1. Introduction
Most implantable medical devices involve contact with human blood [
1]. These include prosthetic heart valves and blood vessels, filters, stents, shunts, and many others. However, the requirement for hemocompatibility has a much larger group of materials, including externally attached devices, as well as contact with blood ex vivo. In connection with the increasing volume of this group, the ISO 10993-4 was developed [
2]. It considers the various applications of products that require compliance with hemocompatibility, and therefore suspends various types of tests. They are included in the following categories: study of thrombosis, coagulation, hematological indications and hemolysis, the behavior of platelets, and the complement system. Depending on the product group, two to five types of tests are required; however, a hemolysis study is the most common, which is why it has been studied in this work. Red blood cell hemolysis is accompanied by the release of hemoglobin molecules into the blood plasma. The intensity of hemolysis can be judged by the degree of change in the optical density of the plasma at wavelengths corresponding to the absorption peaks of hemoglobin [
3]. In this work, changes in the optical density of blood plasma over a long time (60 min) of contact between sorbent and human blood were investigated.
Currently, a lot of research is being done to develop materials based on chitosan and study its interaction with the body. Chitosan and chitin are natural polysaccharides that have many promising properties for medicine, including biocompatibility, biodegradation, and lack of cytotoxicity [
4]. Despite active research on these two polymers, there are different and sometimes opposite opinions in the literature regarding their hemocompatibility. Some studies tell about the interactions between chitosan amino groups and blood cells, which leads to a thrombogenic response and, thus, hemostatic action. This is related to the activation of both complement and blood coagulation systems [
5]. It has been shown that complement activation by chitosan correlates with the number of NH
2 groups [
6], that is the degree of deacetylation indicator and the main difference between chitin and chitosan. Moreover, chitosan enhances platelet adhesion and aggregation [
7]—one of the significant components of hemostasis. On the other hand, other studies show the hemocompatibility of chitosan in experiments with hemolytic activity, platelet aggregation, coagulation, and cytokine induction [
8]. Many researchers have shown the improved and enhanced hemocompatibility of chitosan with additions, such as alginate [
9] or polyethersulfone [
10], or by chitosan modification: sulfonation [
11] or carboxylation [
12].
Despite similar structures of chitin and chitosan, and the absence of amino groups in the chitin, which, as has been shown, play a crucial role in the hemostasis process, there is no research on the simultaneous usage of both polymers for the processing of hemocompatible material. The purpose of this work is to develop composite fibers based on chitosan and chitin nanofibrils with subsequent research on its physical and mechanical properties, along with hemocompatibility.
To be used in the biomedical field, it is necessary to ensure the absence of material toxicity. Since chitosan is a chitin derivative, there is the possibility of endotoxin presence, which could result in inflammation [
13]. There is information on the anti-inflammatory effect of the complex of chitosan and lipopolysaccharide, which is the most common endotoxin, [
14] as well as about its partial dissociation [
15] on interaction with chitosan. Moreover, the treatment of endotoxins with acids and alkalis (more than 0.1 M) results in partial destruction [
16]. Nevertheless, because of different chitosan characteristics, the presence of endotoxin may vary; thus, a toxicity study should be done. One of the methods used is hemolysis analysis [
17,
18], which has been done in this research.
2. Materials and Methods
2.1. Materials
Chitosan by Biolog Heppe (Landsberg, Germany) with molecular mass 164 kDa and degree of deacetylation 92%, and chitin nanofibrils (CNF) by SRL Mavi Sud (Aprilia, Italy) were used to prepare the composite fibers.
The venous blood of healthy donors was used as the biological material in the experiment. It was obtained from the blood transfusion station at Almazov National Medical Research Centre (Saint-Petersburg, Russia). Nine 9 mL of blood was taken from the ulnar vein and put into a vacuum tube with lithium heparin.
A carbon hemosorbent was chosen as a reference sorbent; it is successfully used in clinical practice and is a standard for the study of other hemosorbents [
19].
2.2. Fiber Processing
The first stage of obtaining composite fibers was the preparation of solutions. The optimal concentration of chitosan in terms of the rheological properties of the solution and its further processing in fiber is 4 wt.% [
20]; thus, this concentration was used in this work. In order to study the effect of the introduction of chitin nanofibrils on the properties of the resulting product, a large range of nanofibrils concentrations was selected, ranging from 0.5 wt.% to 50 wt.%. To achieve maximum homogeneity of the solution and split possible aggregates, the suspension was subjected to dispersion in a rod ultrasonic disperser UZV-1.3 (Saint-Petersburg, Russia) for 10 minutes at a frequency of 23.4 kHz. The polymers were dissolved in 2% acetic acid solution with constant stirring for 90 min. The solutions were kept at a temperature of 4 °C for a day, after which they were filtered and de-aired at a pressure of 0.1 atm.
The processing of composite fibers from solutions was carried out using the wet spinning method [
20]. The solution was fed through a die hole with a diameter of 0.6 mm into a precipitation bath consisting of a mixture of 10% aqueous NaOH solution and ethanol in a ratio of 1:1, resulting in solidification of the solution. Passing through the system of rollers that regulate the die and plasticizing extraction, the factor of orientation drawing λ, %, of the monofilament in the coagulation bath, was varied from 0% up to +100%. The fiber was washed with distilled water and dried at a temperature of 40 °C.
2.3. Study of Mechanical Characteristics
The measurements of the mechanical properties of the processed fibers were carried out with Instron 5943 at room temperature and load speed of 10 mm/min; the basic length of the fibers was 100 mm. Before testing, the fibers were maintained under normal climatic conditions (66% relative humidity and 20 ± 2 °C) for at least 24 h.
2.4. Study of the Influence of Relative Water Vapor Pressure on Mechanical Properties
In the human body, conditions such as temperature and humidity differ from normal and depend on the tissue in which the material is placed. To study its influence on the mechanical characteristics of composite fibers, they were placed in desiccators with relative pressure (p/p0) ranging from 0.10 to 0.98; relative air humidity in desiccators was created using saturated solutions of salts. The desiccators were thermostated at 25 ± 1 °C. Before measurements, the samples were dried in a drying chamber until constant weight was achieved; moisture content was controlled by the gravimetric method. The samples were exposed at each value of relative water vapor pressure until sorption equilibrium was established (3–7 weeks); following which the mechanical properties were measured.
2.5. Study of Hemocompatibility
In bench conditions, the effect of blood contact with chitosan fibers on erythrocyte hemolysis was studied. The following sorbents were investigated: pure chitosan fibers (without chitin nanofibrils), fibers with 0.5% CNF, fibers with 5% CNF, fibers with 30% CNF, and fibers with 50% CNF. Ten experiments were carried out on pure chitosan fibers; on all other sorbents, six experiments each. In addition, 10 experiments were carried out on carbon hemosorbent.
The intensity of hemolysis can be estimated by the degree of changes in optical density of blood plasma at wavelengths corresponding to the peak absorption of hemoglobin [
21]. To evaluate the level of the hemolytic activity, human blood and sorbent were bought into contact for 60 minutes in a rotating mode. Samples were taken after certain time intervals to estimate the influence of contact duration on hemolysis level. Bench experiments were carried out in 20-mL disposable blood columns. To filter the samples from the sorbent after the experiment, a nonwoven filter and nylon mesh were placed in the syringe and secured with a pressure ring.
Chitosan fibers were cut into fragments 0.5–1.0 cm long, 100 mg each, and put into columns. Before starting the experiment, all fibers were soaked in physiological saline for 30 minutes until complete swelling. The columns were then washed three times with sterile saline, and then another three times with saline by adding heparin (20 units/mL). After that, heparinized donor blood from a vacuum tube was placed in the syringe column (the ratio of sorbent to blood is 1:4). Previously, a control blood sample was taken from the same tube before contact. Then, the columns were placed horizontally on a rotary mixer and a constant rotation mode was switched on at a speed of 10 rpm. After 5, 20, 40, and 60 min, blood samples of 1.8–2.0 mL were taken.
Blood samples were centrifuged for 10 min at 3500 rpm and diluted 30 times with saline. Optical density was measured at wavelengths of 414 and 540 nm (maximum absorption of hemoglobin [
3]) on a UNICO 280 (S) spectrophotometer (UNICO, Dayton, NJ, USA). With change in the optical density of the plasma in the visible part of the spectrum (300–700 nm) in post-contact samples compared to the sample before contact, the hemolytic activity of sorbents was estimated.
2.6. Statistical Data Processing
Statistical analysis of the results was carried out using Statistica 7.0 and Excel 2013 application packages. Statistically significant changes in indicators within the groups were evaluated using the Student t-test for pairwise-related samples and Wilcoxon’s test for pairwise comparisons, statistically significant differences between the groups using t- Student’s test for independent samples, and the Mann–Whitney U-test. The differences were considered statistically significant at P < 0.05.
3. Results
3.1. Testing of Mechanical Characteristics
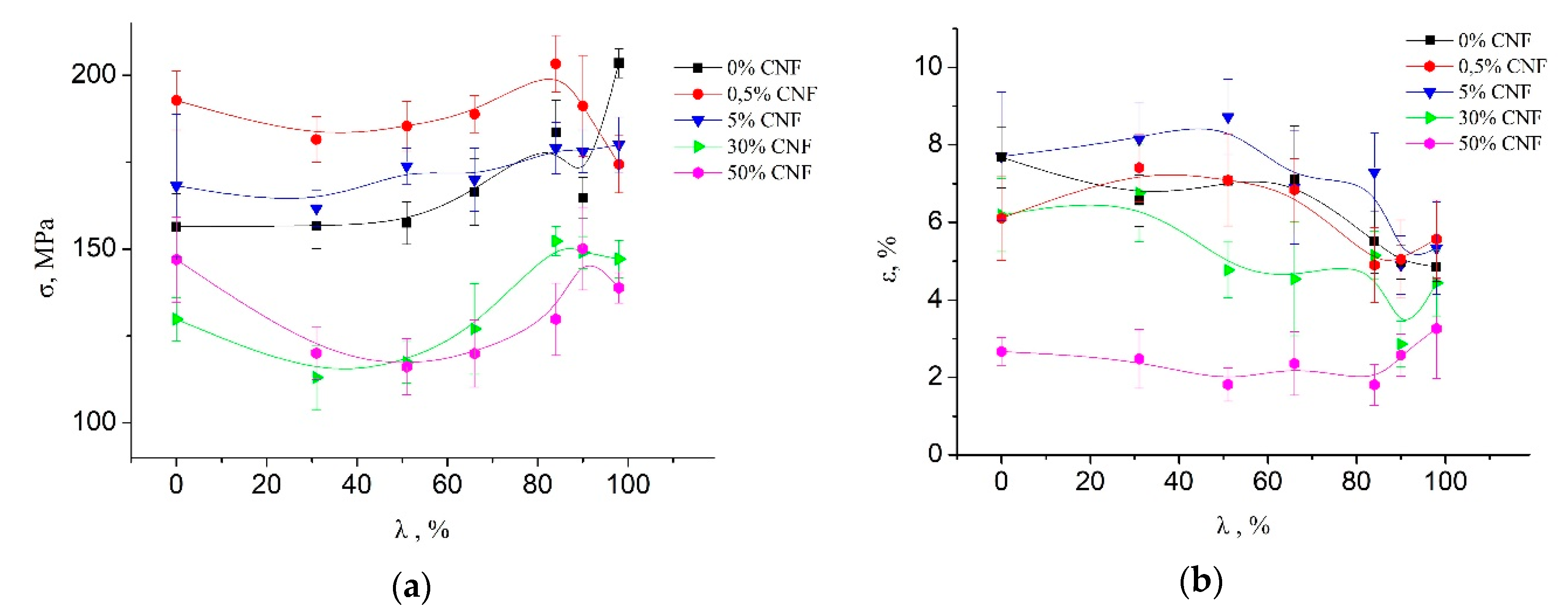
The mechanical properties (tensile strength and elongation at break) of the processed fibers are presented at
Figure 1. It is seen that the addition of a low concentration of CNF (up to 5%) leads to an increase in tensile strength. However, when CNF content exceeds 5%, the tensile strength of the composite fibers significantly decreases. Maximal strength of the composite fibers is observed at CNF content as 0.5%, caused (as shown before [
22]) by a change in the morphology of the composite fibers and the additional orientation of the chitosan macromolecules on the CNF surface along the fiber axis. Strong interaction between chitin and chitosan and the orientation of the chitosan macromolecules on the surface of the CNF was confirmed by the energy minimization and molecular dynamics simulation of systems containing one chitosan molecule on the chitin nanocrystallite surface [
22]. When CNF exceeds 1–1.5% (corresponding to a percolation threshold), a cluster structure of CNF is formed, which reduces chitosan macromolecular mobility and leads to a decrease in the mechanical properties of composite fibers.
3.2. Study of Relative Water Vapor Pressure Influence on Mechanical Properties
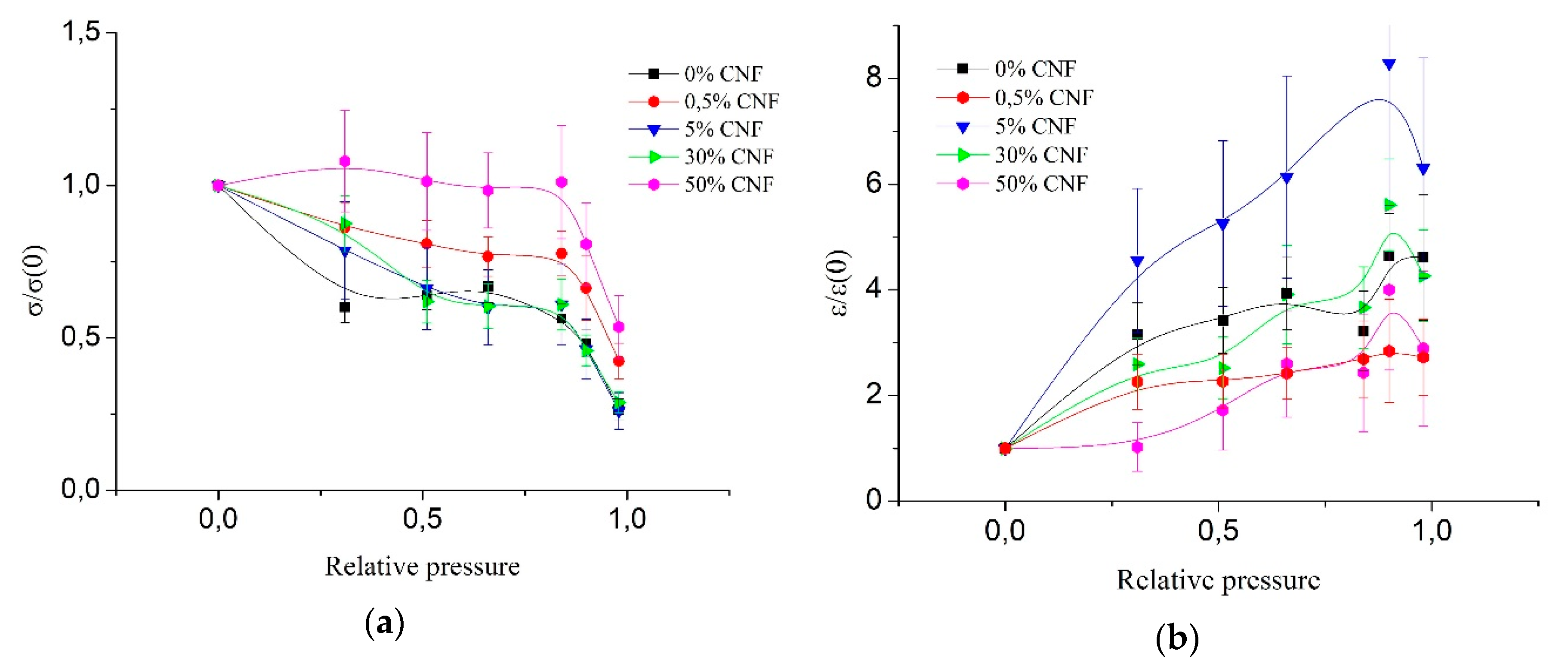
Figure 2 presents the dependence of the ratio of the tensile strength and elongation at break of the wet fibers to the tensile strength and elongation at break of the absolutely dry fibers (σ/σ(0) and ε/ε(0), respectively) for different concentrations of CNF on relative water vapor pressure. It is seen that an increase in the concentration of CNF leads to an increase in relative strength due to less moisture absorption by the fiber [
23]. The strongest resistance to humidity impact is seen for fibers with 50% CNF content, where there is no tensile strength loss up to 84% of relative pressure. Further increase in relative pressure leads to a decrease in mechanical characteristics for all materials, regardless of the content of the filler. It should be noted that the 0.5% concentration of CNF stands out due to its low strength loss, while the other concentrations of CNF (5% and 30%) differ slightly from each other and from pure chitosan. This could be explained by the strong interaction between chitosan and CNF and their ability to form strong hydrogen bonds in composite fibers when CNF content is 0.5%. As a result, these fibers possess an optimal supermolecular structure.
3.3. Study of Hemocompatibility
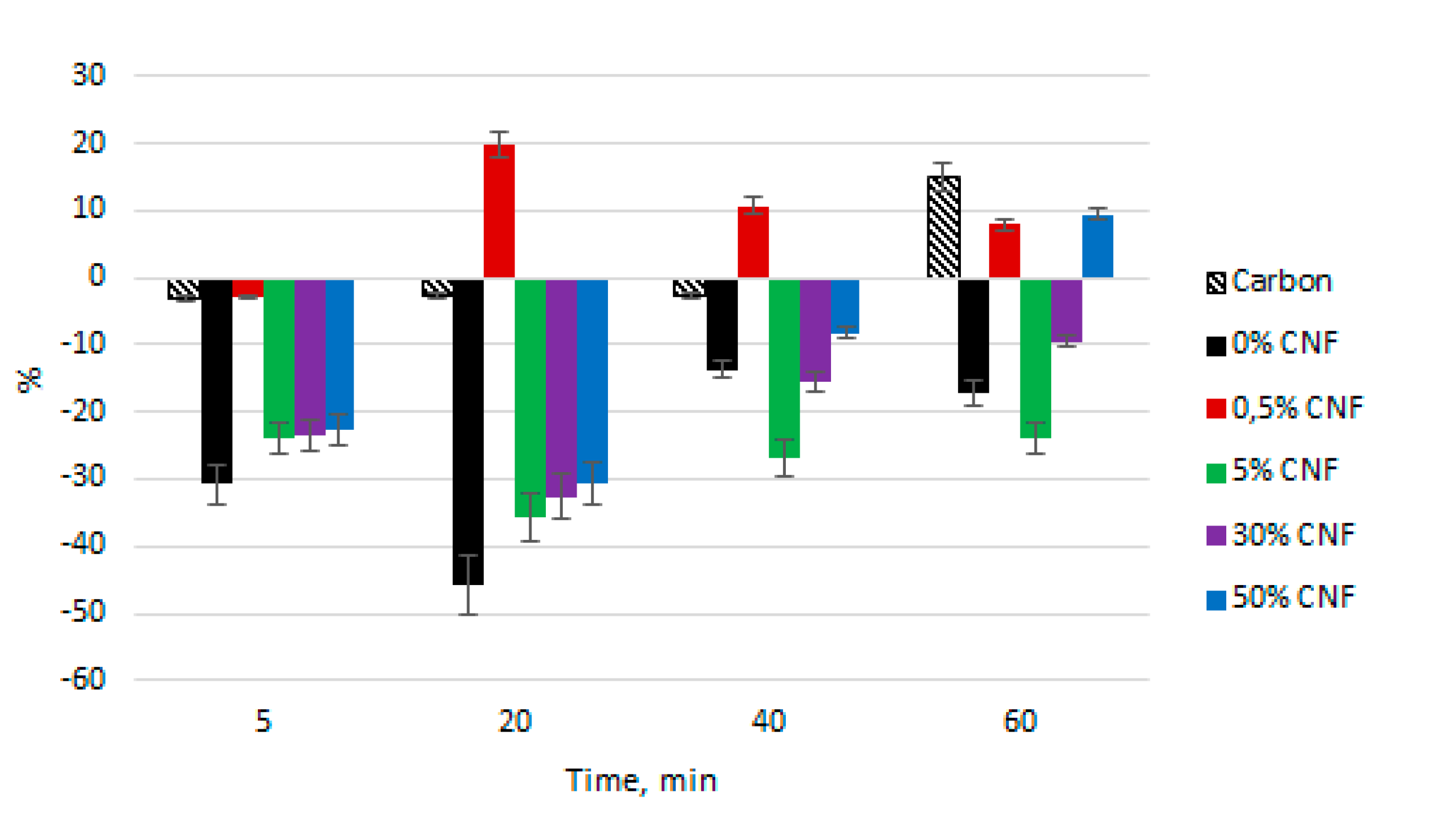
For each of the five sorbents, optical density (OD) of the plasma was measured at wavelengths of 414 nm (
Figure 3) and 540 nm (
Figure 4). Upon contact of blood with all types of chitosan fibers, the OP values in the “5 min” samples decreased, in comparison to the “before contact” (control) samples.
At λ = 414 nm, the maximum sorption activity for all fibers, except for “0.5% CNF”, was observed in the “20 min” samples. Subsequently, there was a decrease in the sorption process, which may be associated with partial saturation of the sorbents and a decrease in their sorption capacity. In this case, the sorption activity decreased in the order of 0% CNF < 5% CNF < 30% CNF < 50% CNF; and for 50% CNF, by the end of the experiment, the OD value was higher than the OD of the control sample plasma (+9.7% compared to the “before contact” sample). All of these sorbents had a lower OD value than a reference sorbent (carbon) at each time point.
A 0.5% CNF sorbent was strongly distinguished from this regularity. Hemoglobin sorption occurred only in the first five minutes of the experiment, and the OD of all the other samples was higher than the OD of the control sample, reaching a maximum (+19.8% compared to the “before contact” sample) in the “20 minutes” sample. This indicates the predominance of hemolysis processes over sorption processes on 0.5% CNF.
At a wavelength of 540 nm, all OD parameters for all the sorbents, except 0.5% CNF, had a negative value, relative to the control samples. The only exception was the “60 min” test for the 50% CNF sorbent, in which erythrocyte hemolysis prevailed over sorption processes (OD + 3.45% compared to the control sample).
4. Discussion
Composite fibers with different CNF content were obtained and its mechanical characteristics were measured. Fiber strengthening with the addition of low concentrations of CNF could be explained as follows. When the polymer solution passes through the die hole, the orientation of the chitosan macromolecules along the jet takes place, which also occurs when the orientation drawing of the resulting fiber increases. As a result, there is an increase in mechanical characteristics, namely tensile strength. The introduction of small concentrations of CNF contributes to the additional orientation of the fiber due to the location of chitosan molecules along the nanofibrils. At the same time, further increasing the filler concentration leads to the formation of a rigid mesh, inhibiting the orientation of chitosan macromolecules, which causes the decrease of the strength properties of the fibers and relative deformation up to rupture. Thus, the addition of 0.5% CNF turns out to be the optimal filler content to obtain the strongest fiber.
It has been shown [
23] that the addition of CNF to chitosan solutions leads to the decrease of material sorption characteristics due to an increase in orderliness in composite materials, the formation of a denser packing of chitosan macromolecules in the adsorption layer on the surface of chitin nanofibrils with the formation of a liquid crystal type mesophase from chitosan macromolecules. This could be the explanation for the reduced susceptibility of fibers with the addition of CNF to relative pressure, which leads to a decrease of tensile loss with relative pressure increase for fibers with a high concentration of CNF. The explanation of the distinction of 0.5% CNF concentration could be the existence of a percolation barrier at 1% [
22], which is related to the best interaction between CNF and chitosan macromolecules.
As for hemocompatibility of the fibers, which was rated by the hemolysis study, the decrease in optical density at λ = 414 nm in most samples compared to the control samples may be associated with the sorption of hemoglobin molecules on the sorbent, since chitosan fibers have strong sorption properties. Since sorption activity decreased with increasing CNF concentration, it can be concluded that the addition of the CNF into chitosan fibers reduces the hemocompatibility of the materials. Nevertheless, chitosan fibers with a 0.5% CNF are the least hemocompatible in comparison to the rest of the studied materials due to their low sorption activity. Likewise, at a wavelength of 540 nm, the maximum sorption activity, and therefore the best hemocompatibility, was found for the chitosan fibers without CNF. Nevertheless, it should be noted that all the fibers of chitosan, except for 0.5% CNF, showed sorption rather than hemolytic properties, and, therefore, all studied sorbents are well hemocompatible and do not negatively affect human blood. Moreover, the use of the studied fibers caused a comparable or even lesser hemolysis than the reference hemosorbent. In addition, due to the low hemolysis value, it is possible to make a preliminary conclusion about the absence of fiber toxicity, which could be connected to the alkali treatment during its processing. Further advanced studies should be conducted to confirm this hypothesis.
5. Conclusions
The addition of low CNF concentrations (about 0.5%) leads to fiber strengthening due to a strong interaction between chitosan and CNF, and additional orientation of chitosan macromolecules on the CNF surface. However, further concentration of CNF causes the formation of a cluster structure (rigid network) and, respectively, the decrease of mechanical characteristics of the composite fibers. On the other hand, the use of high concentrations of CNF results in greater resistance to high humidity, which is important because the developed fibers are planned to be used in the human body, especially in contact with blood, where conditions are different from normal. To study the hemocompatibility of fibers, the optical density of blood plasma was measured. All chitosan fibers, except 0.5% CNF, showed better hemocompatibility than the reference carbon sorbent, even on prolonged contact with human blood. The addition of CNF to chitosan fibers led to a decrease in plasma optical density, which could be interpreted as a decline in hemoglobin molecules sorption. Thus, the highest hemocompatibility was found in chitosan fibers without CNF addition.
Author Contributions
Data curation, E.N.M., O.P.K., and S.I.K.; Formal analysis, O.P.K.; Investigation, E.N.M., O.P.K., and E.N.D.; Resources, P.M.; Supervision, S.IK. and V.V.Y.; Writing—original draft, E.N.M.; Writing—review & editing, E.N.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the RSF, grant number 19-73-30003.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Werner, C.; Maitz, M.F.; Sperling, C. Current strategies towards hemocompatible coatings. J. Mater. Chem. 2007, 17, 3376. [Google Scholar] [CrossRef]
- Hasirci, V.; Hasirci, N. Fundamentals of Biomaterials; Springer: New York, NY, USA, 2018. [Google Scholar]
- Pan, T.; Huang, W.; Liu, Z.; Yao, L. Near-infrared spectroscopic analysis of hemoglobin with stability based on human hemolysates samples. Am. J. Anal. Chem. 2012, 03, 19–23. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Balan, V.; Verestiuc, L. Strategies to improve chitosan hemocompatibility: A review. Eur. Polym. J. 2014, 53, 171–188. [Google Scholar] [CrossRef]
- Suzuki, Y.; Miyatake, K.; Okamoto, Y.; Muraki, E.; Minami, S. Influence of the chain length of chitosan on complement activation. Carbohydr. Polym. 2003, 54, 465–469. [Google Scholar] [CrossRef]
- Chou, T.-C.; Fu, E.; Wu, C.-J.; Yeh, J.-H. Chitosan enhances platelet adhesion and aggregation. Biochem. Biophys. Res. Commun. 2003, 302, 480–483. [Google Scholar] [CrossRef]
- Nadesh, R.; Narayanan, D.P.R.S.; Vadakumpully, S.; Mony, U.; Koyakkutty, M.; Nair, S.V.; Menon, D. Hematotoxicological analysis of surface-modified and -unmodified chitosan nanoparticles. J. Biomed. Mater. Res. Part A 2013, 101, 2957–2966. [Google Scholar] [CrossRef]
- Notara, M.; Scotchford, C.A.; Grant, D.M.; Weston, N.; Roberts, G.A.F. Cytocompatibility and hemocompatibility of a novel chitosan-alginate gel system. J. Biomed. Mater. Res. Part A 2009, 89, 854–864. [Google Scholar] [CrossRef]
- Xue, J.; Zhao, W.; Nie, S.; Sun, S.; Zhao, C. Blood compatibility of polyethersulfone membrane by blending a sulfated derivative of chitosan. Carbohydr. Polym. 2013, 95, 64–71. [Google Scholar] [CrossRef]
- Shelma, R.; Sharma, C.P. Development of lauroyl sulfated chitosan for enhancing hemocompatibility of chitosan. Colloids Surf. B: Biointerfaces 2011, 84, 561–570. [Google Scholar] [CrossRef]
- Rahman, M.A.; Ochiai, B. Fabrication and hemocompatibility of carboxy-chitosan stabilized magnetite nanoparticles. Microsyst. Technol. 2018, 24, 669–681. [Google Scholar] [CrossRef]
- Scherließ, R.; Buske, S.; Young, K.; Weber, B.; Rades, T.; Hook, S. In vivo evaluation of chitosan as an adjuvant in subcutaneous vaccine formulations. Vaccine 2013, 31, 4812–4819. [Google Scholar] [CrossRef]
- Yoon, H.J.; Moon, M.E.; Park, H.S.; Im, S.Y.; Kim, Y.H. Chitosan oligosaccharide (COS) inhibits LPS-induced inflammatory effects in RAW 264.7 macrophage cells. Biochem. Biophys. Res. Commun. 2007, 358, 954–959. [Google Scholar] [CrossRef]
- Ermak, I.M.; Davydova, V.N.; Gorbach, V.I.; Berdyshev, E.L.; Kuznetsova, T.A.; Ivanushko, I.A.; Gazha, A.K.; Smolina, T.P.; Zaporozhets, T.S.; Soloveva, T.F. Modification of biological activity of lipopolysaccharide in the complex with chitosan. Bull. Exp. Biol. Med. 2004, 137, 379–381. [Google Scholar] [CrossRef]
- Dzung, N.; Hà, N.; Van, D.; Phuong, N.; Quynh, N.; Hiep, D.; Hiep, L. Chitosan nanoparticles as a novel delivery system for H1N1 influenza vaccine: Safe properties and immunogenicity in mice. Int. J. Biotechnol. Bioeng. 2011, 5, 915–922. [Google Scholar]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Tramer, F.; da Ros, T.; Passamonti, S. Screening of fullerene toxicity by hemolysis assay. In Nanotoxicity; Humana Press: Totowa, NJ, USA, 2012; pp. 203–217. [Google Scholar]
- Kirichuk, O.P.; Maevskaia, E.N.; Burkova, N.V.; Dresvyanina, E.N.; Kuznetsov, S.I.; Dobrovolskaya, I.P.; Yudin, V.E. Comparative characteristics of the reaction of the cellular elements of venous blood in contact with the carbon hemosorbent and fiber of chitosan in vitro. Tsitologiya 2019, 61, 864–871. [Google Scholar]
- Dresvyanina, E.; Yudenko, A.; Yevlampieva, N.; Maevskaya, E.; Yudin, V.; Gubarev, A.; Slyusarenko, M.; Heppe, K. The molecular mass effect on mechanical properties of chitosan fibers. Vlak. A Text. 2018, 25, 27–31. [Google Scholar]
- Burkova, N.V.; Kirichuk, O.P.; Romanchuk, E.V.; Davankov, V.A.; Postnov, V.N.; Kuznetsov, S.I. Changes in plasma spectral characteristics during the in vitro contact of human venous blood with granulated sorbents. Alm. Clin. Med. 2018, 46, 772–777. [Google Scholar] [CrossRef]
- Yudin, V.E.; Dobrovolskaya, I.P.; Neelov, I.M.; Dresvyanina, E.N.; Popryadukhin, P.V.; Ivankova, E.M.; Elokhovskii, V.Y.; Kasatkin, I.A.; Okrugin, B.M.; Morganti, P. Wet spinning of fibers made of chitosan and chitin nanofibrils. Carbohydr. Polym. 2014, 108, 176–182. [Google Scholar] [CrossRef]
- Smotrina, T.V.; Dresvyanina, E.N.; Grebennikov, S.F.; Kazakov, М.О.; Maslennikova, T.P.; Dobrovolskaya, I.P.; Yudin, V.E. Interaction between water and the composite materials based on chitosan and chitin nanofibrils. Polymer 2020, 189, 122166. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).