Iron Gall Ink Revisited: Natural Formulation for Black Hair-Dyeing
Abstract
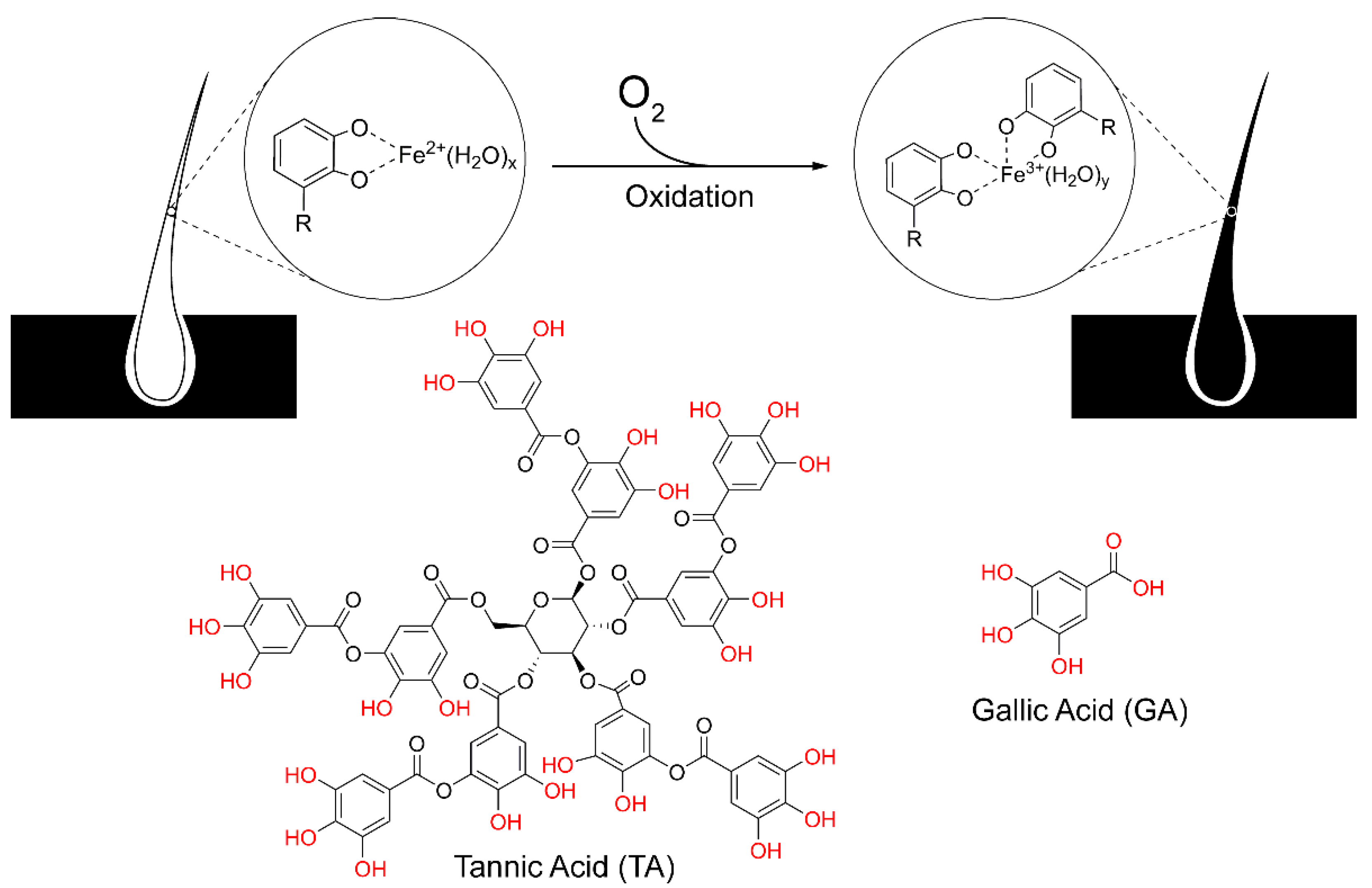
1. Introduction
2. Materials and Methods
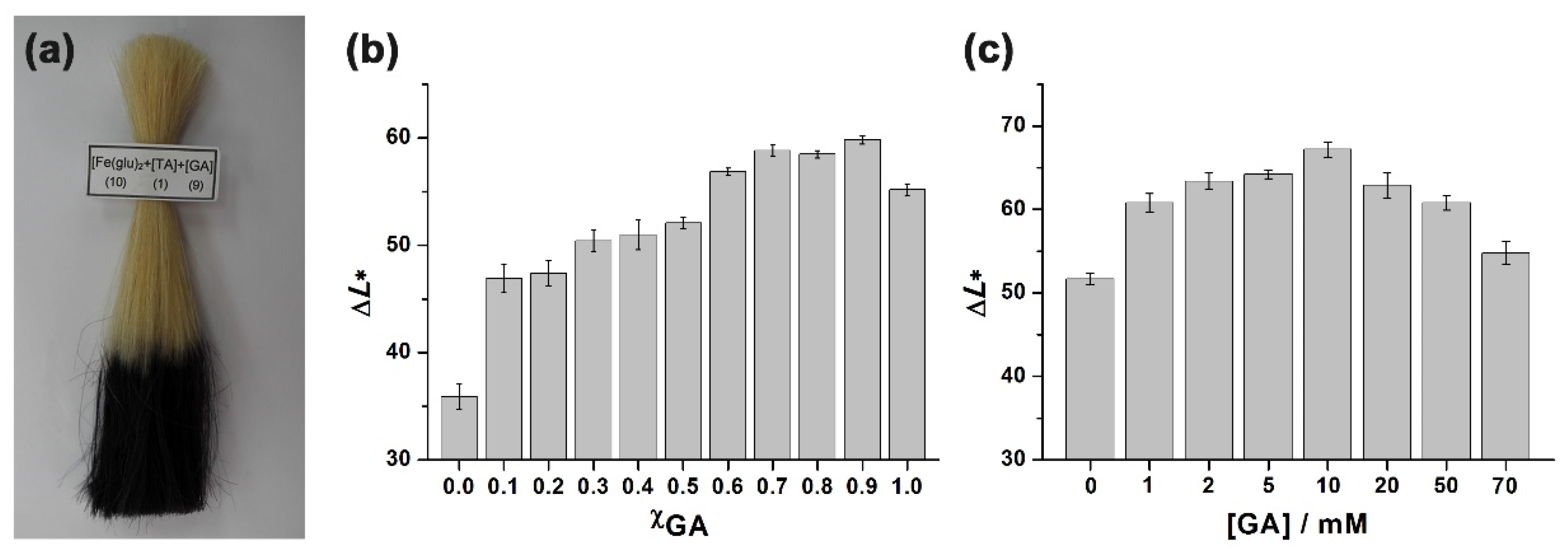
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, T.; Kim, J.Y.; Cho, H.; Moon, H.C.; Kim, B.J.; Park, J.H.; Hong, D.; Park, J.; Choi, I.S. Artificial spores: Immunoprotective nanocoating of red blood cells with supramolecular ferric ion-tannic acid complex. Polymers 2017, 9, 140. [Google Scholar] [CrossRef]
- Lee, J.; Cho, H.; Choi, J.; Kim, D.; Hong, D.; Park, J.H.; Yang, S.H.; Choi, I.S. Chemical sporulation and germination: Cytoprotective nanocoating of individual mammalian cells with a degradable tannic acid-FeIII complex. Nanoscale 2015, 7, 18918–18922. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Bing, W.; Huang, S.; Ren, J.; Qu, X. Mussel byssus-like reversible metal-chelated supramolecular complex used for dynamic cellular surface engineering and imaging. Adv. Funct. Mater. 2015, 24, 3775–3784. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, K.; Lee, J.; Choi, J.Y.; Hong, D.; Yang, S.H.; Caruso, F.; Lee, Y.; Choi, I.S. A cytoprotective and degradable metal-polyphenol nanoshell for single-cell encapsulation. Angew. Chem. Int. Ed. 2014, 53, 12420–12425. [Google Scholar] [CrossRef]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; van Koeverden, M.P.; Such, G.K.; Cui, J.; Caruso, F. One-step assembly of coordination complexes for versatile film and particle engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Bat-Yehouda, M.Z. Les Encres Noires au Moyen Age (jusqu’à 1600) [Black Inks in the Middle Ages (until 1600)]; Centre National de la Recherche Scientifique: Paris, France, 1983; pp. 96–97.
- Pone, A.; Brostoff, L.B.; Gibbons, S.K.; Zavalij, P.; Virgah, C.; Hooper, J.; Alnemart, S.; Gaskell, K.J.; Eichhorn, B. Elucidation of the Fe(III) gallate structure in historical iron gall ink. Anal. Chem. 2016, 88, 5152–5258. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Wang, H.C.; DeGuire, S.N.; Jenkins, M.; Lawson, M.; Brumaghim, J.L. Kinetics of iron oxidation upon polyphenol binding. Dalton Trans. 2010, 39, 9982–9987. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Hodges, J.N.; Jenkins, M.; Brumaghim, J.L. Predicting how polyphenol antioxidants prevent DNA damage by binding to iron. Inorg. Chem. 2008, 47, 6153–6161. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, W.I.; Youn, W.; Park, T.; Lee, S.; Kim, T.-S.; Mano, J.F.; Choi, I.S. Iron gall ink revisited: in situ oxidation of Fe(II)-tannin complex for fluidic-interface engineering. Adv. Mater. 2018, 30, 1805091. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Lee, J.K.; Choi, I.S. Iron gall ink revisited: hierarchical formation of Fe(III)-tannic acid coacervate particles in microdroplets for protein condensation. Chem. Commun. 2019, 55, 2142–2145. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Welcomme, E.; Hallégot, P.; Zaluzec, N.J.; Deeb, C.; Castaing, J.; Veyssière, P.; Bréniaux, R.; Lévêque, J.L.; Tsoucaris, G. Early use of PbS nanotechnology for an ancient hair dyeing formula. Nano Lett. 2006, 6, 2215–2219. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhou, L.; Chiou, K.; Huang, J. Multifunctional graphene hair dye. Chem 2018, 4, 784–794. [Google Scholar] [CrossRef]
- Billmeyer, F.W.; Saltzman, M. Principles of Color Technology, 2nd ed.; Wiley-Interscience: New York, NY, USA, 1981. [Google Scholar]
- Park, T.; Kim, W.I.; Kim, B.J.; Lee, H.; Choi, I.S.; Park, J.H.; Cho, W.K. Salt-induced, continuous deposition of supramolecular iron(III)-tannic acid complex. Langmuir 2018, 34, 12318–12323. [Google Scholar] [CrossRef] [PubMed]
- Covington, A.D. Modern tanning chemistry. Chem. Soc. Rev. 1997, 26, 111–126. [Google Scholar] [CrossRef]
- Andersen, A.; Krogsgaard, M.; Birkedal, H. Mussel-inspired self-healing double-cross-linked hydrogels by controlled combination of metal coordination and covalent cross-linking. Biomacromolecules 2018, 19, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Cuerra, E.; Llompart, M.; Garcia-Jares, C. Analysis of dyes in cosmetics: challenges and recent developments. Cosmetics 2018, 5, 47. [Google Scholar]
- Goossens, A. Cosmetic contact allergens. Cosmetics 2016, 3, 5. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, S.; Moon, H.C.; Seo, H.; Kim, J.Y.; Hong, S.-P.; Lee, B.S.; Kang, E.; Lee, J.; Ryu, D.H.; et al. Antimicrobial spray nanocoating of supramolecular Fe(III)-tannic acid metal-organic coordination complex: applications to shoe insoles and fruits. Sci. Rep. 2017, 7, 6980. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Ebato, A.; Napolitano, A.; Koike, K. The late stages of melanogenesis: Exploring the chemical facets and the application opportunities. Int. J. Mol. Sci. 2018, 19, 1753. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
| χGA | Before Dyeing | After Dyeing | Sunlight Exposure | |||
|---|---|---|---|---|---|---|
| 1 d | 2 d | 5 d | 90 d | |||
| 0.0 | 82.1 ± 0.4 | 46.2 ± 0.8 | 46.8 ± 0.7 | 47.1 ± 0.5 | 49.3 ± 0.6 | 49.3 ± 0.5 |
| 0.9 | 80.9 ± 0.5 | 21.1 ± 0.4 | 21.3 ± 0.3 | 21.3 ± 0.3 | 22.3 ± 0.5 | 22.5 ± 0.3 |
| 1.0 | 80.0 ± 0.5 | 24.8 ± 0.5 | 25.0 ± 0.7 | 25.4 ± 1.0 | 25.9 ± 1.2 | 25.8 ± 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.Y.; Hong, S.-P.; Kang, E.K.; Kim, B.J.; Lee, H.; Kim, W.I.; Choi, I.S. Iron Gall Ink Revisited: Natural Formulation for Black Hair-Dyeing. Cosmetics 2019, 6, 23. https://doi.org/10.3390/cosmetics6020023
Han SY, Hong S-P, Kang EK, Kim BJ, Lee H, Kim WI, Choi IS. Iron Gall Ink Revisited: Natural Formulation for Black Hair-Dyeing. Cosmetics. 2019; 6(2):23. https://doi.org/10.3390/cosmetics6020023
Chicago/Turabian StyleHan, Sang Yeong, Seok-Pyo Hong, Eunhye K. Kang, Beom Jin Kim, Hojae Lee, Won Il Kim, and Insung S. Choi. 2019. "Iron Gall Ink Revisited: Natural Formulation for Black Hair-Dyeing" Cosmetics 6, no. 2: 23. https://doi.org/10.3390/cosmetics6020023
APA StyleHan, S. Y., Hong, S.-P., Kang, E. K., Kim, B. J., Lee, H., Kim, W. I., & Choi, I. S. (2019). Iron Gall Ink Revisited: Natural Formulation for Black Hair-Dyeing. Cosmetics, 6(2), 23. https://doi.org/10.3390/cosmetics6020023






