 64, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Berdick, M. The role of fats and oils in cosmetics. J. Am. Oil Chem. Soc. 1972, 49, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Sanford, J.A.; Zhang, L.J.; Williams, M.R.; Gangoiti, J.A.; Huang, C.M.; Gallo, R.L. Inhibition of HDAC8 and HDAC9 by microbial short-chain fatty acids breaks immune tolerance of the epidermis to TLR ligands. Sci. Immunol. 2016, 1, eaah4609. [Google Scholar] [CrossRef] [PubMed]
- Kasparavičienė, G.; Bajoriūnaitė, D.; Velžienė, S.; Kalvėnienė, Z. Natural vegetable oils potent antioxidants for natural cosmetic. In The Vital Nature Sign [elektroninis išteklius]: 7th International Scientific Conference; Vytautas Magnus University: Kaunas, Lithuania, 2013. [Google Scholar]
- Ab Latip, R.; Lee, Y.Y.; Tang, T.-K.; Phuah, E.-T.; Lee, C.-M.; Tan, C.P.; Lai, O.M.; Liu, Y. Palm-based diacylglycerol fat dry fractionation: Effect of crystallisation temperature, cooling rate and agitation speed on physical and chemical properties of fractions. PeerJ 2013, 1, e72. [Google Scholar] [CrossRef] [PubMed]
- Brud, W.; Konopacka-Brud, I. Essential oils as active substances in cosmetics. Herb Messages 1998, 7, 8–10. [Google Scholar]
- Kaczmarczyk, D.; Strub, D.; Polowy, A.; Wilk, K.; Lochyński, S. Selected Essential Oils in Cosmetic Emulsions: Process Oriented Stability Studies and Antimicrobial Activity. Nat. Vol. Ess. Oils 2016, 27–39. [Google Scholar]
- Kim, K.N.; Son, J.H.; Kim, H.S.; Yun, J.S. Fermented Vegetable Oil and Composition including Same. U.S. Patent Application No. EP2749651A2, 2 July 2014. [Google Scholar]
- Lalitha, C.; Rao, P.P.V.V. Antimicrobial Efficacy of Preservatives used in Skin Care Products on Skin Microbiota. Int. J. Sci. Res. 2015, 4, 366–369. [Google Scholar]
- Gao, Z.; Perez-Perez, G.I.; Chen, Y.; Blaser, M.J. Quantitation of Major Human Cutaneous Bacterial and Fungal Populations. J. Clin. Microbiol. 2010, 48, 3575–3581. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a Prokaryotic Universal Primer for Simultaneous Analysis of Bacteria and Archaea Using Next-Generation Sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef]
- Apprill, A.; McNally, S.; Parsons, R.; Weber, L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat. Microb. Ecol. 2015, 75, 129–137. [Google Scholar] [CrossRef]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A. Improved Bacterial 16S rRNA Gene (V4 and V4-5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. mSystems 2016, 1, e00009-15. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, N.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- He, N.; Warner, K.S.; Chantasart, D.; Shaker, D.S.; Higuchi, W.I.; Li, S.K. Mechanistic study of chemical skin permeation enhancers with different polar and lipophilic functional groups. J. Pharm. Sci. 2004, 93, 1415–1430. [Google Scholar] [CrossRef]
- Raya, S.A.; Saaid, I.M.; Ahmed, A.A.; Umar, A.A. A critical review of development and demulsification mechanisms of crude oil emulsion in the petroleum industry. J. Pet. Explor. Prod. Technol. 2020, 10, 1711–1728. [Google Scholar] [CrossRef]
- Agredo, P.; Rave, M.C.; Echeverri, J.D.; Romero, D.; Salamanca, C.H. An Evaluation of the Physicochemical Properties of Stabilized Oil-In-Water Emulsions Using Different Cationic Surfactant Blends for Potential Use in the Cosmetic Industry. Cosmetics 2019, 6, 12. [Google Scholar] [CrossRef]
- Addor, F.A.S. Antioxidants in dermatology. An. Bras. Dermatol. 2017, 92, 356–362. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Esmael, A.; Hassan, M.G.; Amer, M.M.; Abdelrahman, S.; Hamed, A.M.; Abd-raboh, H.A.; Foda, M.F. Antimicrobial activity of certain natural-based plant oils against the antibiotic-resistant acne bacteria. Saudi J. Biol. Sci. 2020, 27, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Białoń, M.; Krzyśko-Łupicka, T.; Nowakowska-Bogdan, E.; Wieczorek, P.P. Chemical Composition of Two Different Lavender Essential Oils and Their Effect on Facial Skin Microbiota. Molecules 2019, 24, 3270. [Google Scholar] [CrossRef]
- Habashy, R.R.; Abdel-Naim, A.B.; Khalifa, A.E.; Al-Azizi, M.M. Anti-inflammatory effects of jojoba liquid wax in experimental models. Pharmacol. Res. 2005, 51, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Trytek, M.; Paduch, R.; Fiedurek, J.; Kandefer-Szerszeń, M. Monoterpenes–Old compounds, new applications and biotechnological methods of their production. Biotechnologia 2007, 76, 135–155. [Google Scholar]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef]
- Chak, K.F.; Hsiao, C.Y.; Chen, T.Y. A Study of the Effect of Shiunko, a Traditional Chinese Herbal Medicine, on Fibroblasts and Its Implication on Wound Healing Processes. Adv. Wound Care 2013, 2, 448–455. [Google Scholar] [CrossRef]
- Park, J.Y.; Shin, M.S.; Hwang, G.S.; Yamabe, N.; Yoo, J.E.; Kang, K.S.; Kim, J.C.; Lee, J.G.; Ham, J.; Lee, H.L. Beneficial Effects of Deoxyshikonin on Delayed Wound Healing in Diabetic Mice. Int. J. Mol. Sci. 2018, 19, 3660. [Google Scholar] [CrossRef]
- Yang, J.H.; Lee, E.; Lee, B.; Cho, W.K.; Ma, J.Y.; Park, K.I. Ethanolic Extracts of Artemisia apiacea Hance Improved Atopic Dermatitis-Like Skin Lesions In Vivo and Suppressed TNF-Alpha/IFN-Gamma⁻Induced Proinflammatory Chemokine Production In Vitro. Nutrients 2018, 10, 806. [Google Scholar] [CrossRef]
- Yu, J.; Wang, G.; Jiang, N. Study on the Repairing Effect of Cosmetics Containing Artemisia annua on Sensitive Skin. J. Cosmet Dermatol Sci. Appl. 2013, 10, 8–19. [Google Scholar]
- Zadeh, J.B.; Kor, Z.M.; Goftar, M.K. Licorice (Glycyrrhiza glabra Linn) As a Valuable Medicinal Plant. Int. J. Adv. Biol. Biom. Res. 2013, 1, 1281–1288. [Google Scholar]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M.B.P.P. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef] [PubMed]
- Haahtela, T.A. Biodiversity hypothesis. Allergy 2019, 74, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L.; Larcombe, D.-L.; Logan, A.C.; West, C.; Burks, W.; Caraballo, L.; Levin, M.; Van Etten, E.; Horwitz, P.; Kozyrskyj, A.; et al. The skin microbiome: Impact of modern environments on skin ecology, barrier integrity, and systemic immune programming. World Allergy Organ. J. 2017, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Shibagaki, N.; Suda, W.; Clavaud, C.; Bastien, P.; Takayasu, L.; Iioka, E.; Kurokawa, R.; Yamashita, N.; Hattori, Y.; Shindo, C.; et al. Aging-related changes in the diversity of women’s skin microbiomes associated with oral bacteria. Sci. Rep. 2017, 7, 10567. [Google Scholar] [CrossRef] [PubMed]
- Wilantho, A.; Deekaew, P.; Srisuttiyakorn, C.; Tongsima, S.; Somboonna, N. Diversity of bacterial communities on the facial skin of different age-group Thai males. PeerJ 2017, 5, e4084. [Google Scholar] [CrossRef]
- Nejrup, R.G.; Licht, T.R.; Hellgren, L.I. Fatty acid composition and phospholipid types used in infant formulas modifies the establishment of human gut bacteria in germ-free mice. Sci. Rep. 2017, 7, 3975. [Google Scholar] [CrossRef]
- Nakagawa, S.; Hillebrand, G.G.; Nunez, G. Rosmarinus officinalis L. (Rosemary) Extracts Containing Carnosic Acid and Carnosol are Potent Quorum Sensing Inhibitors of Staphylococcus aureus Virulence. Antibiotics 2020, 9, 149. [Google Scholar] [CrossRef]
- Diard, M.; Hardt, W.D. Evolution of bacterial virulence. FEMS Microbiol. Rev. 2017, 41, 679–697. [Google Scholar] [CrossRef]
| Identifying Name | INCI * Name | Manufacturer/Distributor | |
|---|---|---|---|
| Active | F-Shiunko | Pseudozyma epicola/ apricot kernel oil/olive fruit oil/sunflower seed oil/sweet almond oil/(Angelica gigas/Lithospermum erythrorhizon) root extract ferment filtrate | LABIO Co., Ltd., Korea |
| F-Artemisia | Pseudozyma epicola/ apricot kernel oil/olive fruit oil/sweet almond oil/sunflower seed oil/Artemisia princeps extract ferment extract filtrate | LABIO Co., Ltd., Korea | |
| F-Glycyrrhiza | Pseudozyma epicola/ apricot kernel oil/olive fruit oil/sweet almond oil/sunflower seed oil/licorice root extract ferment extract filtrate | LABIO Co., Ltd., Korea | |
| Preservative | Euxyl K712® | Aqua, sodium benzoate, potassium sorbate | Schuelke & Meyr, Italy |
| Emulsifier | Pemulen TR-1® | Acrylates c10-30 alkyl acrylates crosspolymer | Lubrizol, USA |
| Carbopol Ultrez 21® | Acrylates c10-30 alkyl acrylates crosspolymer | Lubrizol, USA | |
| Others | Glicerina | Glycerin | Acef, Italy |
| EDTA bisodico | Disodium EDTA | Acef, Italy | |
| Emultop Velvet IP® | Lecithin | Labio LAB, Italy |
| Metric | Samples | p-Value 1 |
|---|---|---|
| Observed OTU number | T1-FS | 0.0007 |
| T1-FA | 0.0217 | |
| T1-FG | <0.0001 | |
| T1-FSAG | 0.0027 | |
| Chao-estimated OTU number | T1-FS | 0.0012 |
| T1-FA | 0.0116 | |
| T1-FG | <0.0001 | |
| T1-FSAG | 0.0031 | |
| Shannon Index | T1-FS | 0.7991 |
| T1-FA | 0.5369 | |
| T1-FG | 0.2997 | |
| T1-FSAG | 0.4258 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciardiello, T.; Pinto, D.; Marotta, L.; Giuliani, G.; Rinaldi, F. Effects of Fermented Oils on Alpha-Biodiversity and Relative Abundance of Cheek Resident Skin Microbiota. Cosmetics 2020, 7, 34. https://doi.org/10.3390/cosmetics7020034
Ciardiello T, Pinto D, Marotta L, Giuliani G, Rinaldi F. Effects of Fermented Oils on Alpha-Biodiversity and Relative Abundance of Cheek Resident Skin Microbiota. Cosmetics. 2020; 7(2):34. https://doi.org/10.3390/cosmetics7020034
Chicago/Turabian StyleCiardiello, Tiziana, Daniela Pinto, Laura Marotta, Giammaria Giuliani, and Fabio Rinaldi. 2020. "Effects of Fermented Oils on Alpha-Biodiversity and Relative Abundance of Cheek Resident Skin Microbiota" Cosmetics 7, no. 2: 34. https://doi.org/10.3390/cosmetics7020034
APA StyleCiardiello, T., Pinto, D., Marotta, L., Giuliani, G., & Rinaldi, F. (2020). Effects of Fermented Oils on Alpha-Biodiversity and Relative Abundance of Cheek Resident Skin Microbiota. Cosmetics, 7(2), 34. https://doi.org/10.3390/cosmetics7020034






