The Potential Application of Ecklonia cava Extract in Scalp Protection
Abstract
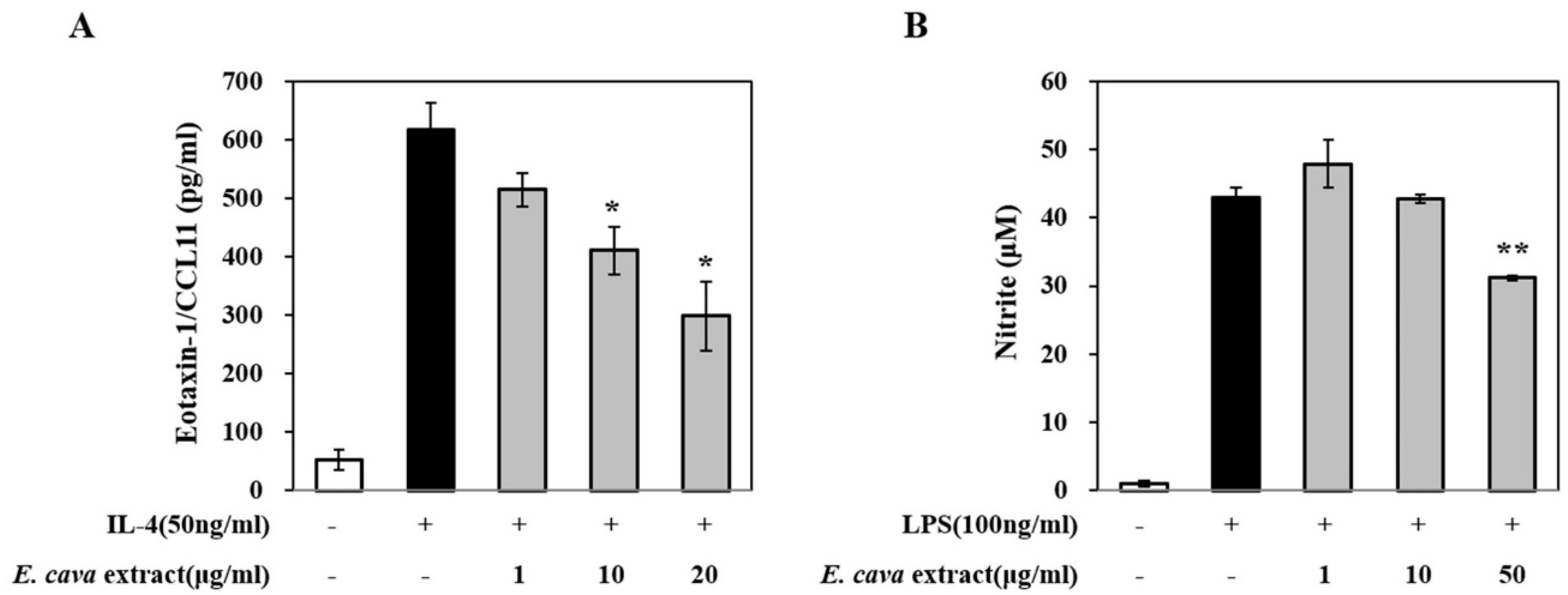
1. Introduction
2. Materials and Methods
2.1. E. cava Extraction
2.2. Cell Culture
2.3. Reagents
2.4. Evaluation of Cytotoxicity of HFDPC
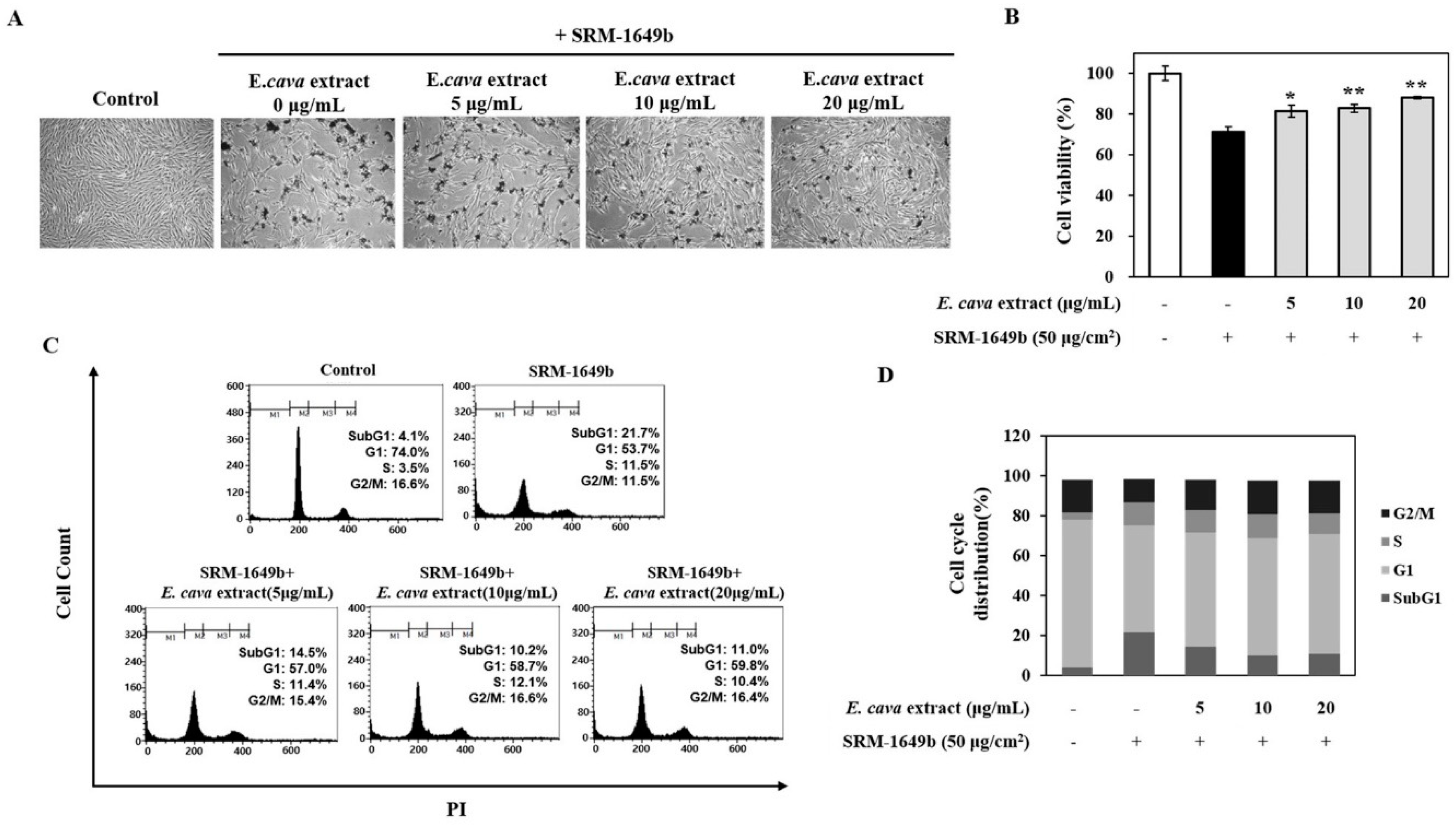
2.5. Cell Cycle Analysis
2.6. Western Blotting
2.7. Ex Vivo Study
2.8. Clinical Study
2.9. Statistics
3. Results
3.1. E. cava Extract Protects against Pollutant-Induced Cytotoxicity
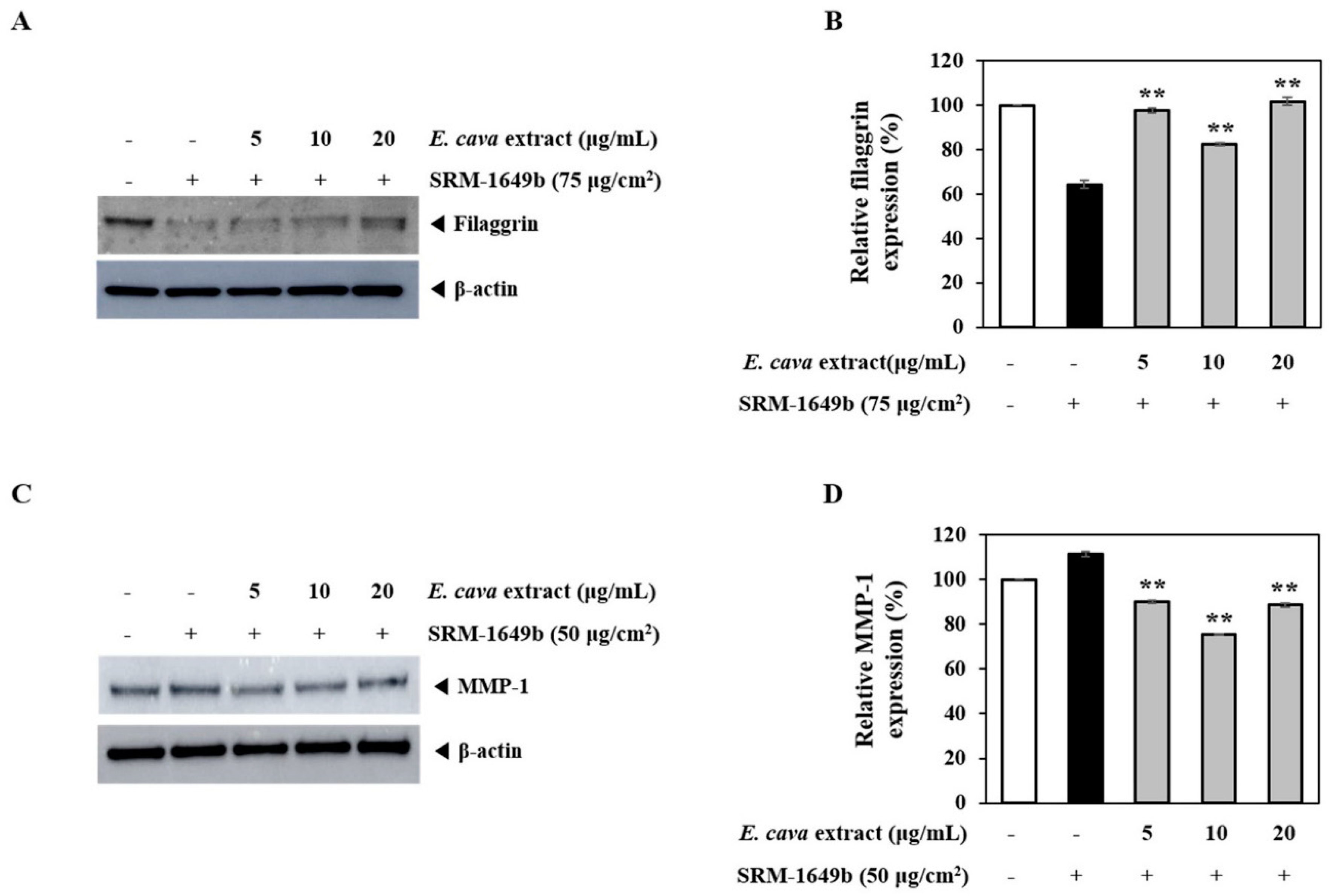
3.2. E. cava Extract Attenuates Pollutant-Induced Loss of Filaggrin and Increased MMP-1 Protein Expression In Vitro
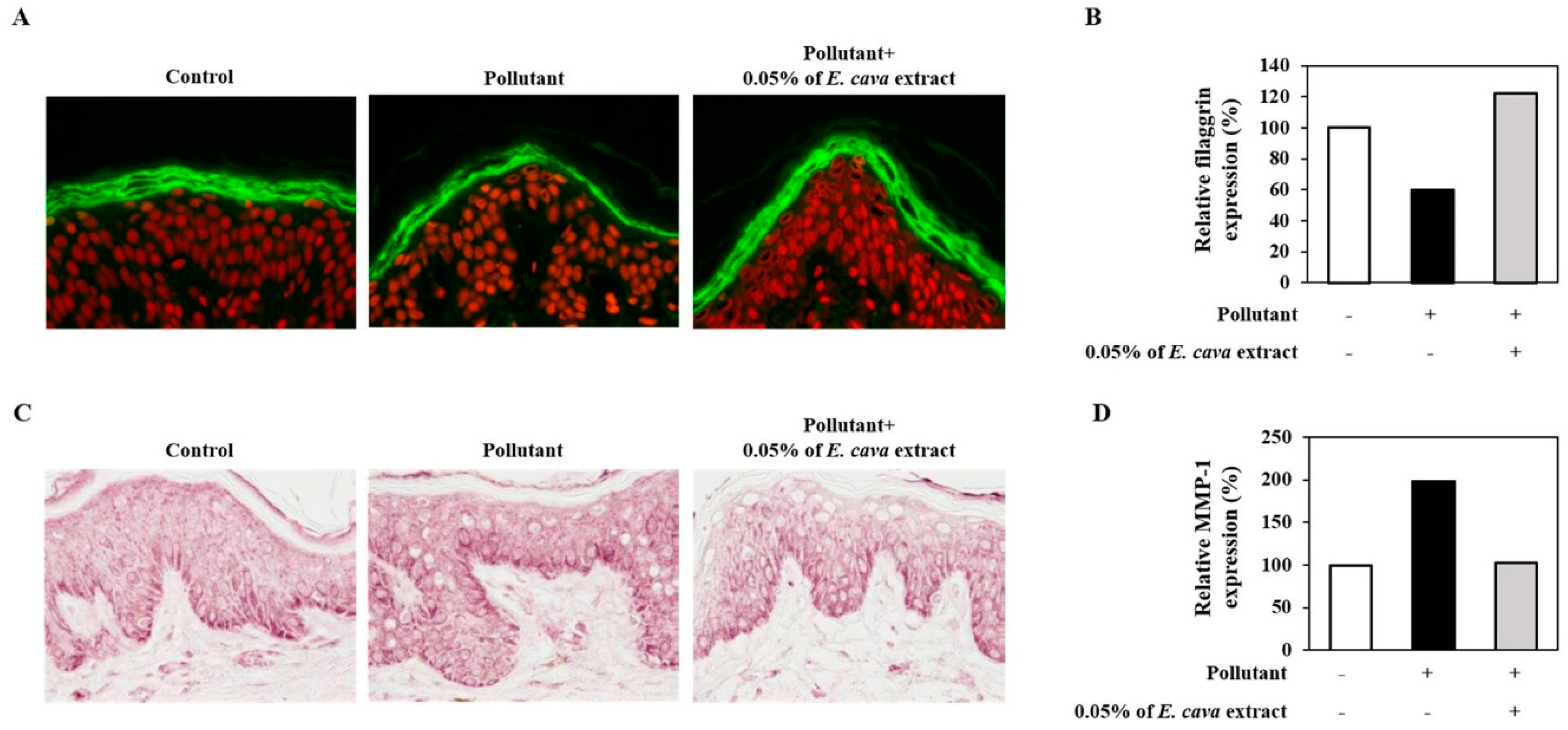
3.3. E. cava Extract Attenuates the Pollutant-Induced Loss of Filaggrin and Increase in MMP-1 Protein Expression Ex-Vivo
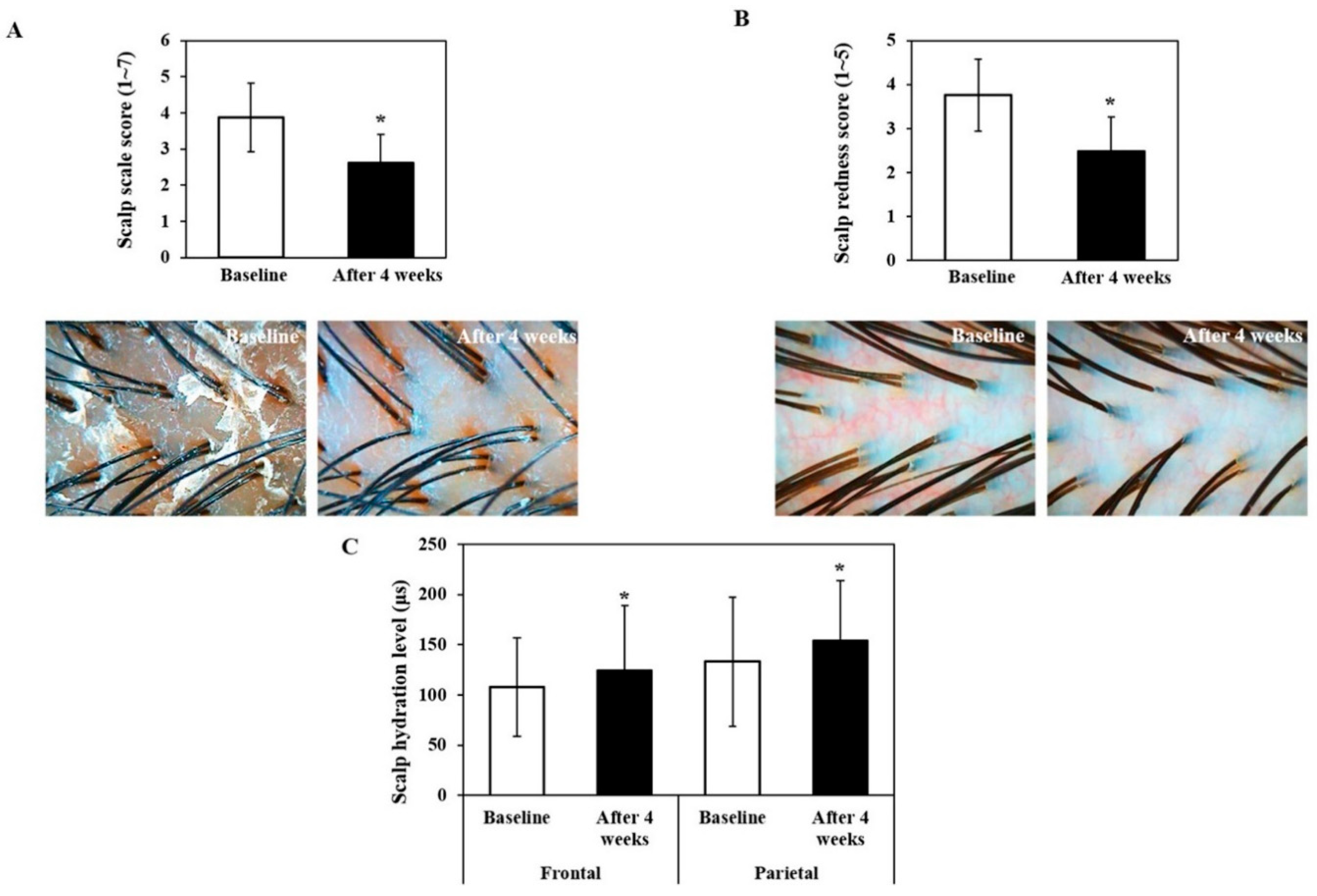
3.4. Analysis of Scalp Scale, Redness and Hydration
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Zouboulis, C.C. Sebaceous Gland in Human Skin-The Fantastic Future of a Skin Appendage. J. Investig. Dermatol. 2003, 120, 14–15. [Google Scholar] [CrossRef] [PubMed]
- Ro, B.I.; Dawson, T.L. The Role of Sebaceous Gland Activity and Scalp Microfloral Metabolism in the Etiology of Seborrheic Dermatitis and Dandruff. J. Investig. Dermatol. Symp. Proc. 2005, 10, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Holme, J.A.; Rosas, I.; Schwarze, P.E.; Alfaro-Moreno, E. Recent advances in particulate matter and nanoparticle toxicology: A review of the in vivo and in vitro studies. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Kolli, H. Examining the Relationship between Particulate Matter, Nitrogen Oxide, Carbon Oxides, Sulfur Dioxide and Hypertension in Urban Areas in India: A Review. The Journal of Undergraduate Research and Creative Scholarship. July 2013. Available online: https://scholarscompass.vcu.edu/cgi/viewcontent.cgi?article=1020&context=auctus (accessed on 16 January 2020).
- Sharma, S.B.; Jain, S.; Khirwadkar, P.; Kulkarni, S. The effects of air pollution on the environment and human health. Indian J. Res. Pharm. Biotechnol. 2013, 1, 391–396. [Google Scholar]
- Hamra, G.B.; Guha, N.; Cohen, A.; Laden, F.; Raaschou-Nielsen, O.; Samet, J.M.; Vineis, P.; Forastiere, F.; Saldiva, P.; Yorifuji, T.; et al. Outdoor Particulate Matter Exposure and Lung Cancer: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2014, 122, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K. The role of air pollutants in atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Morita, A.; Torii, K.; Maeda, A.; Yamaguchi, Y. Molecular basis of tobacco smoke-induced premature skin aging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 53–55. [Google Scholar] [CrossRef]
- Ono, Y.; Torii, K.; Fritsche, E.; Shintani, Y.; Nishida, E.; Nakamura, M.; Shirakata, Y.; Haarmann-Stemmann, T.; Abel, J.; Krutmann, J.; et al. Role of the aryl hydrocarbon receptor in tobacco smoke extract–induced matrix metalloproteinase-1 expression. Exp. Dermatol. 2013, 22, 349–353. [Google Scholar] [CrossRef]
- Lee, C.W.; Lin, Z.C.; Hu, S.C.; Chiang, Y.C.; Hsu, L.F.; Lin, Y.C.; Lee, I.T.; Tsai, M.H.; Fang, J.Y. Urban particulate matter down-regulates filaggrin via COX2 expression/PGE2 production leading to skin barrier dysfunction. Sci. Rep. 2016, 6, 27995. [Google Scholar] [CrossRef]
- Jin, S.P.; Li, Z.; Choi, E.K.; Lee, S.; Kim, Y.K.; Seo, E.Y.; Chung, J.H.; Cho, S. Urban particulate matter in air pollution penetrates into the barrier-disrupted skin and produces ROS-dependent cutaneous inflammatory response in vivo. J. Dermatol. Sci. 2018. [Google Scholar] [CrossRef]
- Ramos, P.M.; Brianezi, G.; Martins, A.C.P.; Silva, M.G.D.; Marques, M.E.A.; Miot, H.A. Aryl hydrocarbon receptor overexpression in miniaturized follicles in female pattern hair loss. An. Bras. Dermatol. 2017, 92, 430–431. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.A.; Jeon, Y.J. Exploiting biological activities of brown seaweed Ecklonia cava for potential industrial applications: A review. Int. J. Food Sci. Nutr. 2012, 63, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.I.; Kim, S.C.; Kim, M.K.; Boo, H.J.; Jeon, Y.J.; Koh, Y.S.; Yoo, E.S.; Kang, S.M.; Kang, H.K. Effect of Dieckol, a Component of Ecklonia cava, on the Promotion of Hair Growth. Int. J. Mol. Sci. 2012, 13, 6407–6423. [Google Scholar] [CrossRef] [PubMed]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.H. Filaggrin in the frontline: Role in skin barrier function and disease. J. Cell Sci. 2009, 122 Pt 9, 1285–1294. [Google Scholar] [CrossRef]
- Simakou, T.; Butcher, J.P.; Reid, S.; Henriquez, F.L. Alopecia areata: A multifactorial autoimmune condition. J. Autoimmun. 2018, 98, 74–85. [Google Scholar] [CrossRef]
- Inami, Y.; Sasaki, A.; Andoh, T.; Kuraishi, Y. Surfactant-induced Chronic Pruritus: Role of L-Histidine Decarboxylase Expression and Histamine Production in Epidermis. Acta Derm. Venereol. 2014, 94, 645–650. [Google Scholar] [CrossRef]
- Pedrosa, A.F.; Lisboa, C.; Branco, J.; Pellevoisin, C.; Miranda, I.M.; Rodrigues, A.G. Malassezia interaction with a reconstructed human epidermis: Keratinocyte immune response. Mycoses 2019, 62, 932–936. [Google Scholar] [CrossRef]
- Drakaki, E.; Dessinioti, C.; Antoniou, C.V. Air pollution and the skin. Environ. Sci. 2014, 2, 11. [Google Scholar] [CrossRef]
- D’Agostini, F.; Balansky, R.; Pesce, C.; Fiallo, P.; Lubet, R.A.; Kelloff, G.J.; De Flora, S. Induction of alopecia in mice exposed to cigarette smoke. Toxicol. Lett. 2000, 114, 117–123. [Google Scholar] [CrossRef]
- Kezic, S.; Jakasa, I. Filaggrin and Skin Barrier Function. Curr. Probl. Dermatol. 2016, 49, 1–7. [Google Scholar]
- Schultz, G.S.; Gibson, D.J. Measurement of biomarkers for impaired healing in fluids and tissues. In Measurements in Wound Healing, 1st ed.; Mani, R., Romanelli, M., Shukla, V., Eds.; Springer: London, UK, 2013; pp. 243–258. [Google Scholar]
- Hwang, J.H.; Kim, K.J.; Lee, B.Y. Crude Ecklonia cava Flake Extracts Attenuate Inflammation through the Regulation of TLR4 Signaling Pathway in LPS-Induced RAW264.7 Cells. Molecules 2017, 22, 777. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.W.; Song, H.; Hong, S.S.; Boo, Y.C. Marine Alga Ecklonia cava Extract and Dieckol Attenuate Prostaglandin E2 Production in HaCaT Keratinocytes Exposed to Airborne Particulate Matter. Antioxidants 2019, 21, 8. [Google Scholar] [CrossRef] [PubMed]
| Grade | Description Criteria |
|---|---|
| 1 | No |
| 2 | Slight recognition |
| 3 | Perceptible but not clearly identified light pink |
| 4 | Clear red |
| 5 | Dark red or purple with epidermal damage |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Woo, H.; Shin, S.; Park, D.; Jung, E. The Potential Application of Ecklonia cava Extract in Scalp Protection. Cosmetics 2020, 7, 9. https://doi.org/10.3390/cosmetics7010009
Kim H, Woo H, Shin S, Park D, Jung E. The Potential Application of Ecklonia cava Extract in Scalp Protection. Cosmetics. 2020; 7(1):9. https://doi.org/10.3390/cosmetics7010009
Chicago/Turabian StyleKim, Hayeon, Hyunju Woo, Seoungwoo Shin, Deokhoon Park, and Eunsun Jung. 2020. "The Potential Application of Ecklonia cava Extract in Scalp Protection" Cosmetics 7, no. 1: 9. https://doi.org/10.3390/cosmetics7010009
APA StyleKim, H., Woo, H., Shin, S., Park, D., & Jung, E. (2020). The Potential Application of Ecklonia cava Extract in Scalp Protection. Cosmetics, 7(1), 9. https://doi.org/10.3390/cosmetics7010009





