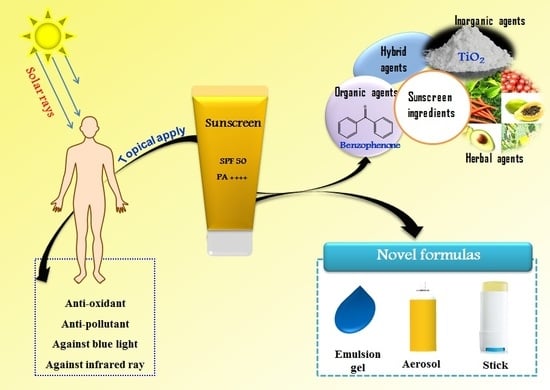
Recent Trends of Sunscreen Cosmetic: An Update Review
Abstract
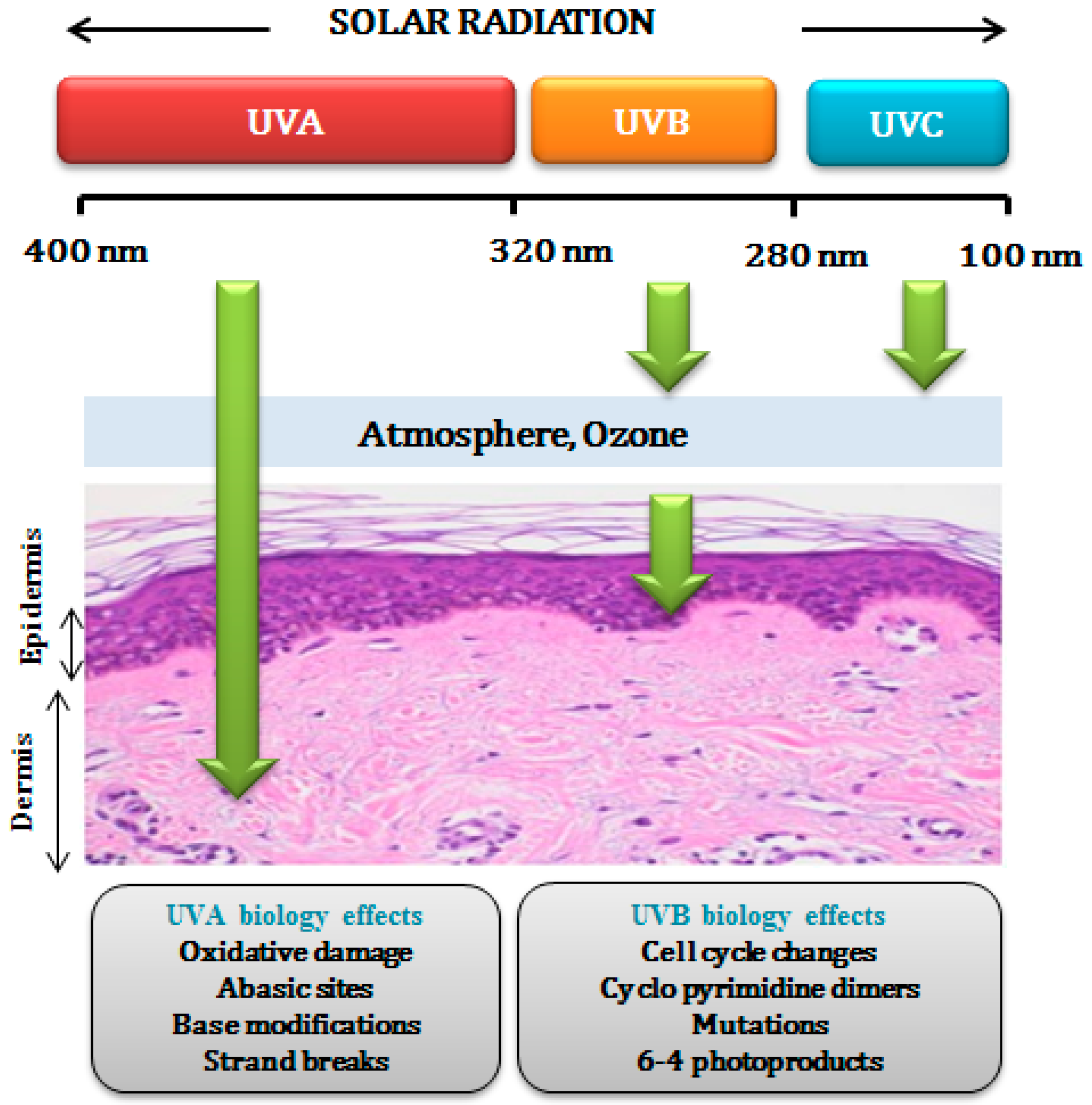
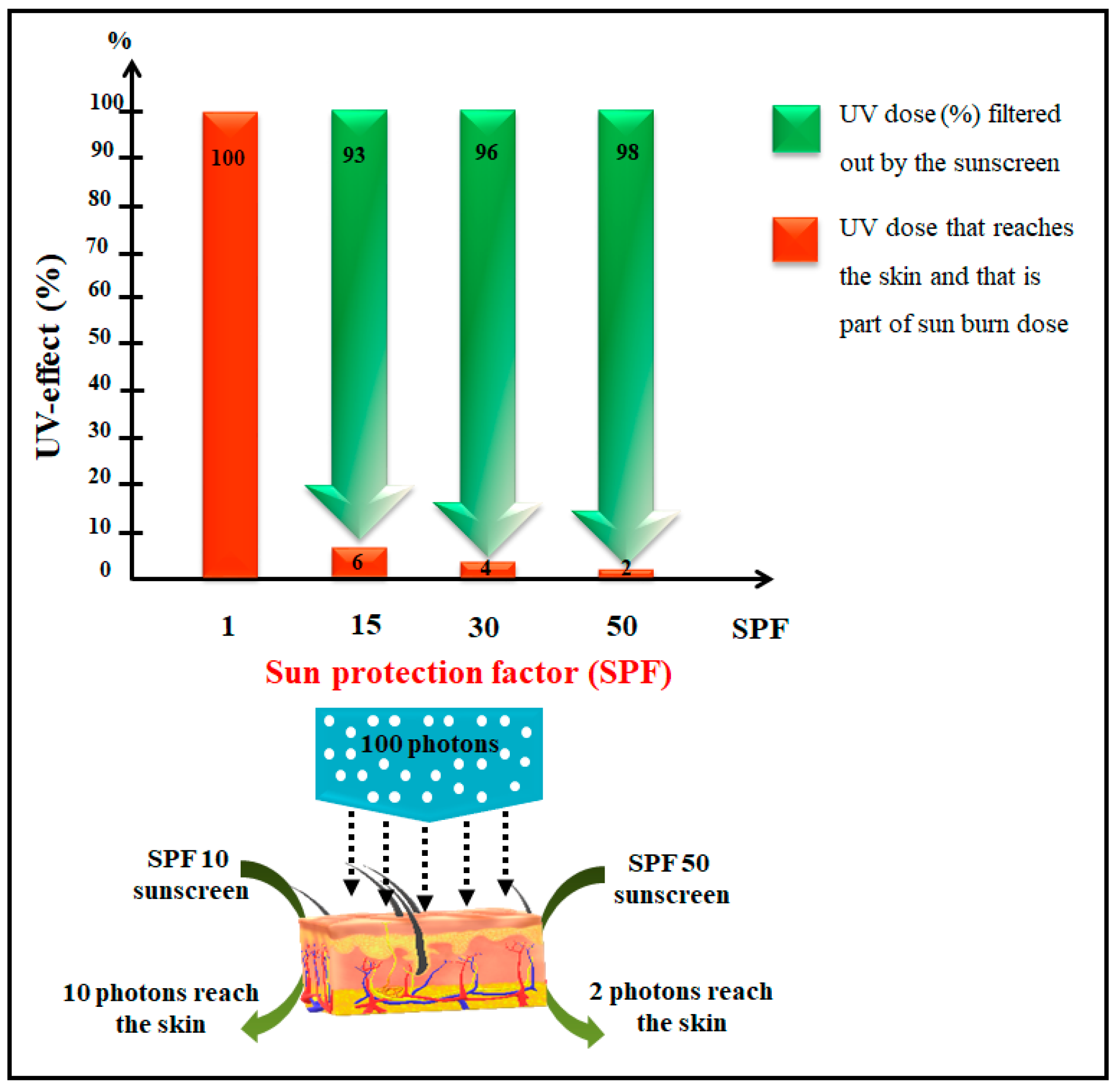
1. Introduction
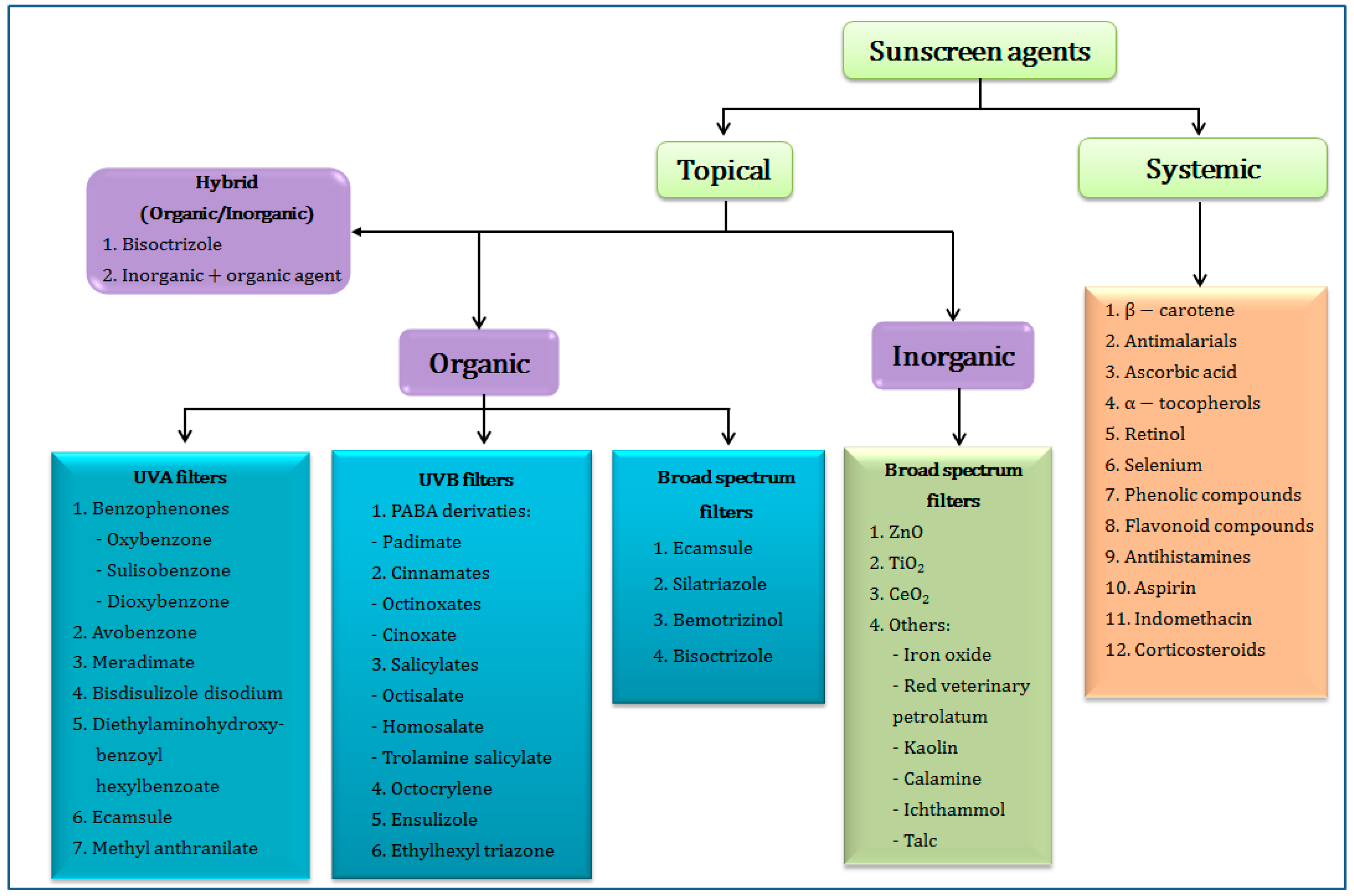
2. Classification of Sunscreen Agents
2.1. Organic UV Filters
2.2. Inorganic UV Filters
2.3. Hybrid UV Filters (Organic/Inorganic Agents)
2.4. Botanical Agents
2.5. Safety and Health Hazards of Sunscreen Agents
3. Sunscreen Formulations
3.1. Emulsion Sunscreen
3.2. Gel Sunscreen
3.3. Aerosol Sunscreen
3.4. Sun Stick
4. Novel Properties of Commercial Sun Protection Products
4.1. Sunscreen with Antioxidants and Anti-Aging
4.2. Sunscreen Combined with DNA Repair Enzymes
4.3. Sunscreen Against Environmental Pollutants
4.4. Sunscreen Against Blue Light
4.5. Sunscreen against Thermal IR
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Hidaka, H.; Horikoshi, S.; Serpone, N.; Knowland, J. In vitro photochemical damage to DNA, RNA and their bases by an inorganic sunscreen agent on exposure to UVA and UVB radiation. J. Photochem. Photobiol. A Chem. 1997, 111, 205–213. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Juzeniene, A.; Moan, J. Beneficial effects of UV radiation other than via vitamin D production. Dermato-Endocrinol. 2012, 4, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T.; Litopoulou-Tzanetaki, E.; Davies, R.; Robinson, R.K. The antimicrobial effects of long-wave ultra-violet light and furocoumarins on some micro-organisms that occur in cheese brines. Food Microbiol. 2000, 17, 687–695. [Google Scholar] [CrossRef]
- Fleury, N.; Geldenhuys, S.; Gorman, S. Sun exposure and its effects on human health: Mechanisms through which sun exposure could reduce the risk of developing obesity and cardiometabolic dysfunction. Int. J. Environ. Res. Public Health 2016, 13, 999. [Google Scholar] [CrossRef]
- Weller, R.B. Sunlight has cardiovascular benefits independently of vitamin D. Blood Purif. 2016, 41, 130–134. [Google Scholar] [CrossRef]
- Taylor, B.R. Ultraviolet radiation and the eye: An epidemiologic study. Tr. Am. Ophth. Soc. 1989, 87, 802. [Google Scholar]
- Hitchin, V.M.; Withrow, T.J.; Olvey, K.M.; Harleston, B.A.; Ellingson, O.L.; Bostrom, A.R.G. The cytotoxic and mutagenic effects of UVA radiation on L5178Y mouse lymphoma cells. J. Photochem. Photobiol. 1986, 44, 53–57. [Google Scholar] [CrossRef]
- Sambandan, D.R.; Ratner, D. Sunscreens: An overview and update. J. Am. Acad. Dermatol. 2011, 64, 748–758. [Google Scholar] [CrossRef]
- Manikrao Donglikar, M.; Laxman Deore, S. Sunscreens: A review. Pharmacogn. J. 2016, 8, 171–179. [Google Scholar] [CrossRef]
- Palm, M.D.; O’Donoghue, M.N. Update on photoprotection. Dermatol. Ther. 2007, 20, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.; Karrer, S.; Berneburg, M. Modern sun protection. Curr. Opin. Pharmacol. 2019, 46, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Mistry, N. Guidelines for formulating anti-pollution products. Cosmetics 2017, 4, 57. [Google Scholar] [CrossRef]
- Lee, S.-H. New Technical Developments in Sun Care and Blue Light Defense; SUNJIN Beauty Science: Gyeonggi-do, Korea, 2018; p. 134. [Google Scholar]
- Ham, W.T.; Mueller, H.A.; Sliney, D.H. Retinal sensitivity to damage from short wavelength light. Nature 1976, 260, 153–155. [Google Scholar] [CrossRef]
- Schieke, S.M.; Schroeder, P.; Krutmann, J. Cutaneous effects of infrared radiation: From clinical observations to molecular response mechanisms. Photodermatol. Photoimmunol. Photomed. 2003, 19, 228–234. [Google Scholar] [CrossRef]
- Schalka, S.; Reis, V.M.S.d. Sun protection factor: Meaning and controversies. An. Bras. Dermatol. 2011, 86, 507–515. [Google Scholar] [CrossRef]
- Osterwalder, U.; Herzog, B. Sun protection factors: World wide confusion. Br. J. Dermatol. 2009, 161, 13–24. [Google Scholar] [CrossRef]
- Moyal, D. UVA protection labeling and in vitro testing methods. Photochem. Photobiol. Sci. 2010, 9, 516–523. [Google Scholar] [CrossRef]
- Wang, S.Q.; Stanfield, J.W.; Osterwalder, U. In vitro assessments of UVA protection by popular sunscreens available in the United States. J. Am. Acad. Dermatol. 2008, 59, 934–942. [Google Scholar] [CrossRef]
- Lademann, J.; Schanzer, S.; Jacobi, U.; Schaefer, H.; Pflucker, F.; Driller, H.; Beck, J.; Meinke, M.; Roggan, A.; Sterry, W. Synergy effects between organic and inorganic UV filters in sunscreens. J. Biomed. Opt. 2005, 10, 14008. [Google Scholar] [CrossRef]
- Vergou, T.; Patzelt, A.; Richter, H.; Schanzer, S.; Zastrow, L.; Golz, K.; Doucet, O.; Antoniou, C.; Sterry, W.; Lademann, J. Transfer of ultraviolet photon energy into fluorescent light in the visible path represents a new and efficient protection mechanism of sunscreens. J. Biomed. Opt. 2011, 16, 105001. [Google Scholar] [CrossRef] [PubMed]
- Latha, M.S.; Martis, J.; Shobha, V.; Shinde, R.S.; Banera, S.; Krishnankutty, B.; Bellary, S.; Varughese, S.; Rao, P.; Kumar, B.R.N. Sunscreening agents. J. Clin. Aesthet. Dermatol. 2013, 6, 16–26. [Google Scholar] [PubMed]
- Serpone, N.; Dondi, D.; Albini, A. Inorganic and organic UV filters: Their role and efficacy in sunscreens and sun care products. Inorganica Chim. Acta 2007, 360, 794–802. [Google Scholar] [CrossRef]
- Pathak, M.A. Sunscreens: Topical and systemic approaches for protection of human skin against harmful effects of solar radiation. J. Am. Acad. Dermatol. 1982, 7, 285–311. [Google Scholar] [CrossRef]
- Administration, U.F.A.D. Sunscreen drug products for over-the-counter human use. In Final Monograph; Food and Drug Administration: Rockville, MD, USA, 2000. [Google Scholar]
- Siller, A.; Blaszark, S.C.; Lazar, M. Update about the effects of the sunscreen ingredients oxybenzone and octinoxate on humans and the environment. Plast. Surg. Nurs. 2018, 38, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Paris, C.; Lhiaubet-Vallet, V.; Jiménez, O.; Trullas, C.; Miranda, M.A. A blocked diketo form of avobenzone: Photostability, photosensitizing properties and triplet quenching by a triazine-derived UVB-filter. Photochem. Photobiol. 2019, 85, 178–184. [Google Scholar] [CrossRef]
- Karlsson, I.; Hillerstrom, L.; Stenfeldt, A.-L.; Mårtensson, J.; Borje, A. Photodegradation of dibenzoylmethanes: Potential cause of photocontact allergy to sunscreens. Chem. Res. Toxicol. 2009, 22, 1881–1892. [Google Scholar] [CrossRef]
- Gaspar, L.R.; Maia Campos, P.M.B.G. Evaluation of the photostability of different UV filter combinations in a sunscreen. Int. J. Pharm. 2006, 307, 123–128. [Google Scholar] [CrossRef]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef]
- Giacomoni, P.U. Sun Protection in Man; Elsevier: Amsterdam, The Netherlands, 2001; Volume 3, pp. 495–519. [Google Scholar]
- Jacobs, J.F.; van de Poel, I.; Osseweijer, P. Sunscreens with titanium dioxide (TiO2) nano-particles: A societal experiment. Nanoethics 2010, 4, 103–113. [Google Scholar] [CrossRef]
- Gonzalez, A.D.; Pechko, A.H.; Kalafsky, R.E. Photostable Sunscreen Compositions and Methods of Stabilizing. US6440402B1, 27 August 2002. [Google Scholar]
- Schroeder, P.; Lademann, J.; Darvin, M.E.; Stege, H.; Marks, C.; Bruhnke, S.; Krutmann, J. Infrared radiation-induced matrix metalloproteinase in human skin: Implications for protection. J. Investig. Dermatol. 2008, 128, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Addor, F.A.S. Antioxidants in dermatology. An. Bra. Dermatol. 2017, 92, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef]
- Pouillot, A.; Polla, L.L.; Tacchini, P.; Neequaye, A.; Polla, A.; Polla, B. Natural antioxidants and their effects on the skin. In Formulating, Packaging, and Marketing of Natural Cosmetic Products; Dayan, N., Kromidas, L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 239–257. [Google Scholar]
- Anitha, D.; Reddy, K.Y.; Venkatesh, P.; Raani, M.J. A review-herbal sunscreen agents on skin protection. Eur. J. Pharm. Med. Res. 2016, 3, 308–313. [Google Scholar]
- Sopyan, I.; Gozali, D.; Tiassetiana, S. Formulation of tomato extracts (Solanum lycopersicum L.) as a sunscreen lotion. Natl. J. Physiol. Pharm. Pharmacol. 2017, 8, 453–458. [Google Scholar] [CrossRef]
- Afaq, F.; Zaid, M.A.; Khan, N.; Dreher, M.; Mukhtar, H. Protective effect of pomegranate-derived products on UVB-mediated damage in human reconstituted skin. Exp. Dermatol. 2009, 18, 553–561. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Elmets, C.A. Green tea polyphenolic antioxidants and skin photoprotection (review). Int. J. Oncol. 2001, 18, 1307–1313. [Google Scholar]
- Maheshwar, G.H.; Patil, B.S.; Prashant, D. Comparative sun protection factor determination fo fresh fruits extract of cucumber vs marketed cosmetic formulation. Res. J. Pharm. Biol. Chem. Sci. 2010, 1, 55–59. [Google Scholar]
- Shenoy, P.; Khot, S.; Chavan, M.; Takawale, J.; Singh, S. Study of sunscreen activity of aqueous, methanol and acetone extracts of leaves of Pongamia pinnata (L.) pierre, fabaceae. Int. J. Green Pharm. 2010, 4, 270. [Google Scholar] [CrossRef]
- Patil, V.; Patil, S.B.; Kondawar, M.S.; Naikwade, N.S.; Magdum, C.S. Study of methanolic extract of flower of Spathodea campanulata L. as an anti-solar. Int. J. Green Pharm. 2009, 3, 248. [Google Scholar] [CrossRef]
- Park, K.; Choi, H.S.; Hong, Y.H.; Jung, E.Y.; Suh, H.J. Cactus cladodes (Opuntia humifusa) extract minimizes the effects of UV irradiation on keratinocytes and hairless mice. Pharm.Biol. 2017, 55, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Hyun, T.K.; Ko, Y.-J.; Kim, E.-H.; Chung, I.-M.; Kim, J.-S. Anti-inflammatory activity and phenolic composition of Dendropanax morbifera leaf extracts. Ind. Crop. Prod. 2015, 74, 263–270. [Google Scholar] [CrossRef]
- Chen, L.; Hu, J.Y.; Wang, S.Q. The role of antioxidants in photoprotection: A critical review. J. Am. Acad. Dermatol. 2012, 67, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- McVean, M.; Liebler, D.C. Prevention of DNA photodamage by vitamin E compounds and sunscreens: Roles of ultraviolet absorbance and cellular uptake. Mol. Carcinog. 1999, 24, 169–176. [Google Scholar] [CrossRef]
- Dzialo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef]
- José, M.T.d.A.F.; Pedrita, A.S.; Emanuella, C.V.P.; Raimundo, G.d.O.J.; Fabrício, S.S.; Jackson, R.G.d.S.A.; Larissa, A.R.; Xirley, P.N.; Edigênia, C.d.C.A. Flavonoids as photoprotective agents: A systematic review. J. Med. Plants Res. 2016, 10, 848–864. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Beta-carotene and other carotenoids in protection from sunlight. Am. J. Clin. Nutr. 2012, 96, 1179S–1184S. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). 127 newFDA Rules Regulations for Sunscreen. 2012. Available online: https://smartshield.com/news/reviews/54-resources/127-new-fda-rules-regulations-for-sunscreen (accessed on 20 April 2019).
- Kerr, A.; Ferguson, J. Photoallergic contact dermatitis. Photodermatol. Photoimmunol. Photomed. 2010, 26, 56–65. [Google Scholar] [CrossRef]
- Faurschou, A.; Beyer, D.M.; Schmedes, A.; Bogh, M.K.; Philipsen, P.A.; Wulf, H.C. The relation between sunscreen layer thickness and vitamin D production after ultraviolet B exposure: A randomized clinical trial. Brit. J. Dermatol. 2012, 167, 391–395. [Google Scholar] [CrossRef]
- Gorham, E.D.; Mohr, S.B.; Garland, C.F.; Chaplin, G.; Garland, F.C. Do sunscreens increase risk of melanoma in populations residing at higher latitudes? Annals Epidemiol. 2007, 17, 956–963. [Google Scholar] [CrossRef]
- Autier, P. Sunscreen abuse for intentional sun exposure. Brit. J. Dermatol. 2009, 161 (Suppl. 3), 40–45. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Lee, W.; Slutsky, L.; Clark, R.A.; Permodet, N.; Rafailovich, M.H. Adverse effects of titanium dioxide nanoparticles on human dermal fibroblasts and how to protect cells. Small 2009, 5, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Filipe, P.; Silva, J.N.; Silva, R.; Cirne de Castro, J.L.; Marques Gomes, M.; Alves, L.C.; Santus, R.; Pinheiro, T. Stratum corneum is an effective barrier to TiO2 and ZnO nanoparticle percutaneous absorption. Skin Pharmacol. Phys. 2009, 22, 266–275. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: A millenium perspective. J. Cell Biochem. 2003, 88, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Marks, R.; Foley, P.A.; Jolley, D.; Knight, K.R.; Thompson, S.C. The effect of regular sunscreen use on vitamin D levels in an Australian population. Arch. Dermatol. 1995, 131, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Klein, K. Sunscreen products: Formulation and regulatory considerations. Cosmet. Sci. Technol. Ser. 1997, 285–312. [Google Scholar]
- Schröder, B.; Ohrmann, R.; Issleib, M.; Endlein, E. O/W-Emulsifiers, O/W-Emulsions and Methods of Manufacture Thereof. US8961943B2, 24 February 2015. [Google Scholar]
- Smaoui, S.; Ben Hlima, H.; Ben Chobba, I.; Kadri, A. Development and stability studies of sunscreen cream formulations containing three photo-protective filters. Arab. J. Chem. 2017, 10, S1216–S1222. [Google Scholar] [CrossRef]
- Bara, I.; Mellul, M. New Cosmetic or Dermopharmaceutical Compositions in the Form of Aqueous Gels Modified by the Addition of Expanded Microspheres. U.S. Patent 5593680, 14 January 1997. [Google Scholar]
- Teng, J.; Lucas, J.M.; Stubits, M.C. Sunscreen Gel. U.S. Patent 4193989, 18 March 1980. [Google Scholar]
- Diec, K.H.; Gersbarlag, H.; Klier, M.; Schreiber, J.; Wolf, F. Cosmetic or Dermatological Gels Based on Microemulsions. U.S. Patent 6607733B1, 19 August 2003. [Google Scholar]
- Strobridge, J.R. Gel-Type Sunscreen Composition. U.S. Patent 4917882, 17 April 1990. [Google Scholar]
- Hougaz, L. Sunscreen Aerosol Spray. U.S. Patent 20090061001A1, 5 March 2009. [Google Scholar]
- Hanson, J.E.; Antonacci, C. Natural Sunscreen Composition. U.S. Patent 9056063, 16 June 2015. [Google Scholar]
- Nieuwenhuijsen, B. Composition of a Water-Soluble Sunscreen Preparation for Acne Rosacea. U.S. Patent 8216555, 10 July 2012. [Google Scholar]
- Rosenthal, A.; Stoddard, M.; Chipps, L.; Herrmann, J. Skin cancer prevention: A review of current topical options complementary to sunscreens. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1261–1267. [Google Scholar] [CrossRef]
- Megna, M.; Lembo, S.; Balato, N.; Monfrecola, G. “Active” photoprotection: Sunscreens with DNA repair enzymes. G. Ital. Dermatol. Venereol. 2017, 152, 302–307. [Google Scholar]
- Emanuele, E.; Altabas, V.; Altabas, K.; Berardesca, E. Topical application of preparations containing DNA repair enzymes prevents ultraviolet-induced telomere shortening and c-FOS proto-oncogene hyperexpression in human skin: An experimental pilot study. J. Drugs Dermatol. 2013, 12, 1017–1021. [Google Scholar]
- Carducci, M.; Pavone, P.S.; De, G.M.; Lovati, S.; Altabas, V.; Altabas, K.; Emanuele, E. Comparative effects of sunscreens alone vs sunscreens plus DNA repair enzymes in patients with actinic keratosis: Clinical and molecular findings from a 6-month, randomized, clinical study. J. Drugs Dermatol. 2015, 14, 986–990. [Google Scholar] [PubMed]
- Kuraoka, I. Diversity of endonuclease V: From DNA repair to RNA editing. Biomolecules 2015, 5, 2194–2206. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, M.; Herrmann, J.; Moy, L.; Moy, R. Improvement of actinic keratoses using topical DNA repair enzymes: A randomized placebo-controlled trial. J. Drugs Dermatol. 2017, 16, 1030–1034. [Google Scholar] [PubMed]
- Liu, Z.; Tan, C.; Guo, X.; Kao, Y.-T.; Li, J.; Wang, L.; Sancar, A.; Zhong, D. Dynamics and mechanism of cyclobutane pyrimidine dimer repair by DNA photolyase. Proc. Natl. Acad. Sci. USA 2011, 108, 14831–14836. [Google Scholar] [CrossRef] [PubMed]
- Stege, H.; Roza, L.; Vink, A.A.; Grewe, M.; Ruzicka, T.; Grether-Beck, S.; Krutmann, J. Enzyme plus light therapy to repair DNA damage in ultraviolet-B-irradiated human skin. Proc. Natl. Acad. Sci. USA 2000, 97, 1790–1795. [Google Scholar] [CrossRef] [PubMed]
- Berardesca, E.; Bertona, M.; Altabas, K.; Altabas, V.; Emanuele, E. Reduced ultraviolet-induced DNA damage and apoptosis in human skin with topical application of a photolyase-containing DNA repair enzyme cream: Clues to skin cancer prevention. Mol. Med. Rep. 2012, 5, 570–574. [Google Scholar]
- Huang, X.X.; Scolyer, R.A.; Abubakar, A.; Halliday, G.M. Human 8-oxoguanine-DNA glycosylase-1 is downregulated in human basal cell carcinoma. Mol. Genet. Metab. 2012, 106, 127–130. [Google Scholar] [CrossRef]
- Wulff, B.C.; Schick, J.S.; Thomas-Ahner, J.M.; Kusewitt, D.F.; Yarosh, D.B.; Oberyszyn, T.M. Topical treatment with OGG1 enzyme affects UVB-induced skin carcinogenesis. Photochem. Photobiol. 2008, 84, 317–321. [Google Scholar] [CrossRef]
- Kuse, Y.; Ogawa, K.; Tsuruma, K.; Shimazawa, M.; Hara, H. Damage of photoreceptor-derived cells in culture induced by light emitting diode-derived blue light. Sci. Rep. 2014, 4, 5223. [Google Scholar] [CrossRef]
- Mark, J.R.; Gelpi-Hammerschmidt, F.; Trabulsi, E.J.; Gomella, L.G. Blue light cystoscopy for detection and treatment of non-muscle invasive bladder cancer. Can. J. Urol. 2012, 19, 6227–6231. [Google Scholar]
- Brown, S.B.; Brown, E.A.; Walker, I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004, 5, 497–508. [Google Scholar] [CrossRef]
- Barolet, D.; Christiaens, F.; Hamblin, M.R. Infrared and skin: Friend or foe. J. Photochem. Photobiol. B 2016, 155, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Bae, J.; Lee, S.E.; Lee, J.B.; Park, C.H.; Lim, D.H.; Park, M.S. A novel in vivo test method for evaluating the infrared radiation protection provided by sunscreen products. Skin Res. Technol. 2019, 0, 1–6. [Google Scholar]
- Alan, K. FDA Regulations for Sunscreens. 2016. Available online: https://www.sansoleil.com/FDA-Regulations-for-Sunscreens_b_2.html (accessed on 14 February 2019).
| Compounds | Protection Mechanism |
|---|---|
| Vitamin C | |
| Vitamin E |
|
| Phenolic compounds | |
| Flavonoid compounds | |
| Carotenoids |
| DNA Repair Enzymes | Proposal Mechanism and Proven Effects |
|---|---|
| Topical T4 endonuclease | |
| Photolyase |
|
| 8-Oxoguanine glycosylase |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngoc, L.T.N.; Tran, V.V.; Moon, J.-Y.; Chae, M.; Park, D.; Lee, Y.-C. Recent Trends of Sunscreen Cosmetic: An Update Review. Cosmetics 2019, 6, 64. https://doi.org/10.3390/cosmetics6040064
Ngoc LTN, Tran VV, Moon J-Y, Chae M, Park D, Lee Y-C. Recent Trends of Sunscreen Cosmetic: An Update Review. Cosmetics. 2019; 6(4):64. https://doi.org/10.3390/cosmetics6040064
Chicago/Turabian StyleNgoc, Le Thi Nhu, Vinh Van Tran, Ju-Young Moon, Minhe Chae, Duckshin Park, and Young-Chul Lee. 2019. "Recent Trends of Sunscreen Cosmetic: An Update Review" Cosmetics 6, no. 4: 64. https://doi.org/10.3390/cosmetics6040064
APA StyleNgoc, L. T. N., Tran, V. V., Moon, J.-Y., Chae, M., Park, D., & Lee, Y.-C. (2019). Recent Trends of Sunscreen Cosmetic: An Update Review. Cosmetics, 6(4), 64. https://doi.org/10.3390/cosmetics6040064