Analysis of Heavy Metal Content in Conventional and Herbal Toothpastes Available at Maltese Pharmacies
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Reagents and Solutions
2.3. Preliminary Treatment of Samples
2.4. Measurements
2.5. Statistical Methods
3. Results and Discussion
3.1. Heavy Metal Analysis
3.1.1. Silver
3.1.2. Chromium
3.1.3. Copper
3.1.4. Nickel
3.1.5. Lead
3.1.6. Tin
3.1.7. Zinc
3.1.8. Iron
3.1.9. Manganese
3.1.10. Cadmium and Mercury
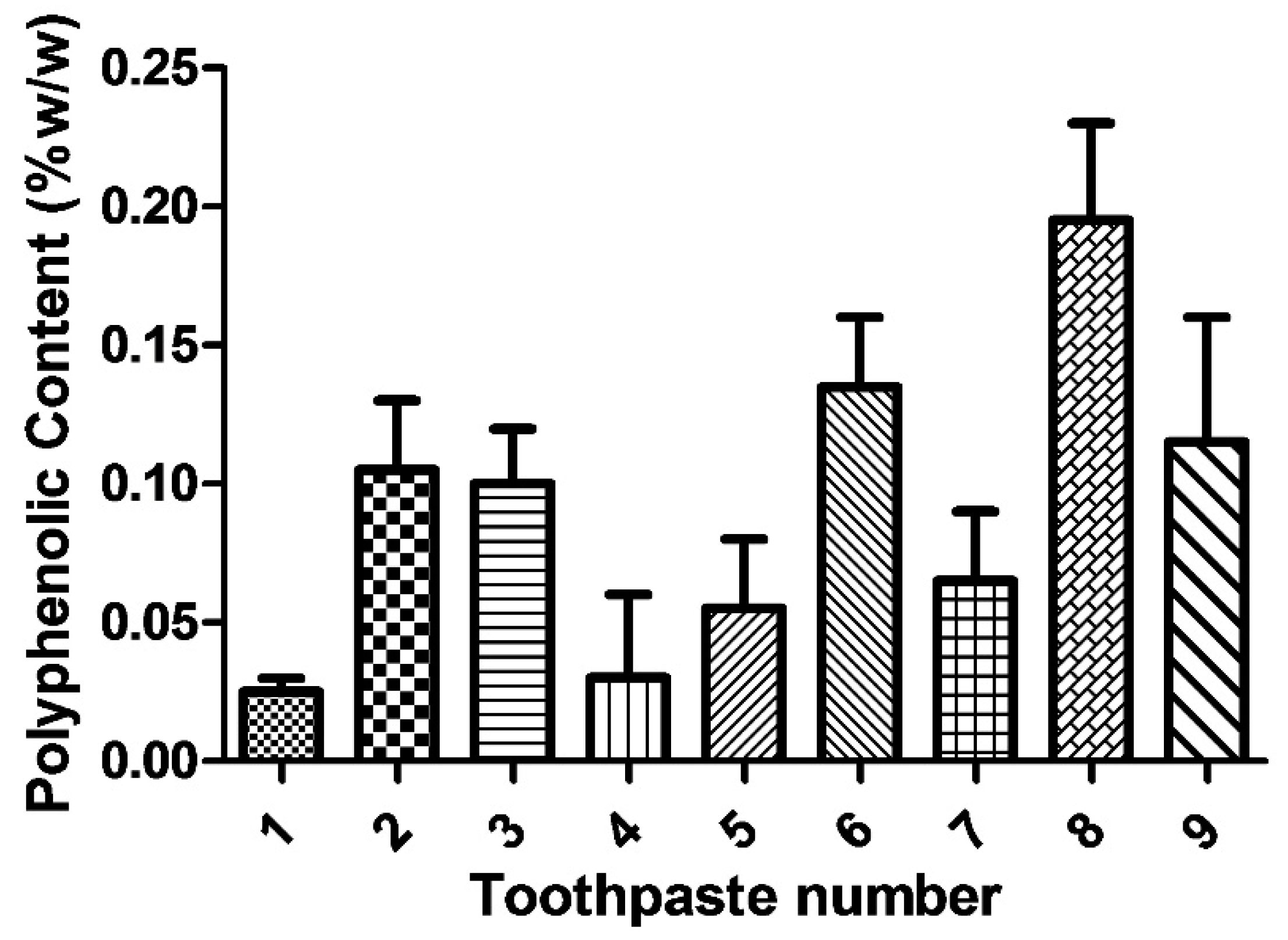
3.2. Polyphenolic Content
3.3. Correlation between Presence of Polyphenols and Heavy Metals in Toothpaste
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Łodyga-Chruścińska, E.; Sykuła, A.; Więdłocha, M. Hidden Metals in Several Brands of Lipstick and Face Powder Present on Polish Market. Cosmetics 2018, 5, 57. [Google Scholar] [CrossRef]
- Marinovich, M.; Boraso, M.S.; Testai, E.; Galli, C.L. Metals in cosmetics: An a posteriori safety evaluation. Regul. Toxicol. Pharmacol. 2014, 69, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Bocca, B.; Pino, A.; Alimonti, A.; Forte, G. Toxic metals contained in cosmetics: A status report. Regul. Toxicol. Pharmacol. 2014, 68, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Ratz-Łyko, A.; Arct, J.; Majewski, S.; Pytkowska, K. Influence of polyphenols on the physiological processes in the skin. Phytother. Res. 2015, 29, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Anunciato, T.P.; da Rocha Filho, P.A. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J. Cosmet. Dermatol. 2012, 11, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, D.A.; Tillman, M.D.; Hubbs, L.M. Limit of detection (LOD)/limit of quantitation (LOQ): Comparison of the empirical and the statistical methods exemplified with GC-MS assays of abused drugs. Clin. Chem. 1994, 40, 1233–1238. [Google Scholar] [PubMed]
- Attard, E. A rapid microtitre plate Folin-Ciocalteu method for the assessment of polyphenols. Open Life Sci. 2013, 8, 48–53. [Google Scholar] [CrossRef]
- Duffus, J.H. “Heavy metals” A meaningless term? Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef]
- Tsankov, I.U.; Iordanova, I.; Lolova, D.; Uzunova, S.; Dinoeva, S. Hygienic evaluation of the content of heavy metals (lead and copper) in cosmetic products. Probl. Khigienata 1982, 7, 127–136. [Google Scholar]
- Filon, F.L.; D’Agostin, F.; Crosera, M.; Adami, G.; Bovenzi, M.; Maina, G. In vitro absorption of metal powders through intact and damaged human skin. Toxicol. In Vitro 2009, 23, 574–579. [Google Scholar] [CrossRef]
- Larese, F.; Gianpietro, A.; Venier, M.; Maina, G.; Renzi, N. In vitro percutaneous absorption of metal compounds. Toxicol. Lett. 2007, 170, 49–56. [Google Scholar] [CrossRef]
- Official Journal of the European Union. Regulation (EC) No 1881/2006 of 19 December 2006 on Setting Maximum Levels for Certain Contaminants in Foodstuffs. 2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1881&from=EN (accessed on 29 March 2019).
- Joint FAO/WHO Expert Committee on Food Additives. Meeting. (57th: Rome, Italy, 2001). Safety Evaluation of Certain Food Additives and Contaminants/Prepared by the Fifty-Seventh Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); World Health Organization: Geneva, Switzerland, 2002; Available online: https://www.who.int/foodsafety/areas_work/chemical-risks/jecfa/en/ (accessed on 29 March 2019).
- Chan, E. Health Canada Published Guidelines on Heavy Metal Impurities in Cosmetics. Sparkle Volume 632. 2012. Available online: http://www.intertek.co.th/uploadedFiles/Intertek/Divisions/Consumer_Goods/Media/PDFs/Sparkles/2012/sparkle632.pdf (accessed on 29 March 2019).
- Whitehouse, L. Germany Reduces Heavy Metal Limits in Cosmetics. 2017. Available online: https://www.cosmeticsdesign-europe.com/Article/2017/07/25/Germany-reduces-heavy-metal-limits-in-cosmetics (accessed on 29 March 2019).
- U.S. Food and Drug Administration. FDA’s Testing of Cosmetics for Arsenic, Cadmium, Chromium, Cobalt, Lead, Mercury, and Nickel Content. 2018. Available online: https://www.fda.gov/Cosmetics/ProductsIngredients/PotentialContaminants/ucm452836.htm (accessed on 29 March 2019).
- Yee, R.; Holmgren, C.; Mulder, J.; Lama, D.; Walker, D.; van Palenstein Helderman, W. Efficacy of silver diamine fluoride for arresting caries treatment. J. Dent. Res. 2009, 88, 644–647. [Google Scholar] [CrossRef]
- Umar, M.A.; Caleb, H. Analysis of metals in some cosmetic products in FCT-Abuja, Nigeria. Int. J. Cosmet. Sci. 2013, 3, 14–18. [Google Scholar]
- Ideriah, T.J.; Odunwo, C.C.; Tamuno-opurbo, D.E. Assessment of Fluoride and Heavy Metals Concentrations in Toothpastes Marketed in Port Harcourt Nigeria. Open Access J. Sci. 2018, 2, 233–236. [Google Scholar]
- Odukudu, F.B.; Ayenimo, J.G.; Adekunle, A.S.; Yusuff, A.M.; Mamba, B.B. Safety evaluation of heavy metals exposure from consumer products. Int. J. Consum. Stud. 2014, 38, 25–34. [Google Scholar] [CrossRef]
- Orisakwe, O.E.; Okolo, K.O.; Igweze, Z.N.; Ajaezi, G.C.; Udowelle, N.A. Potential hazards of toxic metals found in toothpastes commonly used in Nigeria. Rocz. Panstw. Zakl. Hig. 2016, 67, 197–204. [Google Scholar] [PubMed]
- Hepp, N.M.; Mindak, W.R.; Gasper, J.W.; Thompson, C.B.; Barrows, J.N. Survey of cosmetics for arsenic, cadmium, chromium, cobalt, lead, mercury, and nickel content. J. Cosmet. Sci. 2014, 65, 125–145. [Google Scholar] [PubMed]
- Official Journal of the European Union. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. 2009. Available online: Eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:342:0059:0209:en:PDF (accessed on 29 March 2019).
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Guidelines for Carcinogen Risk Assessment. 2016. Available online: https://www.epa.gov/risk/guidelines-carcinogen-risk-assessment (accessed on 29 March 2019).
- Jan, A.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef]
- Rao, R.N.; Rao, T.N. Determination of heavy metals in toothpastes containing tin as an active ingredient. Indian J. Chem. Technol. 2014, 21, 238–243. [Google Scholar]
- Mahurpawar, M. Effects of heavy metals on human health. Int. J. Res. Granthaalayah 2015, 1, 7. [Google Scholar]
- Gunsolley, J.C. A meta-analysis of six-month studies of antiplaque and antigingivitis agents. J. Am. Dent. Assoc. 2006, 137, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
- Lynch, R.J. Zinc in the mouth, its interactions with dental enamel and possible effects on caries; a review of the literature. Int. Dent. J. 2011, 61, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Neal, A.P.; Guilarte, T.R. Mechanisms of lead and manganese neurotoxicity. Toxicol. Res. 2013, 2, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, B.R.; Garai, A.; Deb, M.; Bhattacharya, S. Herbal toothpaste: A possible remedy for oral cancer. J. Nat. Prod. 2013, 6, 44–55. [Google Scholar]
- Anghileri, L.J.; Thouvenot, P. Natural polyphenols-iron interaction. Biol. Trace Elem. Res. 2000, 73, 251–258. [Google Scholar] [CrossRef]
- Official Journal of the European Union. Directive (EEC) No 76/768 of 27 July 1976 on the Approximation of the Laws of the Member States Relating to Cosmetic Products. 1976. Available online: https://publications.europa.eu/en/publication-detail/-/publication/5a1f70d8-77f7-4a4f-9de7-d5fb9913d91f/language-en (accessed on 29 March 2019).
| Element | Wavelength (nm) | R2 | LOD (mg/kg) | LOQ (mg/kg) |
|---|---|---|---|---|
| Ag | 328.068 | 0.9746 | 0.0549 | 0.1665 |
| Cd | 228.802 | 0.9993 | 0.0067 | 0.0204 |
| Cr | 425.433 | 0.9975 | 0.0005 | 0.0014 |
| Cu | 324.754 | 0.9996 | 0.0007 | 0.0022 |
| Fe | 259.940 | 1.0000 | 0.0037 | 0.0113 |
| Hg | 253.652 | 0.9968 | 0.0789 | 0.2391 |
| Mn | 403.076 | 0.9288 | 0.0042 | 0.0127 |
| Ni | 352.454 | 0.9987 | 0.0056 | 0.0169 |
| Pb | 405.781 | 1.0000 | 0.0169 | 0.0511 |
| Sn | 317.505 | 0.9852 | 0.0375 | 0.1137 |
| Zn | 213.857 | 1.0000 | 0.0301 | 0.0912 |
| Nebulizer | One-neb (concentric) |
| Spray chamber | Cyclonic spray chamber, single pass |
| Calibration Correlation Coefficient Limit | 0.9999 |
| Pump speed (rpm) | 15 |
| Number of replicates | 3 |
| Stabilization time (s) | 15 |
| Uptake time (s) | 15 |
| Available International Standards | Lead | Cadmium | Mercury | Nickel | Chromium |
|---|---|---|---|---|---|
| EU* [12] | 0.1 | 0.05 | - | - | - |
| WHO [13] | 2 | 2 | - | - | - |
| Canada [14] | 10 | 3 | 3 | - | - |
| Germany [15] | 0.5 | 0.1 | 0.1 | - | - |
| US FDA [16] | 10 | - | 1 | - | 50 |
| Toothpaste Category and Sample No. | Mean Value Ag (ppm) | Mean Value Cd (ppm) | Mean Value Cr (ppm) | Mean Value Cu (ppm) | Mean Value Hg (ppm) | Mean Value Ni (ppm) | Mean Value Pb (ppm) | Mean Value Sn (ppm) | Mean Value Zn (ppm) | Mean Value Fe (ppm) | Mean Value Mn (ppm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Conventional 1 | 5.29 ± 0.018 | 0.00 ± 0.000 | 1.42 ± 0.012 | 1.35 ± 0.006 | 0.00 ± 0.000 | 1.96 ± 0.007 | 3.26 ± 0.152 | 134.6 ± 0.253 | 1842 ± 3.550 | 1.76 ± 0.012 | 0.20 ± 0.015 |
| Conventional 2 | 3.36 ± 0.017 | 0.00 ± 0.000 | 7.35 ± 0.019 | 3.68 ± 0.003 | 0.00 ± 0.000 | 1.31 ± 0.006 | 8.83 ± 0.019 | 9671 ± 8.400 | 2417 ± 0.859 | 17.68 ± 0.0088 | 0.72 ± 0.003 |
| Conventional 3 | 3.26 ± 0.035 | 0.00 ± 0.000 | 1.35 ± 0.026 | 0.84 ± 0.003 | 0.00 ± 0.000 | 1.34 ± 0.020 | 2.37 ± 0.064 | 104.7 ± 1.47 | 7.80 ± 1.69 | 12.33 ± 0.000 | 0.66 ± 0.023 |
| Herbal 4 | 5.12 ± 0.020 | 0.00 ± 0.000 | 0.72 ± 0.010 | 1.40 ± 0.003 | 0.00 ± 0.000 | 0.43 ± 0.0033 | 12.04 ± 0.079 | 178.3 ± 0.412 | 2.90 ± 0.072 | 4.50 ± 0.058 | 0.52 ± 0.006 |
| Herbal 5 | 2.23 ± 0.089 | 0.00 ± 0.000 | 0.67 ± 0.012 | 1.26 ± 0.006 | 0.00 ± 0.000 | 1.55 ± 0.021 | 2.23 ± 0.050 | 86.69 ± 1.280 | 3.66 ± 0.051 | 7.84 ± 0.017 | 1.70 ± 0.015 |
| Herbal 6 | 3.10 ± 0.081 | 0.00 ± 0.000 | 0.65 ± 0.015 | 1.16 ± 0.000 | 0.00 ± 0.000 | 1.99 ± 0.032 | 4.33 ± 0.133 | 110.0 ± 0.386 | 6.32 ± 0.076 | 9.68 ± 0.009 | 1.54 ± 0.020 |
| Children 7 | 2.00 ± 0.015 | 0.00 ± 0.000 | 0.4 ± 0.023 | 0.81 ± 0.000 | 0.00 ± 0.000 | 1.73 ± 0.013 | 2.64 ± 0.117 | 82.99 ± 0.307 | 0.31 ± 0.01 | 8.52 ± 0.017 | 2.07 ± 0.031 |
| Children 8 | 3.44 ± 0.029 | 0.00 ± 0.000 | 0.28 ± 0.010 | 0.73 ± 0.003 | 0.00 ± 0.000 | 1.15 ± 0.009 | 4.76 ± 0.062 | 104.4 ± 0.113 | 3.12 ± 0.074 | 10.20 ± 0.009 | 1.13 ± 0.027 |
| Children 9 | 3.82 ± 0.052 | 0.00 ± 0.000 | 0.86 ± 0.009 | 1.37 ± 0.003 | 0.00 ± 0.000 | 2.54 ± 0.035 | 4.72 ± 0.065 | 99.82 ± 0.798 | 0.00 ± 0.000 | 7.56 ± 0.007 | 1.20 ± 0.023 |
| Variables | Cr | Cu | Ni | Pb | Sn | Zn | Fe | Mn | PolyP |
|---|---|---|---|---|---|---|---|---|---|
| Ag | 0.037 | 0.115 | −0.201 | 0.526 | −0.044 | 0.340 | −0.513 | −0.867 | −0.337 |
| Cr | 0.960 | −0.119 | 0.397 | 0.985 | 0.825 | 0.680 | −0.337 | 0.019 | |
| Cu | −0.097 | 0.521 | 0.958 | 0.799 | 0.548 | −0.310 | −0.073 | ||
| Ni | −0.617 | −0.160 | 0.032 | −0.113 | 0.312 | 0.127 | |||
| Pb | 0.438 | 0.236 | 0.078 | −0.434 | −0.135 | ||||
| Sn | 0.769 | 0.723 | −0.226 | 0.096 | |||||
| Zn | 0.236 | −0.538 | −0.221 | ||||||
| Fe | 0.154 | 0.562 | |||||||
| Mn | 0.262 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vella, A.; Attard, E. Analysis of Heavy Metal Content in Conventional and Herbal Toothpastes Available at Maltese Pharmacies. Cosmetics 2019, 6, 28. https://doi.org/10.3390/cosmetics6020028
Vella A, Attard E. Analysis of Heavy Metal Content in Conventional and Herbal Toothpastes Available at Maltese Pharmacies. Cosmetics. 2019; 6(2):28. https://doi.org/10.3390/cosmetics6020028
Chicago/Turabian StyleVella, Andrew, and Everaldo Attard. 2019. "Analysis of Heavy Metal Content in Conventional and Herbal Toothpastes Available at Maltese Pharmacies" Cosmetics 6, no. 2: 28. https://doi.org/10.3390/cosmetics6020028
APA StyleVella, A., & Attard, E. (2019). Analysis of Heavy Metal Content in Conventional and Herbal Toothpastes Available at Maltese Pharmacies. Cosmetics, 6(2), 28. https://doi.org/10.3390/cosmetics6020028